Abstract
C11H21N3, monoclinic, P21/c (no. 14), a = 11.2439(9) Å, b = 9.7110(6) Å, c = 11.9008(9) Å, β = 112.832(9)∘, V = 1197.63(17) Å3, Z = 4, Rgt(F) = 0.0775, wRref(F2) = 0.2318, T = 178 K.
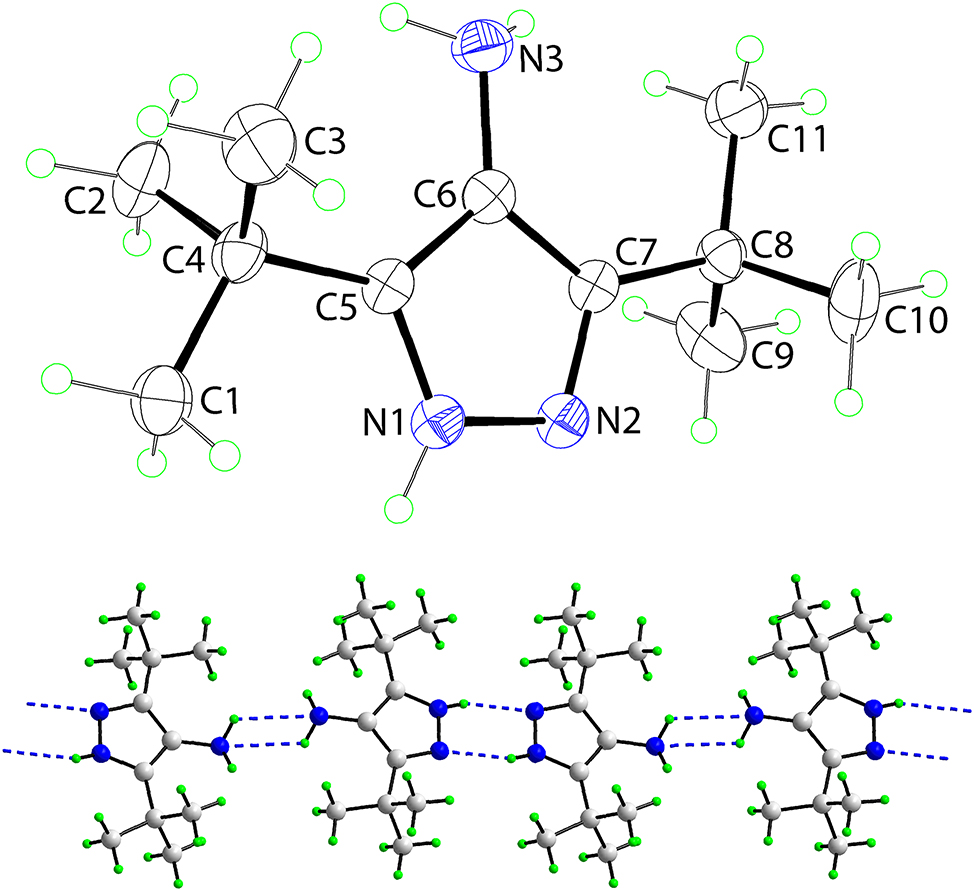
The molecular structure is shown in the figure. Table 1 contains crystallographic data.
1 Source of material
The synthesis of (I) was accomplished in a three-step process. LHpzH, 3,5-bis(t-butyl)-1H-pyrazole, was obtained by a modified literature method, 5 by the reaction of 2,2,6,6-tetramethyl-3,5-heptanedione (10.0 g, 54.3 mmol) with hydrazine monohydrate (8.8 mL, 181 mmol, 3.3 equiv.) in ethanol (35 mL) at 373 K for 8 h. After the reaction, the solution was dried under vacuum. Crystallization was carried out at 273 K from dichloromethane solution to yield colourless crystals (8.71 g, 48.3 mmol, 89 % yield). 1H NMR (CDCl3, 500 MHz): δ 1.30 (s, 18H, C(CH3)3), 5.90 (s, 1H, pz-4H), amine-NH not observed. IR (KBr, cm−1): 3234 s ν(N–H), 2966 s ν(C–H), 2904 s ν(C–H), 2866 s ν(C–H), 1570 s ν(C=N). LNO 2 pzH, 3,5-bis(t-butyl)-1H-pyrazol-4-nitro, was obtained by a modified literature method. 6 The nitration reaction of LHpzH (5.00 g, 27.7 mmol) was achieved using a mixture of concentrated nitric acid (8 mL) and concentrated sulfuric acid (18 mL). After heating at 373 K for 3 h, the cooled reaction mixture was neutralised with sodium hydroxide solution. After the filtration of the precipitated yellow-white powder, the obtained powder was carefully washed with distilled water. Crystallization from dichloromethane solution at 273 K yielded pale-yellow crystals (4.38 g 19.4 mmol, 70 % yield). Anal. calcd. for C11H19N3O2: C 58.64, H 8.50, N 18.65 %. Found: C 58.43, H 8.68, N 18.70 %. 1H NMR (CDCl3, 500 MHz): δ 1.40 (s, 18H, C(CH3)3), NH not observed. IR (KBr, cm−1): 3288 s ν(N–H), 2965 s ν(C–H), 2932 s ν(C–H), 2872 s ν(C–H), 1561 s ν(C=N). LNH2pzH 3,5-bis(t-butyl)-1H-pyrazol-4-amine (I), was obtained by a hydrogenation reaction with palladium on carbon (10 %) catalyst under H2 (1 atm). After drying, LNO2pzH (1.00 g, 4.44 mmol) and Pd/C catalyst (1.0 g) were suspended in methanol (30 mL), and the atmosphere was changed from Ar to H2. The reaction was performed for 45 h at room temperature. The obtained reaction mixture was filtered through Celite to remove undissolved solids. The filtrate was crystallized at 243 K to yield a colourless powder (0.54 g, 2.76 mmol, 62 % yield). Crystals suitable for single crystal analysis were obtained from its methanol solution held at 243 K. Anal. calcd. for C11H21N3·1/3(H2O) (bulk material): C 65.63, H 10.85, N 20.87 %. Found: C 65.86, H 10.84, N 20.64 %. 1 H NMR (CDCl3, 500 MHz): δ 1.30 (s, 18H, C(CH3)3), NH not observed. IR (KBr, cm−1): 3424 s ν(NH2), 3284 s ν(N–H), 2962 s ν(C–H), 2930 s ν(C–H), 2867 s ν(C–H), 1577 s ν(C=N).
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.39 × 0.24 × 0.17 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.07 mm−1 |
| Diffractometer, scan mode: | Rigaku XtaLAB P200, ω scan |
| θmax, completeness: | 27.5°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 8,825, 2,739, 0.037 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1971 |
| N(param)refined: | 158 |
| Programs: | CrysAlisPRO, 1 Il Milone 2 , Shelx 3 , WinGx 4 |
2 Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.98 Å) and refined as riding with Uiso (H) = 1.5Ueq (C). The coordinates of the N-bound H atom were refined freely and with Uiso (H) = 1.2Ueq (N). Near the conclusion of the refinement, three residual electron density peaks, in positions rotated by approximately 60∘ to the C8-t-butyl group were evident. These were refined isotropically. The second orientation of the C8-t-butyl group converged with a site occupancy = 0.151(6). Owing to poor agreement, one reflection, i.e. (−1 3 3), was omitted from the final cycles of refinement.
3 Discussion
The functionalisation of pyrazole, an important five-membered heterocycle containing two adjacent nitrogen atoms, is an attractive research area with much work accomplished over the last few decades. Recent work has focussed upon incorporating an amino group as a functional substituent in the ring. Thus, the structure of 3,5-bis(propan-2-yl)-1H-pyrazol-4-amine (II) (denoted as L1NH2pzH) 7 and its cobalt dichlorido complex [CoCl2(L1NH2pzH)2] 8 were reported recently. Herein, the synthesis and structure of the title pyrazole, 3,5-bis(t-butyl)-1H-pyrazol-4-amine, LNH2pzH, (I), with more bulky tert-butyl substituents in the 3- and 5-positions of the pyrazolyl ring, are described.
After the nitration reaction of LHpzH with a mixture of concentrated nitric acid and concentrated sulfuric acid, the known compound LNO2pzH was obtained in 70 % yield. 6 The hydrogenation reaction of LNO2pzH with palladium on carbon (10 %) catalyst under an H2 (1 atm) atmosphere yielded a colourless powder of LNH2pzH in 62 % yield. The related compound (II) was also obtained from L1NO2pzH by the hydrogenation reaction with palladium on carbon (10 %) catalyst in 76 % yield and also through reduction of L1NO2pzH by iron powder/NH4Cl. 7
The IR spectrum of (I) features a characteristic absorption band at 3424 cm−1, assigned to amine–NH2 stretching and a strong absorption band at 3284 cm−1 is assigned to pyrazolyl-N-H stretching. The expected signal in the 1H NMR spectrum of (I) was 1.37 ppm (methyl-H), which was shifted compared to those of the precursors, LNO2pzH, at 1.40 ppm, and LHpzH, at 1.30 ppm. The solid-state structure determination of (I) was achieved through X-ray crystallography.
The molecular structure of (I) is illustrated in the upper view of the figure (35 % displacement ellipsoids; the disorder component of the C8-t-butyl group is omitted for clarity). The molecule comprises a planar pyrazolyl ring with the maximum deviation from the least-squares plane being 0.005(2) Å for the N2 atom; the amine-N3 atom lies 0.030(3) Å out of the plane. An amine group is bound at the ring-C6 position and the t-butyl groups are connected at the C5 and C7 positions. The confirmation of protonation at the pyrazolyl-N1 atom is manifested by the significantly wider angle at the N1 atom compared with the N2 atom, i.e. C5–N1–N2 = 111.28(19)∘ cf. C7–N2–N1 = 106.98(18)∘. Significant delocalisation of π-electron density over the five-membered ring is indicated by the experimental equivalence of the N1–C5 and N2–C7 [1.336(3) and 1.339(3) Å] and of the C5–C6 and C6–C7 [1.394(3) and 1.400(3) Å] bond lengths. The same conclusions are made for the methyl 9 and i-propyl 7 analogues. The N1–N2 and C6–N3 bond lengths in (I) are 1.353(3) and 1.419(3) Å, respectively.
In the molecular packing, supramolecular chains aligned along [1 0 1] feature N–H⃛N hydrogen-bonding interactions, see the lower view of the figure. Thus, a pair pyrazolyl-N–H⃛N(pyrazolyl) hydrogen bonds [N1–H1⃛N2 i : H1⃛N2 i = 2.10(2) Å, N1–N2 i = 2.902(3) Å with angle at H1n = 152.5(18)∘ for symmetry operation i: 1-x, 2-y, -z] assemble over a centre of inversion to form a six-membered {⃛HNN}2 synthon. Similarly, a pair amine-N–H⃛N(amine) hydrogen bonds [N3–H2⃛N3 ii : H2…N3 ii = 2.53(3) Å, N3⃛N3 ii = 3.051(3) Å with angle at H2n = 119(2)∘ for ii: 2-x, 2-y, 1-z] assemble over a centre of inversion to form a four-membered {⃛HN}2 synthon. The linear chains are connected into a three-dimensional architecture by methyl-C–H⃛π(pyrazolyl) interactions [C1–H1⃛Cg(N1,N2,C5–C7) iii : H1⃛Cg(N1,N2,C5–C7) iii = 2.59 Å; C⃛Cg(N1,N2,C5–C7) iii = 3.545(3) Å with angle at H1a = 166° for iii: 1-x, −1/2+y, 1/2-z] which occur about a 21 screw axis aligned along the b-axis. There is no apparent role in the molecular packing for the amine-H3n atom.
The molecular packing pattern in (I) contrasts those observed in the crystals of the methyl 9 and i-propyl 7 derivatives. In the former, a three-dimensional architecture features N–H⃛N hydrogen bonds operating in three-dimensions as each donor/acceptor site participates in at least one N–H⃛N hydrogen bond. 9 In the crystal of the i-propyl derivative, supramolecular layers feature N–H⃛N hydrogen bonds but one of the amine-H atoms does not participate in a directional intermolecular contact, 7 as in the crystal of (I).
Finally, an analysis of the nature of and relative contributions to the calculated Hirshfeld surfaces and two-dimensional fingerprint plots in the crystals of (I) and the i-propyl and methyl derivatives was undertaken employing CrystalExplorer 10 and standard procedures. 11 For the methyl species, calculations were performed on each of the disorder (60° degree rotation for one of the methyl groups) and the reported percentages are average values. For the t-butyl derivatives, only the major component of the disordered t-butyl group was considered. In each crystal there are only three types of surface contacts evident, namely H⃛H, N⃛H/H⃛N and C⃛H/H⃛C contacts. These were found to systematically follow trends in accord with the number of hydrogen atoms in the molecule. Thus, H⃛H contacts increased in the order of the methyl to i-propyl to t-butyl derivatives, viz. 61.5, 79.9 and 83.9 %, respectively. Consequently, the percentage contributions from the N⃛H/H⃛N (25.5, 15.3 and 12.2 %, respectively) and C⃛H/H⃛C (13.0, 4.7 and 3.9 %, respectively) diminished passing from the methyl to i-propyl to t-butyl.
Funding source: Joint Usage/Research Centre for Catalysis and the Koyanagi Foundation
Acknowledgments
This research was supported by the Joint Usage/Research Centre for Catalysis and the Koyanagi Foundation.
-
Research funding: This study was financially supported by the Joint Usage/Research Centre for Catalysis (Proposals 22DS0143, 23DS0198 and 24ES0584).
-
Author contributions: All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest.
References
1. Rigaku Oxford Diffraction. CrysAlis Pro; Rigaku Corporation: Oxford, UK, 2021.Suche in Google Scholar
2. Burla, M. C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G. L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: a Suite of Computer Programs for Crystal Structure Solution of Proteins. J. Appl. Cryst. 2007, 40, 609–613. https://doi.org/10.1107/S0021889807010941.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/S2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Farrugia, L. J. WinGX and Ortep for Windows: an Update. J. Appl. Cryst. 2012, 45, 849–854. https://doi.org/10.1107/S0021889812029111.Suche in Google Scholar
5. Fujisawa, K.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K. Pyrazolate-bridged Group 11 Metal(I) Complexes: Substituent Effects on the Supramolecular Structures and Physicochemical Properties. Inorg. Chim. Acta 2010, 363, 2977–2989. https://doi.org/10.1016/j.ica.2010.05.014.Suche in Google Scholar
6. Ochandoa, L. E.; Amig, J. M.; Rius, J.; Louër, D.; Fontenas, Ch.; Elguero, J. The Crystal Structure of 3,5-Diisopropyl-4-Nitropyrazole from X-Ray Powder Diffraction Data. J. Mol. Struct. 2001, 562, 11–17. https://doi.org/10.1016/S0022–2860(00)00766–3.10.1016/S0022-2860(00)00766-3Suche in Google Scholar
7. Fujisawa, K.; Ageishi, K.; Okano, M.; Tiekink, E. R. T. The Crystal Structure of 3,5-Bis(propan-2-yl)-1H-pyrazol-4-amine, C9H17N3. Z. Kristallogr. – New Cryst. Struct. 2022, 237, 1055–1057. https://doi.org/10.1515/ncrs-2022–0362.10.1515/ncrs-2022-0362Suche in Google Scholar
8. Fujisawa, K.; Ageishi, K.; Iwai, K.; Okano, M.; Williams–Sekiguchi, R. Y.; Tiekink, E. R. T. Coordination Chemistry of 4-aminopyrazole: Structure and Physicochemical Properties of Cobalt(II) Chlorido Complexes, and Amino Group Reactivity towards a Ketone to Yield an Imine Bond. Inorg. Chim. Acta 2024, 572, 122283. https://doi.org/10.1016/j.ica.2024.122283.Suche in Google Scholar
9. Infantes, L.; Foces–Foces, C.; Claramunt, R. M.; López, C.; Elguero, J. Aminopyrazoles and Their Conjugated Acids. An X-Ray Study of 3,5-Dimethyl-4-Aminopyrazole and the Picrate of 3(5)-aminopyrazole. J. Heterocycl. Chem. 1999, 36, 595–600. https://doi.org/10.1002/jhet.5570360303.Suche in Google Scholar
10. Spackman, P. R.; Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Jayatilaka, D.; Spackman, M. A. CrystalExplorer: a Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. https://doi.org/10.1107/S1600576721002910.Suche in Google Scholar PubMed PubMed Central
11. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T. Utilizing Hirshfeld Surface Calculations, Non-covalent Interaction (NCI) Plots and the Calculation of Interaction Energies in the Analysis of Molecular Packing. Acta Crystallogr. 2019, E75, 308–318. https://doi.org/10.1107/S2056989019001129.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

