Abstract
C24H24I2O2, monoclinic, P21/n (no. 14), a = 11.128(2) Å, b = 5.7775(12) Å, c = 17.607(4) Å, β = 98.87(3)°, V = 1118.4(4) Å3, Z = 2, R gt(F) = 0.0246, wR ref(F 2) = 0.0537, T = 296(2) K.
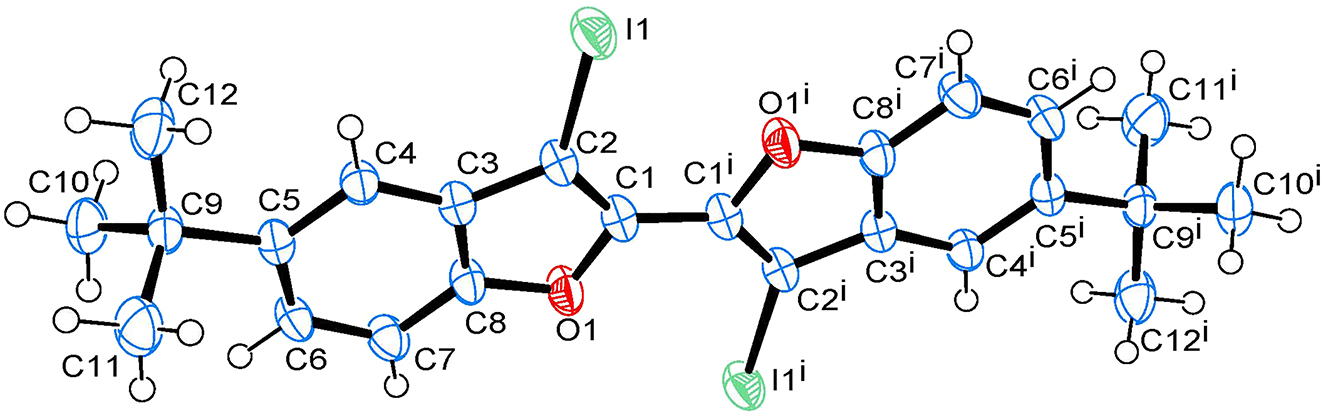
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.23 × 0.21 × 0.19 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 2.83 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 27.9°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 6656, 2641, 0.023 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2189 |
| N(param)refined: | 130 |
| Programs: | Bruker [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.5017 (2) | 0.4394 (5) | −0.03552 (13) | 0.0322 (6) |
| C2 | 0.5701 (2) | 0.2659 (5) | −0.05807 (13) | 0.0308 (6) |
| C3 | 0.5269 (2) | 0.2237 (5) | −0.13819 (13) | 0.0296 (5) |
| C4 | 0.5602 (2) | 0.0753 (5) | −0.19381 (13) | 0.0300 (5) |
| H4 | 0.6228 | −0.0311 | −0.1810 | 0.036* |
| C5 | 0.4987 (2) | 0.0881 (5) | −0.26852 (13) | 0.0297 (5) |
| C6 | 0.4031 (2) | 0.2460 (5) | −0.28461 (13) | 0.0349 (6) |
| H6 | 0.3616 | 0.2527 | −0.3346 | 0.042* |
| C7 | 0.3667 (2) | 0.3926 (5) | −0.23064 (14) | 0.0366 (6) |
| H7 | 0.3017 | 0.4943 | −0.2426 | 0.044* |
| C8 | 0.4330 (2) | 0.3786 (5) | −0.15752 (14) | 0.0326 (6) |
| C9 | 0.5366 (3) | −0.0552 (5) | −0.33396 (14) | 0.0342 (6) |
| C10 | 0.4253 (3) | −0.1699 (6) | −0.38181 (16) | 0.0481 (8) |
| H10A | 0.3838 | −0.2631 | −0.3489 | 0.072* |
| H10B | 0.4514 | −0.2658 | −0.4207 | 0.072* |
| H10C | 0.3713 | −0.0524 | −0.4057 | 0.072* |
| C11 | 0.5951 (3) | 0.1080 (6) | −0.38652 (18) | 0.0551 (9) |
| H11A | 0.5367 | 0.2217 | −0.4080 | 0.083* |
| H11B | 0.6215 | 0.0203 | −0.4273 | 0.083* |
| H11C | 0.6638 | 0.1839 | −0.3572 | 0.083* |
| C12 | 0.6275 (3) | −0.2438 (6) | −0.30448 (16) | 0.0486 (8) |
| H12A | 0.7011 | −0.1741 | −0.2790 | 0.073* |
| H12B | 0.6451 | −0.3353 | −0.3469 | 0.073* |
| H12C | 0.5936 | −0.3409 | −0.2689 | 0.073* |
| I1 | 0.70674 (2) | 0.07277 (3) | 0.00468 (2) | 0.04253 (7) |
| O1 | 0.41526 (17) | 0.5130 (3) | −0.09584 (9) | 0.0373 (4) |
1 Source of materials
The 1,4-bis(5-tert-butyl-2-methoxyphenyl)buta-1,3-diyne (synthesized by the literature reported procedure [4]) (1.20 g, 3.20 mmol) was dissolved in dry dichloromethane (5 mL), and a dichloromethane solution (20 mL) of I2 (3.26 g, 12.82 mmol) was added dropwise to the reaction mixture at room temperature. Reaction progress was detected by TLC for 10 h, the reaction was quenched with saturated sodium sulfite solution (40 mL), extracted with dichloromethane (50 mL × 3), dried with anhydrous magnesium sulfate, filtered and concentrated to obtain crude residues. The yellow solid, namely, 5-tert-butyl-2-(5-tert-butyl-3-iodobenzofuran-2-yl)-3-iodobenzofuran (1.72 g, 90 %), was obtained by silica gel column chromatography. Melting point: 175–176 °C. Crystals of the title compound were obtained in a mixture of dichloromethane and diethyl ether. 1 H NMR (400 MHz, CDCl3) δ 7.50–7.46 (m, 3H), 1.42 (d, J = 1.2 Hz, 9H). 13 C NMR (100 MHz, CDCl3) δ 152.66, 147.53, 146.38, 131.12, 125.03, 118.37, 111.12, 66.20, 35.08, 31.93, 29.85.
2 Experimental details
The crystal structure was solved using the Shelxs [2] structure solution program and refined with the Shelxl [3]. All hydrogen atoms were placed in calculated positions and refined as riding on their parent atom.
3 Comment
3,3′-Diiodo-2,2′-bibenzofuran derivatives are an important class of organic reaction intermediates [4] and used to synthesize heteropentacenes with π-conjugated systems in material science [5], [6], [7]. In this paper, a para-tert-butyl substituted 3,3′-diiodo-2,2′-bibenzofuran derivative, namely, 5-tert-butyl-2-(5-tert-butyl-3-iodobenzofuran-2-yl)-3-iodobenzofuran was synthesized by using the iodine electrophilic cyclization reaction of 1,4-bis(2-methoxy-3,5-ditert-butylphenyl)-1,3-diyne with I2 [4].
The crystal structure of the title compound indicates that it is a centrosymmetric structure with the inversion center of the C–C bond as the center of symmetry. Its molecular structure contains two benzofuran rings, two iodine atoms and two tert-butyl groups. As illustrated in the figure, an anti-arrangement of two benzofuran moieties and two iodine atoms were observed. In the crystal structure, the carbon-carbon double bond length of C1–C2 is 1.355(4) Å, which is typical for carbon-carbon double bond [8]. The bond lengths of O1–C1 and O1–C8 are 1.385(3) and 1.374(3) Å, respectively. The bond distance of C2–I1 is 2.064(2) Å. These bond lengths are within the normal range [9]. In addition, the plane (C3/C4/C8) of benzene ring and the plane (C1/C2/O1) of furan ring are approximately coplanar and their dihedral angle is only 0.81°, which is similar to the reported related structure containing a benzofuran moiety [9]. The two furan rings lie in a highly coplanar arrangement with the dihedral angle of about 0.81° by connection of C1–C1 i .
Funding source: Yan'an High-level Talents Special Foundation
Award Identifier / Grant number: 2019-33
Funding source: College Students Innovation and Entrepreneurship Training Program of Yan’an University
Award Identifier / Grant number: D2021030
Funding source: Scientific Research Program Funded by Shaanxi Provincial Education Department
Award Identifier / Grant number: 23JK0432
Acknowledgments
We gratefully acknowledge the supports of Yan’an University.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Yan’an High-level Talents Special Foundation (Program No. 2019-33); College Students Innovation and Entrepreneurship Training Program of Yan’an University (Program No. D2021030) and Scientific Research Program Funded by Shaanxi Provincial Education Department (Program No. 23JK0432).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Apex2, Sadabs, Xprep and Saint-Plus; Bruker AXS Inc.: Madison, 2004.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Larock, R. C., Mehta, S. Iodine/palladium approaches to the synthesis of polyheterocyclic compounds. J. Org. Chem. 2010, 75, 1652–1658; https://doi.org/10.1021/jo902639f.Suche in Google Scholar PubMed PubMed Central
5. Chen, H., Delaunay, W., Li, J., Wang, Z. Y., Bouit, P. A., Tondelier, D., Geffroy, B., Mathey, F., Duan, Z., Réau, R., Hissler, M. Benzofuran-fused phosphole: synthesis, electronic, and electroluminescence properties. Org. Lett. 2013, 15, 330–333; https://doi.org/10.1021/ol303260d.Suche in Google Scholar PubMed
6. Zhang, F. B., Adachi, Y., Ooyama, Y., Ohshita, J. Synthesis and properties of benzofuran-fused silole and germole derivatives: reversible dimerization and crystal structures of monomers and dimers. Organometallics 2016, 35, 2327–2332; https://doi.org/10.1021/acs.organomet.6b00222.Suche in Google Scholar
7. Imoto, H., Fujii, T., Tanaka, S., Yamamoto, S., Mitsuishi, M., Yumura, T., Naka, K. As-heteropentacenes: an experimental and computational study on a novel class of heteroacenes. Org. Lett. 2018, 20, 5952–5955; https://doi.org/10.1021/acs.orglett.8b02660.Suche in Google Scholar PubMed
8. Wang, M. D., Lu, W. W., Wang, J. L., Li, M. Y., Feng, Y. Y., Ma, J.-Y. The crystal structure of 1-(3-oxo-1-phenyl-3-(p-tolyl)propylidene)-1,3-dihydro-2H – inden-2-one, C25H20O2. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 23–24; https://doi.org/10.1515/ncrs-2023-0418.Suche in Google Scholar
9. Zhang, M., Bai, Y.-B. The crystal structure of (2R,6′R)-2′,7-dichloro-4,6-dimethoxy-6′- methyl-3H-spiro[benzofuran-2,1′-cyclohexan]-2′-ene-3,4′-dione, C16H14Cl2O5. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 185–187; https://doi.org/10.1515/ncrs-2022-0538.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

