Abstract
C26H22CuN4O4, triclinic, P1− (no. 2), a = 9.142(3) Å, b = 10.794(3) Å, c = 12.718(4) Å, α = 69.308(11)°, β = 81.326(10)°, γ = 76.862(10)°, V = 1139.9(6) Å3, Z = 2, Rgt (F) = 0.0666, wRref (F 2) = 0.1966, T = 100(2) K.
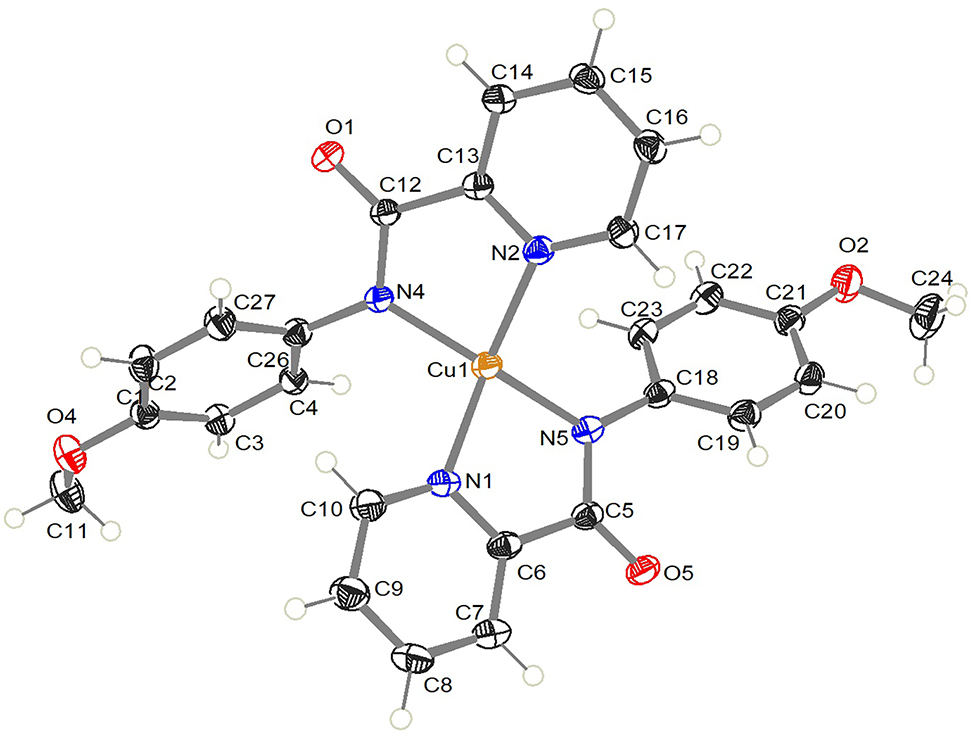
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Black block |
| Size: | 0.26 × 0.14 × 0.04 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.00 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 32.6°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 33191, 8255, 0.084 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 6625 |
| N(param)refined: | 318 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Cu1 | 0.31525 (3) | 0.49835 (3) | 0.27269 (3) | 0.02150 (10) |
| O1 | 0.2102 (3) | 0.7189 (2) | 0.48147 (17) | 0.0287 (4) |
| O2 | −0.0559 (3) | 0.7958 (2) | −0.21288 (19) | 0.0339 (5) |
| O4 | 0.2026 (2) | 0.1282 (2) | 0.82971 (18) | 0.0302 (4) |
| O5 | 0.3829 (2) | 0.2763 (2) | 0.06587 (18) | 0.0265 (4) |
| N1 | 0.4388 (2) | 0.3124 (2) | 0.3242 (2) | 0.0220 (4) |
| N2 | 0.3133 (2) | 0.6964 (2) | 0.20588 (19) | 0.0210 (4) |
| N4 | 0.2645 (3) | 0.5376 (2) | 0.41427 (18) | 0.0211 (4) |
| N5 | 0.2975 (3) | 0.4524 (2) | 0.1403 (2) | 0.0229 (4) |
| C1 | 0.3019 (3) | 0.3200 (3) | 0.7172 (2) | 0.0278 (5) |
| H1 | 0.3547 | 0.3096 | 0.7778 | 0.033* |
| C2 | 0.2049 (3) | 0.2321 (3) | 0.7281 (2) | 0.0229 (4) |
| C3 | 0.1217 (3) | 0.2509 (3) | 0.6385 (2) | 0.0229 (4) |
| H3 | 0.0545 | 0.1946 | 0.6464 | 0.027* |
| C4 | 0.1398 (3) | 0.3554 (2) | 0.5363 (2) | 0.0213 (4) |
| H4 | 0.0844 | 0.3676 | 0.4767 | 0.026* |
| C5 | 0.3732 (3) | 0.3294 (2) | 0.1396 (2) | 0.0214 (4) |
| C6 | 0.4533 (3) | 0.2511 (3) | 0.2455 (2) | 0.0224 (4) |
| C7 | 0.5408 (3) | 0.1241 (3) | 0.2602 (3) | 0.0291 (5) |
| H7 | 0.5481 | 0.0827 | 0.2062 | 0.035* |
| C8 | 0.6173 (4) | 0.0599 (3) | 0.3573 (3) | 0.0325 (6) |
| H8 | 0.6757 | −0.0259 | 0.3695 | 0.039* |
| C9 | 0.6063 (4) | 0.1242 (3) | 0.4359 (3) | 0.0324 (6) |
| H9 | 0.6593 | 0.0836 | 0.5001 | 0.039* |
| C10 | 0.5147 (3) | 0.2504 (3) | 0.4169 (3) | 0.0276 (5) |
| H10 | 0.5056 | 0.2932 | 0.4701 | 0.033* |
| C11 | 0.0898 (4) | 0.0486 (3) | 0.8503 (3) | 0.0356 (6) |
| H11A | 0.1016 | −0.0220 | 0.9218 | 0.053* |
| H11B | 0.1001 | 0.0098 | 0.7917 | 0.053* |
| H11C | −0.0081 | 0.1045 | 0.8512 | 0.053* |
| C12 | 0.2481 (3) | 0.6682 (2) | 0.4056 (2) | 0.0199 (4) |
| C13 | 0.2807 (3) | 0.7573 (2) | 0.2854 (2) | 0.0202 (4) |
| C14 | 0.2775 (3) | 0.8941 (3) | 0.2580 (2) | 0.0256 (5) |
| H14 | 0.2520 | 0.9351 | 0.3132 | 0.031* |
| C15 | 0.3131 (3) | 0.9693 (3) | 0.1463 (2) | 0.0257 (5) |
| H15 | 0.3125 | 1.0611 | 0.1260 | 0.031* |
| C16 | 0.3498 (3) | 0.9050 (3) | 0.0652 (2) | 0.0255 (5) |
| H16 | 0.3749 | 0.9529 | −0.0095 | 0.031* |
| C17 | 0.3479 (3) | 0.7685 (3) | 0.0984 (2) | 0.0237 (4) |
| H17 | 0.3713 | 0.7255 | 0.0445 | 0.028* |
| C18 | 0.2134 (3) | 0.5382 (3) | 0.0464 (2) | 0.0220 (4) |
| C19 | 0.2680 (3) | 0.5547 (3) | −0.0659 (2) | 0.0259 (5) |
| H19 | 0.3630 | 0.5085 | −0.0816 | 0.031* |
| C20 | 0.1809 (3) | 0.6399 (3) | −0.1545 (2) | 0.0260 (5) |
| H20 | 0.2177 | 0.6496 | −0.2287 | 0.031* |
| C21 | 0.0393 (3) | 0.7103 (3) | −0.1316 (2) | 0.0249 (5) |
| C22 | −0.0147 (3) | 0.6964 (3) | −0.0189 (2) | 0.0277 (5) |
| H22 | −0.1086 | 0.7444 | −0.0031 | 0.033* |
| C23 | 0.0720 (3) | 0.6114 (3) | 0.0680 (2) | 0.0247 (5) |
| H23 | 0.0357 | 0.6028 | 0.1421 | 0.030* |
| C24 | −0.0174 (5) | 0.7903 (5) | −0.3242 (3) | 0.0478 (9) |
| H24A | −0.0983 | 0.8425 | −0.3708 | 0.072* |
| H24B | −0.0006 | 0.6983 | −0.3220 | 0.072* |
| H24C | 0.0725 | 0.8263 | −0.3549 | 0.072* |
| C26 | 0.2395 (3) | 0.4408 (2) | 0.5231 (2) | 0.0206 (4) |
| C27 | 0.3192 (3) | 0.4235 (3) | 0.6152 (2) | 0.0260 (5) |
| H27 | 0.3840 | 0.4815 | 0.6080 | 0.031* |
1 Source of materials
The starting material, (4-methoxyphenyl)picolinamide (L), was prepared as follows: 2.46 g (20.0 mmol) of pyridine-2-carboxylic acid solution was prepared by adding it to 12.0 mL of pyridine. To this solution, 2.20 mL (20.0 mmol) of 4-methoxyaniline were added, and the mixture was heated with continuous stirring for 30.0 min. Subsequently, 5.28 mL (20.0 mmol) of triphenylphosphite were introduced into the resulting solution, and the mixture was stirred at 110 °C for 4 h. The cold reaction mixture was washed with 100.0 mL of distilled water, and the resulting white paste was dissolved into 50.0 mL of dichloromethane. It was then extracted with 100.0 mL of 1:1 (v/v) aqueous hydrochloric acid. The aqueous extract was neutralized using solid sodium bicarbonate. The resulting white solid was filtered, thoroughly washed with distilled water, and subsequently crystallized from aqueous methanol, yielding a colorless crystalline solid. The overall yield of the process was 89.0 %. The copper complex was obtained by the following procedures: A 250.0 mL round bottom flask was charged with 693.0 mg (3.04 × 10−3 mmol) of L and added 20.0 mL of absolute ethanol and continued to stirring at room temperature until all the ligands dissolved. Then, 6.0 mL of 28 % NH3 solution and 259.2 mg (1.52 × 10−3 mmol) of CuCl2 · 2H2O were added to the solution and refluxed the rection mixture for 19 h at 80 °C. The final green solid product was separated with filtration and washed with cold ethanol for removing unreacted metal salt and ligand. Finally, the desired product was isolated as green crystalline material by slow evaporation from hot ethanol and diethyl ether mixture. Yield 85.0 %.
2 Experimental details
The chemicals were purchased from Aldrich (Germany), Fluka (Switzerland), and were used without further purification, unless otherwise stated. Single crystals were obtained through crystallization of the pure compound from slow evaporation of EtOH/Et2O. The collected frames were integrated with the Bruker SAINT software package using a narrow-frame algorithm. Data correction were performed for absorption effects using the multi-scan method (SADABS). The structure was solved and refined using the Bruker SHELXTL [3] software package. Using Olex2 [2], the structure was further refined with the ShelXL [4] refinement package using least squares minimization. All H atoms bonded to C atoms were refined as riding, with C–H distances of 0.93 Å (for aromatic ring).
3 Comment
Picolinamide derivatives are known to act as bidentate ligands [5]. The interest in these ligands arises not only from their anticancer activity but also from their ability to form linkage isomers that depend on the acidity of the medium [6]. Furthermore, these compounds play a key role task in the advancement of coordination chemistry that is related to catalysis, magnetism, and molecular design [7]. Copper, one of essential trace elements in biological systems, can be used as a substitute in cisplatin complexes [8]. In view of the interest in the activity spectrum and profile of picolinamide derivatives, herein we present the title structure of picolinamide copper complex.
The asymmetric unit shows one complex. The coordination sphere of copper(II) consists of two picolinamide ligands. The average Cu–N bond is 1.978 Å (Cu–N1 = 2.000(2) Å, Cu–N2 = 1.999(2) Å, Cu–N4 = 1.958(2) Å, and Cu–N5 = 1.953(2) Å) [9]. The copper(II) is distorted from square planar geometry with an average chelate angle equal to 83.5° (N4–Cu–N2 and N5–Cu–N1) and the other cis nitrogen (N4–Cu–N1 and N5–Cu–N2) have an average angle equal to 101.8°. Trans-nitrogens angles (N2–Cu–N1 and N5–Cu–N4) show 147.2° and 161.3°, respectively. Furthermore, the two ligands that are attached to the copper(II) center are not coplanar. The angle between the mean plane that passes through (N1–Cu–N5–C6–C5) and (N2–Cu–N4–C12–C13) is equal 40.7°. The structure of crystal is being stabilized by the presence of shortest hydrogen bonds between the carbonyl-oxygen atoms O5 (−x, −y, −z) and H15 (−x, 1 − y, −z) with distances equal to 2.382 Å. A weaker hydrogen bond interaction is formed between H17 (−1 + x, 1 + y, +z) and the carbonyl-oxygen atom O5 (−x, −y, −z) with distance equal to 2.464 Å.
Funding source: Research Institute of Science and Engineering (RISE), University of Sharjah, Sharjah, UAE
Award Identifier / Grant number: competitive grant number 21021440108 and 22021440127
Funding source: Part of this work has been carried out during sabbatical leave granted to MAK from the University of Jordan during the academic year 2021–2022
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Research Institute of Science and Engineering (RISE), University of Sharjah, Sharjah, UAE, competitive grant number 21021440108 and 22021440127. Part of this work has been carried out during sabbatical leave granted to MAK from the University of Jordan during the academic year 2021–2022.
References
1. Bruker. APEX4, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2021.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Qi, J.-Y., Ma, H.-X., Li, X.-J., Zhou, Z.-Y., Choi, M. C. K., Chan, A. S. C., Yangc, Q.-Y. Synthesis and characterization of cobalt(III) complexes containing 2-pyridinecarboxamide ligands and their application in catalytic oxidation of ethylbenzene with dioxygen. ChemComm 2003, 1294–1295; https://doi.org/10.1002/chin.200337068.Search in Google Scholar
6. Das, A., Peng, S.-M., Lee, G.-H., Bhattacharya, S. Synthesis, structure and electrochemical properties of a group of ruthenium(III) complexes of N-(aryl)picolinamide. New J. Chem. 2004, 28, 712–717; https://doi.org/10.1039/b317018g.Search in Google Scholar
7. Ashraf, A., Hanif, M., Kubanik, M., Soehnel, T., Jamieson, S. M. F., Bhattacharyya, A., Hartinger, C. G. Aspirin-inspired organometallic compounds. Structural characterization and cytotoxicity. J. Organomet. Chem. 2017, 839, 31–37; https://doi.org/10.1016/j.jorganchem.2017.01.016.Search in Google Scholar
8. Khanfar, M. A., Jaber, A. M., AlDamen, M. A., Al-Qawasmeh, R. A. Synthesis, characterization, crystal structure, and DFT study of a new square planar Cu(II) complex containing bulky adamantane ligand. Molecules 2018, 23, 701; https://doi.org/10.3390/molecules23030701.Search in Google Scholar PubMed PubMed Central
9. Ray, M., Mukherjee, R., Richardson, J. F., Mashuta, M. S., Buchanan, R. M. Control of the stereochemistry of four-co-ordinate copper(II) complexes by pyridinecarboxamide ligands: crystal structure, spectral and redox properties. J. Chem. Soc., Dalton Trans. 1994, 965–969; https://doi.org/10.1039/dt9940000965.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

