Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
Abstract
C46H64N2O28S2Zn, monoclinic, P21/c (no. 14), a = 18.155(2) Å, b = 9.0872(11) Å, c = 16.373(2) Å, β = 108.095(13)°, V = 2567.6(6) Å3, Z = 2, R gt(F) = 0.0712, wR ref(F 2) = 0.1295, T = 296.15 K.
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters
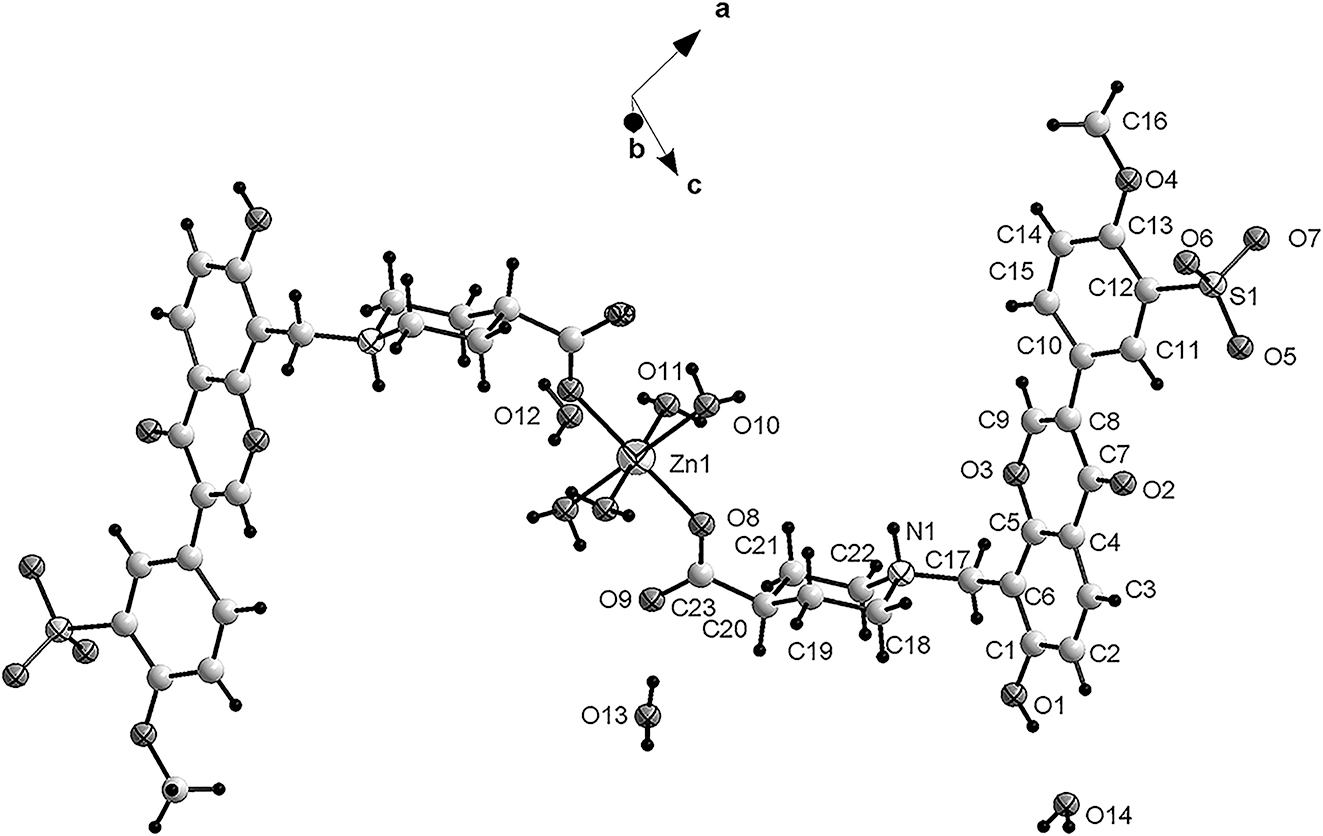
.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.10 × 0.10 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.66 mm-1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θ max, completeness: | 29.2°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 13,199, 6013, 0.067 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3436 |
| N(param)refined: | 364 |
| Programs: | CrysAlis Pro [1], Diamond [2], Shelx [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Zn1 | 0.000000 | 0.500000 | 0.000000 | 0.0304 (2) |
| S1 | 0.75539 (5) | 0.82546 (12) | 0.33094 (6) | 0.0219 (2) |
| O1 | 0.27088 (16) | 0.6209 (4) | 0.58977 (19) | 0.0418 (8) |
| H1 | 0.276774 | 0.676632 | 0.630717 | 0.063* |
| O2 | 0.53742 (15) | 0.8322 (3) | 0.44444 (18) | 0.0350 (8) |
| O3 | 0.39525 (13) | 0.4739 (3) | 0.39284 (16) | 0.0269 (7) |
| O4 | 0.71183 (14) | 0.6375 (3) | 0.17461 (16) | 0.0289 (7) |
| O5 | 0.75224 (14) | 0.8929 (3) | 0.40954 (16) | 0.0327 (7) |
| O6 | 0.75051 (14) | 0.9340 (3) | 0.26400 (16) | 0.0284 (7) |
| O7 | 0.82137 (13) | 0.7277 (3) | 0.34389 (17) | 0.0335 (7) |
| O8 | 0.03109 (14) | 0.5209 (3) | 0.12938 (16) | 0.0373 (8) |
| O9 | −0.07351 (14) | 0.4679 (3) | 0.16621 (16) | 0.0295 (7) |
| O10 | 0.11511 (14) | 0.5784 (3) | 0.00251 (17) | 0.0336 (7) |
| H10A | 0.120474 | 0.567673 | −0.046911 | 0.050* |
| H10B | 0.149754 | 0.523192 | 0.035159 | 0.050* |
| O11 | 0.04526 (15) | 0.2857 (3) | 0.01448 (19) | 0.0471 (9) |
| H11A | 0.017388 | 0.209423 | −0.001498 | 0.071* |
| H11B | 0.069898 | 0.269993 | 0.067072 | 0.071* |
| O12 | −0.08120 (18) | 0.1149 (4) | −0.0391 (2) | 0.0734 (12) |
| H12A | −0.104795 | 0.196783 | −0.043987 | 0.110* |
| H12B | −0.092265 | 0.082803 | −0.090297 | 0.110* |
| O13 | −0.15159 (18) | 0.4441 (3) | 0.2848 (2) | 0.0426 (8) |
| H13A | −0.123708 | 0.459190 | 0.252448 | 0.064* |
| H13B | −0.161939 | 0.529654 | 0.299169 | 0.064* |
| N1 | 0.20833 (16) | 0.4450 (3) | 0.38681 (19) | 0.0188 (7) |
| H1A | 0.225948 | 0.427374 | 0.336817 | 0.023* |
| C1 | 0.3258 (2) | 0.6475 (5) | 0.5525 (2) | 0.0264 (10) |
| C2 | 0.3746 (2) | 0.7703 (5) | 0.5747 (3) | 0.0325 (11) |
| H2 | 0.370210 | 0.835089 | 0.616909 | 0.039* |
| C3 | 0.4289 (2) | 0.7943 (5) | 0.5339 (3) | 0.0299 (10) |
| H3 | 0.460518 | 0.876991 | 0.547923 | 0.036* |
| C4 | 0.43760 (19) | 0.6970 (4) | 0.4717 (2) | 0.0210 (9) |
| C5 | 0.3887 (2) | 0.5760 (4) | 0.4519 (2) | 0.0201 (9) |
| C6 | 0.3310 (2) | 0.5493 (4) | 0.4893 (2) | 0.0225 (9) |
| C7 | 0.4955 (2) | 0.7223 (4) | 0.4275 (2) | 0.0233 (9) |
| C8 | 0.4979 (2) | 0.6123 (4) | 0.3636 (2) | 0.0221 (9) |
| C9 | 0.4493 (2) | 0.4961 (5) | 0.3530 (2) | 0.0272 (10) |
| H9 | 0.453416 | 0.424155 | 0.314262 | 0.033* |
| C10 | 0.55237 (19) | 0.6226 (4) | 0.3121 (2) | 0.0222 (9) |
| C11 | 0.61921 (19) | 0.7095 (4) | 0.3385 (2) | 0.0205 (9) |
| H11 | 0.628776 | 0.765809 | 0.388146 | 0.025* |
| C12 | 0.67134 (19) | 0.7137 (4) | 0.2924 (2) | 0.0174 (8) |
| C13 | 0.6585 (2) | 0.6302 (4) | 0.2174 (2) | 0.0215 (9) |
| C14 | 0.5917 (2) | 0.5463 (5) | 0.1901 (3) | 0.0301 (10) |
| H14 | 0.581699 | 0.491074 | 0.140022 | 0.036* |
| C15 | 0.5398 (2) | 0.5432 (5) | 0.2360 (3) | 0.0288 (10) |
| H15 | 0.495157 | 0.486673 | 0.215727 | 0.035* |
| C16 | 0.7026 (2) | 0.5349 (5) | 0.1054 (3) | 0.0382 (12) |
| H16A | 0.657381 | 0.560399 | 0.058737 | 0.057* |
| H16B | 0.696782 | 0.437370 | 0.125036 | 0.057* |
| H16C | 0.747428 | 0.538526 | 0.086210 | 0.057* |
| C17 | 0.2768 (2) | 0.4211 (5) | 0.4666 (2) | 0.0247 (10) |
| H17A | 0.257749 | 0.400328 | 0.514514 | 0.030* |
| H17B | 0.305358 | 0.335349 | 0.457892 | 0.030* |
| C18 | 0.1746 (2) | 0.5959 (4) | 0.3783 (3) | 0.0280 (10) |
| H18A | 0.215010 | 0.668016 | 0.382825 | 0.034* |
| H18B | 0.152777 | 0.613543 | 0.424519 | 0.034* |
| C19 | 0.1120 (2) | 0.6128 (4) | 0.2927 (2) | 0.0268 (10) |
| H19A | 0.135438 | 0.605860 | 0.247095 | 0.032* |
| H19B | 0.089233 | 0.710023 | 0.289845 | 0.032* |
| C20 | 0.04789 (19) | 0.4981 (4) | 0.2773 (2) | 0.0194 (8) |
| H20 | 0.015682 | 0.520055 | 0.313993 | 0.023* |
| C21 | 0.0821 (2) | 0.3452 (4) | 0.2981 (3) | 0.0280 (10) |
| H21A | 0.041055 | 0.277199 | 0.298731 | 0.034* |
| H21B | 0.102841 | 0.314172 | 0.252979 | 0.034* |
| C22 | 0.1455 (2) | 0.3361 (5) | 0.3834 (3) | 0.0301 (10) |
| H22A | 0.124021 | 0.355975 | 0.429615 | 0.036* |
| H22B | 0.167092 | 0.237521 | 0.391379 | 0.036* |
| C23 | −0.0029 (2) | 0.4971 (4) | 0.1833 (2) | 0.0222 (9) |
| O14 | 0.27237 (16) | 0.7588 (3) | 0.72883 (19) | 0.0491 (9) |
| H14A | 0.231085 | 0.723492 | 0.735122 | 0.074* |
| H14B | 0.269865 | 0.850402 | 0.738162 | 0.074* |
1 Source of materials
All reagents and chemicals were purchased from commercial sources and used without further purification. The educt sodium 5-(8-((4-carboxypiperidin-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-methoxybenzenesulfonate was synthesized via a Mannich reaction. Formaldehyde solution (5 mL, 37 %), sodium 5-(7-hydroxy-4-oxo-4H-chromen-3-yl)-2-methoxybenzenesulfonate (1.85 g, 0.005 mol), water (5 mL) and piperidine-4-carboxylic acid (0.4844 g, 0.00375 mol) were added to ethanol (50 mL, 99 %) and stirred for 0.5 h at 328 K. Then, hot water (20 mL) was added until a transparent solution was obtained. After 12 h reaction time, the mixture was filtered, and the residue was collected. Then the residue was dried at 383 K. Sodium 5-(8-((4-carboxypiperidin-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-methoxybenzenesulfonate (0.6877 g) was obtained. IR spectra (potassium bromide pellet) were recorded on a Nicolet 6700. IR (v/cm−1): 3387, 3079, 2992, 2747, 1733, 1627, 1608, 1494, 1444, 1390, 1368, 1294, 1266, 1215, 1175, 1127, 1094, 1070, 1035, 1015, 989, 957, 923, 903, 843, 829, 800, 720, 689, 628, 603, 581, 548, 489, 469, 447. Zinc nitrate hexahydrate (0.0297 g, 0.1 mmol) and sodium salt described before (0.0256 g, 0.05 mmol) were added to water (10 mL) and sonicated for 10 min. The mixture was heated for 18 h at 363 K. Colorless block crystals of the title complex were obtained. IR spectra (potassium bromide pellet) were recorded on a Nicolet 6700. IR (v/cm−1): 3431, 3023, 2763, 1628, 1604, 1573, 1494, 1450, 1417, 1373, 1361, 1326, 1291, 1266, 1215, 1171, 1096, 1056, 1025, 1004, 959, 926, 897, 824, 797, 780, 722, 637, 622, 550, 516.
2 Experimental details
Carbon-bound H atoms were placed in calculated positions and were included in the refinement in the riding model approximation, with U iso(H) set to 1.2 U eq(C). The oxygen-bound and nitrogen-bound H atoms were located on a difference Fourier map.
3 Comment
In recent years, a series of formononetin derivatives have been synthesized through different procedures to study their pharmacological activities. Some of formononetin derivatives possess various pharmacological activities such as antihypertensive activity and anticancer activity [5], [6], [7]. Our focus was on the design and synthesis of a novel series of formononetin derivatives, with the aim of investigating their potential as effective anticancer agents.
X-ray crystallographic analysis of the Zn(II) complex reveals a mononuclear structure. There is one half of a Zn(II) ion, one organic ligand, two coordinated water molecules and three uncoordinated water molecules in the asymmetric unit of the title structure (cf. the figure, the asymmetric unit is labeled). Zn1, located at an inversion center, is six-coordinated with a distorted octahedral geometry by four coordinated water and two O atoms from two monodentate coordinating ligands. The Zn–O bond distances are in the range of 2.025(2) and 2.196(2) Å. The bond distances and bond angles are in normal ranges [8–10]. The carboxylate groups adopt trans conformations. The pyridine groups adopt chair conformations. The nitrogen atom N1 is protonated. There exist various O–H⋯O and N–H⋯O hydrogen bonds forming a three-dimensional framework. It is obvious that the hydrogen bonds play important roles in the self-assembly and enhance stability of the resultant structure.
Funding source: 2023 Guangxi Zhuang Autonomous Region New Engineering, New Medical, New Agricultural, and New Humanities Research and Practice Project
Award Identifier / Grant number: XYK202319
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financially supported by 2023 Guangxi Zhuang Autonomous Region New Engineering, New Medical, New Agricultural, and New Humanities Research and Practice Project (XYK202319).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku. CrysAlisPro; Rigaku Corporation: Yarnton, Oxfordshire, England, 2023.Suche in Google Scholar
2. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 3.2; Crystal Impact: Bonn, Germany, 2012.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
5. Zuo, S.-J., Ma, D.-L., Li, J., Guo, Q.-H., Zhou, L. Structural modification and antihypertensive activity study of formononetin derivatives. J. Asian Nat. Prod. Res. 2022, 24, 839–848; https://doi.org/10.1080/10286020.2021.2005588.Suche in Google Scholar PubMed
6. Yao, J.-N., Zhang, X.-X., Zhang, Y.-Z., Li, J.-H., Zhao, D.-Y., Gao, B., Zhou, H.-N., Gao, S.-L., Zhang, L.-F. Discovery and anticancer evaluation of a formononetin derivative against gastric cancer SGC7901 cells. Invest. New Drugs 2019, 37, 1300–1308; https://doi.org/10.1007/s10637-019-00767-7.Suche in Google Scholar PubMed
7. Lin, H.-Y., Sun, W.-X., Zheng, C.-S., Han, H.-W., Wang, X., Zhang, Y.-H., Qiu, H.-Y., Tang, C.-Y., Qi, J.-L., Lu, G.-H., Yang, R.-W., Wang, X.-M., Yang, Y.-H. Synthesis, characterization and biological evaluation of formononetin derivatives as novel EGFR inhibitors via inhibiting growth, migration and inducing apoptosis in breast cancer cell line. RSC Adv. 2017, 7, 48404–48419; https://doi.org/10.1039/c7ra09825a.Suche in Google Scholar
8. Chen, H.-L., Li, X., Yao, D.-M., Luo, Z.-P., Wang, Y.-P., Lan, J.-J. Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 1215–1217; https://doi.org/10.1515/ncrs-2023-0412.Suche in Google Scholar
9. Chen, H.-L., Pan, L.-W., Qin, Y.-L., Xie, Y.-J., Zhang, P. Synthesis and crystal structure of trans-tetraaqua-bis(3-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)propanoato-κO)zinc(II) tetrahydrate, C38H48N2O26S2Zn. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 217–219; https://doi.org/10.1515/ncrs-2018-0250.Suche in Google Scholar
10. Inomata, Y., Ando, M., Howell, F. S. Characterization and crystal structures of copper(II), cobalt(II), and nickel(II) complexes with two kinds of piperidine carboxylic acids. J. Mol. Struct. 2002, 616, 201–212; https://doi.org/10.1016/s0022-2860(02)00338-1.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

