Abstract
C26H27BN4, monoclinic, C2/c (no. 15), a = 38.716(3) Å, b = 9.2723(6) Å, c = 27.3977(18) Å, β = 113.4790(10)°, V = 9021.1(11) Å3, Z = 16, Rgt (F) = 0.0520, wRref (F 2) = 0.1440, T = 100 K.
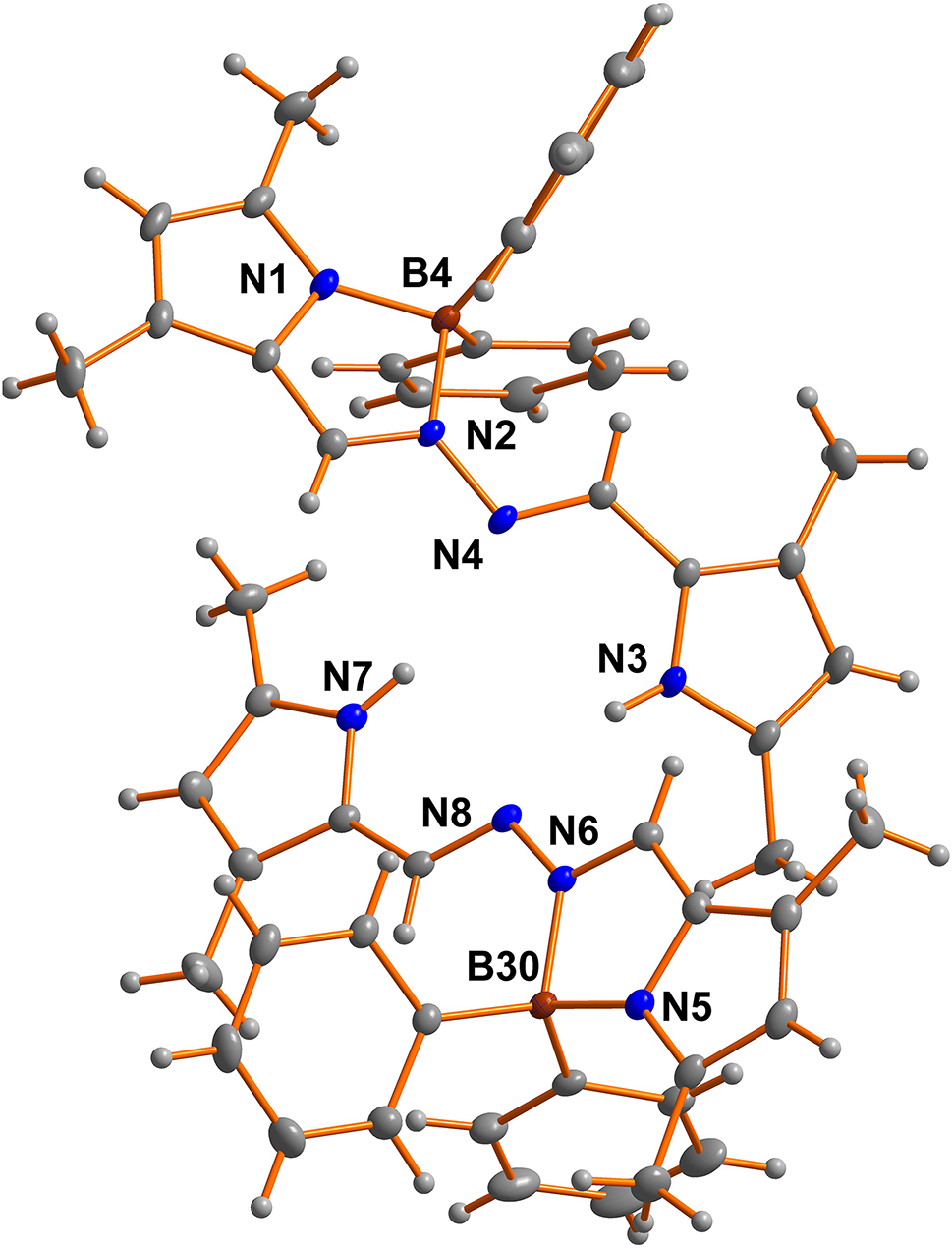
The asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow plate |
| Size: | 0.05 × 0.01 × 0.01 mm |
| Wavelength: | Synchrotron radiation (0.6889 Å) |
| μ: | 0.07 mm−1 |
| Diffractometer, scan mode: | Fluid film devices, φ and ω with 0.1° frames |
| θ max, completeness: | 29.5°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 64,902, 13,737, 0.066 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 10,590 |
| N(param)refined: | 573 |
| Programs: | CrysAlis pro [1], SHELX [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso */U eq |
|---|---|---|---|---|
| N1 | 0.24611 (3) | 0.65896 (11) | 0.34685 (4) | 0.0152 (2) |
| N2 | 0.29702 (3) | 0.49726 (11) | 0.36972 (4) | 0.01283 (18) |
| N3 | 0.40315 (3) | 0.28595 (11) | 0.45073 (4) | 0.01446 (19) |
| H3 | 0.3986 (4) | 0.2773 (18) | 0.4173 (7) | 0.017* |
| N4 | 0.33091 (3) | 0.42149 (11) | 0.39098 (4) | 0.01341 (19) |
| N5 | 0.44494 (3) | −0.15216 (11) | 0.34661 (4) | 0.01451 (19) |
| N6 | 0.41447 (3) | 0.06758 (11) | 0.34683 (4) | 0.01264 (18) |
| N7 | 0.36810 (3) | 0.50307 (11) | 0.31231 (4) | 0.01367 (19) |
| H7A | 0.3627 (4) | 0.4751 (18) | 0.3378 (6) | 0.016* |
| N8 | 0.39911 (3) | 0.20513 (11) | 0.34230 (4) | 0.01326 (18) |
| C1 | 0.21925 (3) | 0.65083 (14) | 0.25624 (5) | 0.0183 (2) |
| C2 | 0.19963 (3) | 0.74828 (14) | 0.27487 (5) | 0.0205 (3) |
| H2 | 0.1788 | 0.8024 | 0.2537 | 0.025* |
| C3 | 0.21657 (3) | 0.75088 (14) | 0.33079 (5) | 0.0187 (2) |
| C4 | 0.27923 (3) | 0.50295 (13) | 0.31761 (5) | 0.0149 (2) |
| H4 | 0.2863 | 0.4515 | 0.2940 | 0.018* |
| C5 | 0.24814 (3) | 0.59657 (13) | 0.30217 (5) | 0.0153 (2) |
| C6 | 0.39737 (3) | 0.33212 (13) | 0.52766 (5) | 0.0162 (2) |
| C7 | 0.43388 (3) | 0.27923 (14) | 0.53864 (5) | 0.0203 (2) |
| H7 | 0.4528 | 0.2653 | 0.5723 | 0.024* |
| C8 | 0.43682 (3) | 0.25154 (14) | 0.49081 (5) | 0.0188 (2) |
| C9 | 0.34281 (3) | 0.39855 (12) | 0.44186 (5) | 0.0130 (2) |
| H9 | 0.3273 | 0.4239 | 0.4591 | 0.016* |
| C10 | 0.37858 (3) | 0.33624 (12) | 0.47234 (4) | 0.0130 (2) |
| C11 | 0.21126 (4) | 0.61055 (17) | 0.19984 (5) | 0.0276 (3) |
| H11A | 0.1864 | 0.5700 | 0.1836 | 0.041* |
| H11B | 0.2294 | 0.5409 | 0.1993 | 0.041* |
| H11C | 0.2128 | 0.6950 | 0.1805 | 0.041* |
| C12 | 0.20565 (4) | 0.83487 (16) | 0.36880 (6) | 0.0271 (3) |
| H12A | 0.1968 | 0.7701 | 0.3887 | 0.041* |
| H12B | 0.1860 | 0.9015 | 0.3494 | 0.041* |
| H12C | 0.2271 | 0.8871 | 0.3928 | 0.041* |
| C13 | 0.38282 (4) | 0.38196 (15) | 0.56799 (5) | 0.0223 (3) |
| H13A | 0.3925 | 0.3204 | 0.5987 | 0.033* |
| H13B | 0.3558 | 0.3781 | 0.5528 | 0.033* |
| H13C | 0.3909 | 0.4793 | 0.5783 | 0.033* |
| C14 | 0.46908 (4) | 0.19499 (18) | 0.47990 (6) | 0.0269 (3) |
| H14A | 0.4664 | 0.0926 | 0.4744 | 0.040* |
| H14B | 0.4922 | 0.2154 | 0.5097 | 0.040* |
| H14C | 0.4694 | 0.2405 | 0.4486 | 0.040* |
| C15 | 0.30577 (3) | 0.72302 (13) | 0.43400 (5) | 0.0145 (2) |
| C16 | 0.32840 (3) | 0.71257 (14) | 0.48853 (5) | 0.0183 (2) |
| H16 | 0.3265 | 0.6307 | 0.5069 | 0.022* |
| C17 | 0.35365 (4) | 0.82052 (15) | 0.51619 (5) | 0.0232 (3) |
| H17 | 0.3683 | 0.8095 | 0.5523 | 0.028* |
| C18 | 0.35698 (4) | 0.94472 (15) | 0.48990 (6) | 0.0227 (3) |
| H18 | 0.3740 | 1.0166 | 0.5081 | 0.027* |
| C19 | 0.33456 (3) | 0.95978 (14) | 0.43616 (5) | 0.0204 (2) |
| H19 | 0.3362 | 1.0429 | 0.4182 | 0.024* |
| C20 | 0.30948 (3) | 0.85026 (14) | 0.40885 (5) | 0.0169 (2) |
| H20 | 0.2948 | 0.8622 | 0.3728 | 0.020* |
| C21 | 0.25614 (3) | 0.49982 (13) | 0.42871 (5) | 0.0153 (2) |
| C22 | 0.24846 (4) | 0.55218 (15) | 0.47120 (5) | 0.0201 (2) |
| H22 | 0.2590 | 0.6395 | 0.4868 | 0.024* |
| C23 | 0.22553 (4) | 0.47721 (16) | 0.49090 (6) | 0.0248 (3) |
| H23 | 0.2208 | 0.5149 | 0.5191 | 0.030* |
| C24 | 0.20988 (4) | 0.34677 (17) | 0.46852 (6) | 0.0264 (3) |
| H24 | 0.1945 | 0.2967 | 0.4815 | 0.032* |
| C25 | 0.21726 (4) | 0.29067 (16) | 0.42660 (6) | 0.0255 (3) |
| H25 | 0.2069 | 0.2027 | 0.4115 | 0.031* |
| C26 | 0.24024 (4) | 0.36678 (14) | 0.40731 (5) | 0.0202 (2) |
| H26 | 0.2451 | 0.3280 | 0.3794 | 0.024* |
| C27 | 0.43375 (3) | −0.28913 (13) | 0.40831 (5) | 0.0175 (2) |
| C28 | 0.45615 (3) | −0.36608 (14) | 0.38775 (5) | 0.0196 (2) |
| H28 | 0.4653 | −0.4590 | 0.3975 | 0.023* |
| C29 | 0.46249 (3) | −0.27972 (13) | 0.34988 (5) | 0.0174 (2) |
| C30 | 0.40967 (3) | −0.02301 (13) | 0.38073 (5) | 0.0144 (2) |
| H30 | 0.3961 | −0.0015 | 0.4013 | 0.017* |
| C31 | 0.42742 (3) | −0.15553 (13) | 0.38206 (5) | 0.0148 (2) |
| C32 | 0.38975 (4) | 0.50208 (13) | 0.24742 (5) | 0.0181 (2) |
| C33 | 0.37060 (4) | 0.63201 (14) | 0.24531 (5) | 0.0205 (2) |
| H33 | 0.3672 | 0.7062 | 0.2210 | 0.025* |
| C34 | 0.35765 (3) | 0.63074 (13) | 0.28564 (5) | 0.0169 (2) |
| C35 | 0.40286 (3) | 0.28191 (13) | 0.30507 (5) | 0.0133 (2) |
| H35 | 0.4162 | 0.2411 | 0.2868 | 0.016* |
| C36 | 0.38830 (3) | 0.42279 (12) | 0.28997 (5) | 0.0134 (2) |
| C37 | 0.41910 (4) | −0.33688 (15) | 0.44863 (6) | 0.0249 (3) |
| H37A | 0.4000 | −0.4088 | 0.4332 | 0.037* |
| H37B | 0.4086 | −0.2557 | 0.4596 | 0.037* |
| H37C | 0.4393 | −0.3767 | 0.4789 | 0.037* |
| C38 | 0.48429 (4) | −0.31359 (16) | 0.31717 (6) | 0.0250 (3) |
| H38A | 0.4675 | −0.3172 | 0.2803 | 0.038* |
| H38B | 0.4965 | −0.4053 | 0.3278 | 0.038* |
| H38C | 0.5029 | −0.2402 | 0.3224 | 0.038* |
| C39 | 0.40815 (5) | 0.45668 (17) | 0.21104 (6) | 0.0298 (3) |
| H39A | 0.4037 | 0.5284 | 0.1839 | 0.045* |
| H39B | 0.3978 | 0.3662 | 0.1948 | 0.045* |
| H39C | 0.4348 | 0.4463 | 0.2311 | 0.045* |
| C40 | 0.33591 (4) | 0.74289 (14) | 0.30055 (6) | 0.0216 (3) |
| H40A | 0.3094 | 0.7282 | 0.2804 | 0.032* |
| H40B | 0.3429 | 0.8369 | 0.2929 | 0.032* |
| H40C | 0.3414 | 0.7357 | 0.3379 | 0.032* |
| C41 | 0.48072 (3) | 0.08365 (13) | 0.34137 (5) | 0.0149 (2) |
| C42 | 0.48956 (3) | 0.19336 (15) | 0.31307 (6) | 0.0208 (2) |
| H42 | 0.4733 | 0.2115 | 0.2780 | 0.025* |
| C43 | 0.52218 (4) | 0.27609 (16) | 0.33629 (7) | 0.0286 (3) |
| H43 | 0.5272 | 0.3493 | 0.3169 | 0.034* |
| C44 | 0.54701 (4) | 0.24960 (18) | 0.38809 (7) | 0.0351 (4) |
| H44 | 0.5684 | 0.3063 | 0.4038 | 0.042* |
| C45 | 0.53984 (4) | 0.13815 (19) | 0.41650 (6) | 0.0311 (3) |
| H45 | 0.5568 | 0.1177 | 0.4510 | 0.037* |
| C46 | 0.50701 (3) | 0.05686 (16) | 0.39314 (5) | 0.0210 (3) |
| H46 | 0.5024 | −0.0175 | 0.4126 | 0.025* |
| C47 | 0.41858 (3) | −0.01123 (12) | 0.25487 (5) | 0.0137 (2) |
| C48 | 0.43772 (4) | −0.04160 (15) | 0.22195 (5) | 0.0200 (2) |
| H48 | 0.4637 | −0.0530 | 0.2374 | 0.024* |
| C49 | 0.41897 (4) | −0.05517 (16) | 0.16701 (5) | 0.0249 (3) |
| H49 | 0.4324 | −0.0759 | 0.1463 | 0.030* |
| C50 | 0.38008 (4) | −0.03782 (16) | 0.14297 (5) | 0.0249 (3) |
| H50 | 0.3674 | −0.0478 | 0.1063 | 0.030* |
| C51 | 0.36033 (4) | −0.00554 (14) | 0.17412 (5) | 0.0212 (3) |
| H51 | 0.3344 | 0.0077 | 0.1583 | 0.025* |
| C52 | 0.37942 (3) | 0.00700 (13) | 0.22923 (5) | 0.0156 (2) |
| H52 | 0.3658 | 0.0281 | 0.2496 | 0.019* |
| B4 | 0.27685 (3) | 0.59844 (14) | 0.39981 (5) | 0.0138 (2) |
| B30 | 0.44094 (3) | −0.00250 (14) | 0.31856 (5) | 0.0132 (2) |
1 Source of materials
Treatment of the tetramethyl bis-(pyrrole imine) hydrazine with one equivalent of BPh3 in toluene for 1 h resulted in formation of the titled compound N-(4,6-dimethyl-1,1-diphenyl-1l4,7l4-pyrrolo[2,1-e][1,3,2]diazaborol-2(1H)-yl)-1-(3,5-dimethyl-1H-pyrrol-2-yl)methanimine. This was isolated by column chromatography in 54 % yield. Good quality single crystals were obtained by slow evaporation from a DCM/hexane mixtures (1:2).
2 Experimental details
The structure of the title compound was solved using SHELXT [2] and refined by SHELXL program [3] through the Olex2 interface [4]. All hydrogen atoms were positioned at calculated coordinates and refined isotropically.
3 Comment
In recent years BOPHY dyes have rapidly found utility in various applications due to their outstanding photophysical characteristics and facile synthesis [5], [6], [7], [8], including energy-transfer cascades [9], cell imaging [10], photosensitizers for solar cells [11], photodynamic therapy [12], [13], and fluorescence sensors [9], [10]. Consequently, there is a need for synthetic modifications capable of modulating or enhancing the photophysical and photochemical properties of BOPHY dyes to extend their applications further [14]. There are two crystallographically independent molecules in the asymmetric unit. Single structural analysis revealed the presence of the five-membered ring. The compound showed a highly twisted conformation of the BOPHY core in solid state owing to the presence of the two phenyl rings with dihedral angles of 85.5° and 61.75° respectively. The two adjacent BOPHY molecules form a dimer structure via hydrogen-bonds.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R76), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. A. K thanks the support of King Abdullah International Medical Research Center (KAIMRC) through start up grant NRC23R/746/11.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlispro; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2015.Suche in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Tamgho, I. S., Hasheminasab, A., Engle, J. T., Nemykin, V. N., Ziegler, C. J. A new highly fluorescent and symmetric pyrrole–BF2 chromophore: BOPHY. J. Am. Chem. Soc. 2014, 136, 5623–5626; https://doi.org/10.1021/ja502477a.Suche in Google Scholar PubMed
6. Wang, L., Tamgho, I. S., Crandall, L. A., Rack, J. J., Ziegler, C. J. Ultrafast dynamics of a new class of highly fluorescent boron difluoride dyes. Phys. Chem. Chem. Phys. 2015, 17, 2349–2351; https://doi.org/10.1039/c4cp04737k.Suche in Google Scholar PubMed
7. Yu, C., Jiao, L., Zhang, P., Feng, Z., Cheng, C., Wei, Y., Mu, X., Hao, E. Highly fluorescent BF2 complexes of hydrazine-Schiff base linked bispyrrole. Org. Lett. 2014, 16, 3048–3051; https://doi.org/10.1021/ol501162f.Suche in Google Scholar PubMed
8. Huaulmé, Q., Mirloup, A., Retailleau, P., Ziessel, R. Synthesis of highly functionalized BOPHY chromophores displaying large Stokes shifts. Org. Lett. 2005, 17, 2246–2249; https://doi.org/10.1021/acs.orglett.5b00858.Suche in Google Scholar PubMed
9. Dai, C., Yang, D., Zhang, W., Bao, B., Cheng, Y., Wang, L. Far-red/near- infrared fluorescent conjugated polymer nanoparticles with size-dependent chirality and cell imaging applications. Polym. Chem. 2015, 6, 3962–3969; https://doi.org/10.1039/c5py00344j.Suche in Google Scholar
10. Zhang, C., Zhao, J. Triplet excited state of diiodo BOPHY derivatives: preparation, study of photophysical properties and application in triplet-triplet annihilation upconversion. J. Mater. Chem. C 2016, 4, 1623–1632; https://doi.org/10.1039/c5tc03193a.Suche in Google Scholar
11. Cui, T.-F., Zhang, J., Jiang, X.-D., Su, Y.-J., Sun, C.-L., Zhao, J.-L. Synthesis dibromo substituted BOPHY dye for the singlet oxygen generation. Chin. Chem. Lett. 2016, 27, 190–194; https://doi.org/10.1016/j.cclet.2015.11.010.Suche in Google Scholar
12. Jiang, X.-D., Su, Y., Yue, S., Li, C., Yu, H., Zhang, H., Sun, C.-L., Xiao, L.-J. Synthesis of mono-(p-dimethylamino)styryl-containing BOPHY dye for a turn-on pH sensor. RSC Adv. 2015, 5, 16735–16739; https://doi.org/10.1039/c4ra15914d.Suche in Google Scholar
13. Li, Y. D., Zhou, H., Yin, S., Jiang, H., Niu, N., Huang, H., Shahzad, S. A., Yu, C. A BOPHY probe for the fluorescence turn-on detection of Cu2+. Sens. Actuators, B 2016, 235, 33–38; https://doi.org/10.1016/j.snb.2016.05.055.Suche in Google Scholar
14. Lv, X., Li, T., Wu, Q., Yu, C., Jiao, L., Hao, E. Polybrominated BOPHY dyes: synthesis, reactivity, and properties. J. Org. Chem. 2018, 83, 1134–1145; https://doi.org/10.1021/acs.joc.7b02415.Suche in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

