Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Abstract
C36H52FNO3, orthorhombic, P212121 (no. 19), a = 7.8101(5) Å, b = 13.1592(7) Å, c = 30.5721(15) Å, V = 3142.0(3) Å3, Z = 4, R gt(F) = 0.0488, wR ref(F 2) = 0.1048, T = 293 K.
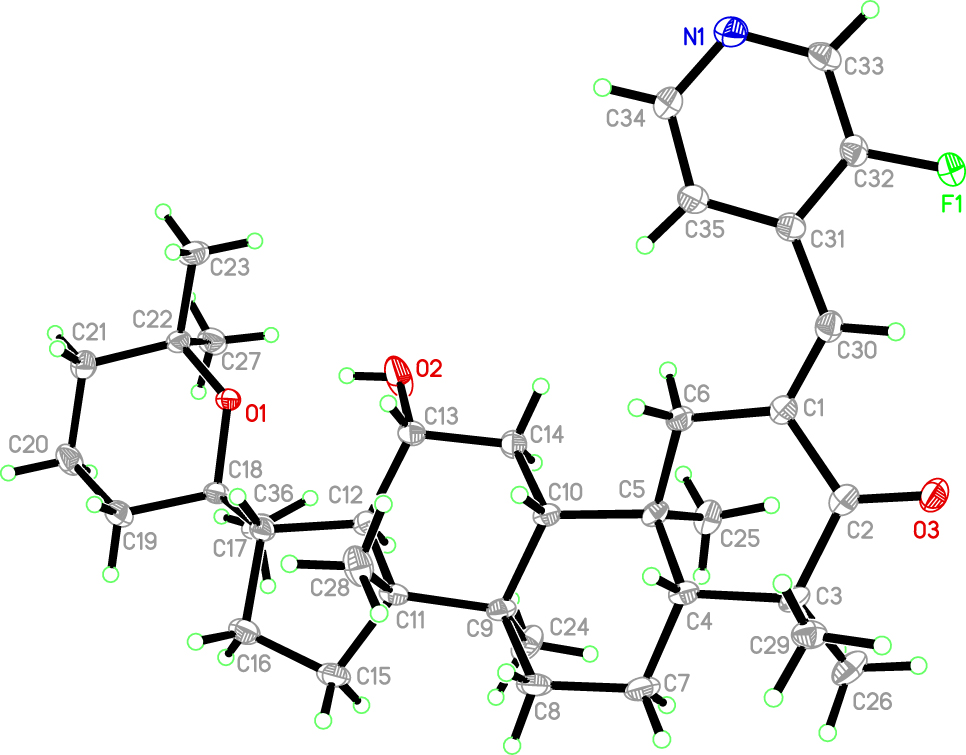
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.14 × 0.13 × 0.10 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | SuperNova |
| θ max, completeness: | 25.5°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 14,083, 5656, 0.040 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 4598 |
| N(param)refined: | 380 |
| Programs: | CrysAlis Pro [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.2736 (4) | −0.3268 (2) | 0.60107 (10) | 0.0250 (7) |
| C2 | 0.3357 (4) | −0.3750 (2) | 0.64263 (10) | 0.0273 (7) |
| C3 | 0.3896 (4) | −0.3084 (2) | 0.68110 (9) | 0.0248 (7) |
| C4 | 0.3414 (4) | −0.1948 (2) | 0.67523 (9) | 0.0248 (7) |
| H4 | 0.434761 | −0.166349 | 0.657657 | 0.030* |
| C5 | 0.1788 (4) | −0.1746 (2) | 0.64796 (9) | 0.0230 (7) |
| C6 | 0.2154 (4) | −0.2176 (2) | 0.60193 (9) | 0.0268 (7) |
| H6A | 0.112144 | −0.211619 | 0.584477 | 0.032* |
| H6B | 0.302740 | −0.175984 | 0.588209 | 0.032* |
| C7 | 0.3467 (5) | −0.1351 (2) | 0.71759 (10) | 0.0361 (9) |
| H7A | 0.452246 | −0.150402 | 0.732964 | 0.043* |
| H7B | 0.251940 | −0.155955 | 0.736044 | 0.043* |
| C8 | 0.3359 (5) | −0.0217 (3) | 0.70952 (10) | 0.0382 (9) |
| H8A | 0.435606 | −0.000437 | 0.692989 | 0.046* |
| H8B | 0.338266 | 0.013350 | 0.737416 | 0.046* |
| C9 | 0.1737 (4) | 0.0098 (2) | 0.68452 (9) | 0.0254 (7) |
| C10 | 0.1569 (4) | −0.0567 (2) | 0.64259 (9) | 0.0230 (7) |
| H10 | 0.253618 | −0.035975 | 0.624202 | 0.028* |
| C11 | 0.1867 (4) | 0.1231 (2) | 0.66841 (9) | 0.0262 (7) |
| C12 | 0.0233 (4) | 0.1541 (2) | 0.64317 (10) | 0.0252 (7) |
| H12 | −0.073750 | 0.142944 | 0.662913 | 0.030* |
| C13 | −0.0052 (5) | 0.0868 (3) | 0.60347 (11) | 0.0388 (9) |
| H13 | 0.084795 | 0.099753 | 0.581837 | 0.047* |
| C14 | −0.0025 (5) | −0.0254 (2) | 0.61640 (12) | 0.0357 (9) |
| H14A | −0.008457 | −0.066389 | 0.590070 | 0.043* |
| H14B | −0.103623 | −0.040044 | 0.633713 | 0.043* |
| C15 | 0.1902 (5) | 0.2064 (2) | 0.70403 (11) | 0.0399 (9) |
| H15A | 0.113487 | 0.189292 | 0.727874 | 0.048* |
| H15B | 0.304927 | 0.214836 | 0.715601 | 0.048* |
| C16 | 0.1307 (5) | 0.3032 (2) | 0.68082 (10) | 0.0351 (8) |
| H16A | 0.227824 | 0.346907 | 0.674720 | 0.042* |
| H16B | 0.050710 | 0.340182 | 0.699141 | 0.042* |
| C17 | 0.0425 (4) | 0.2707 (2) | 0.63743 (10) | 0.0242 (7) |
| H17 | 0.125563 | 0.281737 | 0.613869 | 0.029* |
| C18 | −0.1180 (4) | 0.3348 (2) | 0.62630 (9) | 0.0245 (7) |
| C19 | −0.0723 (5) | 0.4475 (2) | 0.62659 (11) | 0.0324 (8) |
| H19A | −0.063167 | 0.470455 | 0.656640 | 0.039* |
| H19B | 0.038606 | 0.456517 | 0.612860 | 0.039* |
| C20 | −0.2032 (5) | 0.5125 (2) | 0.60289 (11) | 0.0375 (9) |
| H20A | −0.166269 | 0.582899 | 0.602909 | 0.045* |
| H20B | −0.312562 | 0.508568 | 0.617834 | 0.045* |
| C21 | −0.2227 (5) | 0.4751 (2) | 0.55592 (11) | 0.0342 (8) |
| H21A | −0.310335 | 0.514863 | 0.541353 | 0.041* |
| H21B | −0.115732 | 0.485559 | 0.540416 | 0.041* |
| C22 | −0.2707 (4) | 0.3631 (2) | 0.55375 (10) | 0.0271 (8) |
| C23 | −0.2353 (5) | 0.3204 (3) | 0.50834 (10) | 0.0407 (9) |
| H23A | −0.116555 | 0.329633 | 0.501283 | 0.061* |
| H23B | −0.304662 | 0.355371 | 0.487232 | 0.061* |
| H23C | −0.262421 | 0.249241 | 0.507876 | 0.061* |
| C24 | 0.0209 (5) | −0.0033 (3) | 0.71602 (11) | 0.0426 (10) |
| H24A | 0.017282 | −0.072198 | 0.726335 | 0.064* |
| H24B | −0.083650 | 0.012140 | 0.700903 | 0.064* |
| H24C | 0.033974 | 0.041916 | 0.740428 | 0.064* |
| C25 | 0.0182 (4) | −0.2288 (2) | 0.66558 (11) | 0.0341 (8) |
| H25A | −0.080099 | −0.207812 | 0.649044 | 0.051* |
| H25B | 0.002056 | −0.211505 | 0.695803 | 0.051* |
| H25C | 0.032559 | −0.301023 | 0.662878 | 0.051* |
| C26 | 0.3226 (5) | −0.3560 (3) | 0.72356 (10) | 0.0385 (9) |
| H26A | 0.200502 | −0.348153 | 0.724964 | 0.058* |
| H26B | 0.374240 | −0.322590 | 0.748169 | 0.058* |
| H26C | 0.351060 | −0.426917 | 0.724130 | 0.058* |
| C27 | −0.4583 (5) | 0.3454 (3) | 0.56552 (11) | 0.0378 (9) |
| H27A | −0.479512 | 0.273782 | 0.567913 | 0.057* |
| H27B | −0.530075 | 0.373634 | 0.543107 | 0.057* |
| H27C | −0.483262 | 0.377737 | 0.592940 | 0.057* |
| C28 | 0.3478 (4) | 0.1416 (2) | 0.64014 (12) | 0.0391 (9) |
| H28A | 0.357358 | 0.212725 | 0.633615 | 0.059* |
| H28B | 0.338415 | 0.103850 | 0.613372 | 0.059* |
| H28C | 0.447548 | 0.119693 | 0.655865 | 0.059* |
| C29 | 0.5871 (4) | −0.3165 (3) | 0.68134 (10) | 0.0302 (8) |
| H29A | 0.620064 | −0.385251 | 0.687705 | 0.045* |
| H29B | 0.633022 | −0.272005 | 0.703276 | 0.045* |
| H29C | 0.630984 | −0.297406 | 0.653180 | 0.045* |
| C30 | 0.2775 (4) | −0.3863 (2) | 0.56560 (10) | 0.0289 (8) |
| H30 | 0.316251 | −0.452238 | 0.570331 | 0.035* |
| C31 | 0.2294 (4) | −0.3627 (2) | 0.52022 (10) | 0.0271 (8) |
| C32 | 0.1819 (4) | −0.4402 (2) | 0.49228 (10) | 0.0277 (7) |
| C33 | 0.1323 (4) | −0.4243 (3) | 0.44958 (10) | 0.0318 (8) |
| H33 | 0.099547 | −0.479751 | 0.432689 | 0.038* |
| C34 | 0.1780 (6) | −0.2557 (3) | 0.45767 (11) | 0.0436 (10) |
| H34 | 0.178044 | −0.190536 | 0.445946 | 0.052* |
| C35 | 0.2278 (5) | −0.2669 (3) | 0.50075 (11) | 0.0393 (9) |
| H35 | 0.260412 | −0.210137 | 0.516866 | 0.047* |
| C36 | −0.2667 (5) | 0.3112 (3) | 0.65699 (10) | 0.0362 (9) |
| H36A | −0.351862 | 0.363485 | 0.654524 | 0.054* |
| H36B | −0.225632 | 0.308380 | 0.686570 | 0.054* |
| H36C | −0.316076 | 0.246914 | 0.649212 | 0.054* |
| F1 | 0.1806 (3) | −0.53701 (13) | 0.50769 (6) | 0.0389 (5) |
| N1 | 0.1299 (4) | −0.3320 (2) | 0.43173 (9) | 0.0384 (7) |
| O1 | −0.1569 (3) | 0.30433 (14) | 0.58156 (6) | 0.0254 (5) |
| O2 | −0.1682 (5) | 0.10256 (19) | 0.58389 (12) | 0.0840 (12) |
| H2 | −0.183301 | 0.163538 | 0.579934 | 0.126* |
| O3 | 0.3489 (3) | −0.46721 (16) | 0.64538 (7) | 0.0382 (6) |
1 Source of material
Total saponins of ginseng stem and leaves were degraded by 18 % sulfuric acid in ethanol and refluxed for 4 h. Cooled to room temperature, the reaction solution was adjusted to neutral with appropriate amount of sodium carbonate, the residue was extracted with ethyl acetate, dried with anhydrous sodium sulfate, filtered, and concentrated under pressure to obtain the crude product, and the crude product was recrystallized with ethyl acetate to obtain the pure product of 20(R)-panaxadiol. 20(R)–Panaxadiol and PCC were dissolved in 25 mL of dichloromethane solution and purified by silica gel column chromatography after stirring at room temperature for 5 h to obtain 20(R)-3-oxopanaxadiol. 20(R)-3-Oxopanaxadiol (100 mg, 0.22 mmol) and 3-fluoropyridine-4-carboxaldehyde (0.02 mL, 0.22 mmol) were dissolved in 1.4 mL of methanol, 0.72 mL of 25 % aqueous sodium hydroxide solution was added and stirred at room temperature for 5 h. The response endpoint was detected by thin layer chromatography (TLC). At the end of the reaction, it was extracted with ethyl acetate and washed with brine. The product was concentrated under reduced pressure and purified by silica gel column chromatography. The single crystal of the title compound was obtained by recrystallization with ethyl acetate solution.
2 Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d (C–H) = 0.96 Å (methyl), U iso(H) = 1.5U eq(C), and d(C–H) = 0.97 Å (methylene), U iso(H) = 1.2U eq(C), and d(C–H) = 0.98 Å (methyne), U iso(H) = 1.2U eq(C), and d(C–H) = 0.93 Å (aromatic), U iso(H) = 1.2U eq(C). H atoms on hydroxyl group were located in different maps and treated as riding.
3 Comment
Ginsenosides are divided into protopanoxadiol-type sapogenin, protopanoxatriol-type sapogenin and oleanolic acid type sapogenin [4]. The configuration of C20 of 20(S)-protopanoxadiol-type sapogenin is easily converted to R type when treated with an inorganic acid [5]. The synthesis and single crystal X-ray diffraction of 20(S)-propanaxanediol, 20(R)-propanaxanediol, 20(R)-panaxadiol and their derivatives have been studied in our group [6], [7], [8], [9], [10]. The title compound is the 20(R)-panaxadiol derivative.
The title compound has a dammarane type as the parent structure [11], with a pyridine ring structure attached to C(1). An α,β-unsaturated ketone group is formed between the pyridine group and the parent nucleus by the Claisen–Schmidt reaction effect. The angle of twist of C(30)=C(1)–C(2)=O(3) is −16.5(5)°. The bond length of C(30)=C(1) is 1.338(4) Å. The bond length of C(2)=O(3) is 1.221(4) Å. Formation of a Z-configuration between the pyridine ring and the carbonyl group [12]. In addition, the pyridinyl group is substituted with a fluorine atom and the C–F bond length is 1.359(3) Å. The presence of α,β-unsaturated ketone groups and fluorine atoms in the molecule facilitates the enhancement of the biological activity of the molecule [13]. The first ring contains a carbon-oxygen double bond and the bond length of C(2)–O(3) is 1.221(4) Å. The bond length of C(13)–O(2) is 1.422(4) Å. Bond length of C(22)–O(1), C(18)–O(1) in the tetrahydropyran ring are 1.455(3), 1.458(3) Å respectively. The molecules C(5), C(8), C(9), C(10), C(12), C(13), C(14), and C(17) are all chiral carbons, all of which are in the R conformation except C(17), which is in the S conformation. There is a pyran ring substitution at the C17 position, and the oxygen atom on the ring acts as a hydrogen bond acceptor to form an intramolecular O–H⋯O hydrogen bond. In the crystal structure, the pyridine ring has a planar conformation, and all the six-membered rings, except the ketone-containing ring, have a chair conformation with bond lengths and angles within the expected range.
Funding source: Science and Technology Innovation Development Plan of Yantai
Award Identifier / Grant number: 2020XDRH105
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81473104
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Science and Technology Innovation Development Plan of Yantai (No. 2020XDRH105) and the National Natural Science Foundation of China (No. 81473104).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlispro; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Luo, Q., Meng, Q. G., Hou, G. G., Jiang, S., Jin, Y. S., Gao, Y. Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one-water (2/1), C37H56NO4.5. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1223–1226; https://doi.org/10.1515/ncrs-2021-0284.Search in Google Scholar
5. Liu, L., Wang, H. Y., Zhou, X., Zhang, S. N., Chen, X. Q., Zhao, F. L., Meng, Q. G. The crystal structure of (3S,8R,10R,14R)-17-((2S,5S)-5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)-4,4,8,10,14-pentamethyl-12-oxohexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H52O5. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1547–1549; https://doi.org/10.1515/ncrs-2020-0384.Search in Google Scholar
6. Liu, Z., Xu, Y. R., An, X. S., Yang, J. J., Meng, Q. G., Hou, G. G. Synthesis and crystal structure of ocotillol-type metabolites derived from (20R)-protopanaxadiol. J. Chem. Res. 2017, 41, 216–220; https://doi.org/10.3184/174751917x14894997017612.Search in Google Scholar
7. Xu, Y. R., Yang, J. J., Liu, J., Hou, G. G., Meng, Q. G. Synthesis and crystal structures of C24-epimeric 20(R)-ocotillol-type saponins. Acta Crystallogr. 2016, C72, 498–503; https://doi.org/10.1107/s2053229616007270.Search in Google Scholar PubMed
8. Deng, J. Q., Mu, X. D., Zhao, R. L., Liu, Z., Tang, H. J., He, M., Meng, Q. G. Crystal structure of (20R)-20,25-epoxy-dammaran-3,12-dione, C30H48O3. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 145–147; https://doi.org/10.1515/ncrs-2018-0237.Search in Google Scholar
9. Ma, Y., Wang, H. Y., Zhang, X. F., Zhao, F. L., Meng, Q. G. Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 163–166; https://doi.org/10.1515/ncrs-2020-0311.Search in Google Scholar
10. Wang, C. M., Liu, J., Deng, J. Q., Wang, J. Z., Weng, W. Z., Chu, H. X., Meng, Q. G. Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. J. Ginseng Res. 2020, 44, 14–23; https://doi.org/10.1016/j.jgr.2019.01.005.Search in Google Scholar PubMed PubMed Central
11. Zhang, M., Meng, Q. G., Hou, G. G., Jiang, S., Jin, Y. S., Gao, Y. Crystal structure of (8R,10R,14R,Z)-2-((3-fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1139–1142; https://doi.org/10.1515/ncrs-2021-0248.Search in Google Scholar
12. Landge, S. M., Aprahamian, I. A pH activated configurational rotary switch: controlling the E/Z isomerization in hydrazones. J. Am. Chem. Soc. 2009, 131, 18269–18271; https://doi.org/10.1021/ja909149z.Search in Google Scholar PubMed
13. Al-Wahaibi, L. H., Abdelbaky, M. S. M., Garcia-Granda, S., Reiss, G. J., Tiekink, E. R. T., El-Emam, A. A. N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 821–825; https://doi.org/10.1515/ncrs-2023-0195.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

