Abstract
C12H15NO3, monoclinic, P21/c (no. 14), a = 6.7230(2) Å, b = 11.1182(3) Å, c = 14.4996(5) Å, β =
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
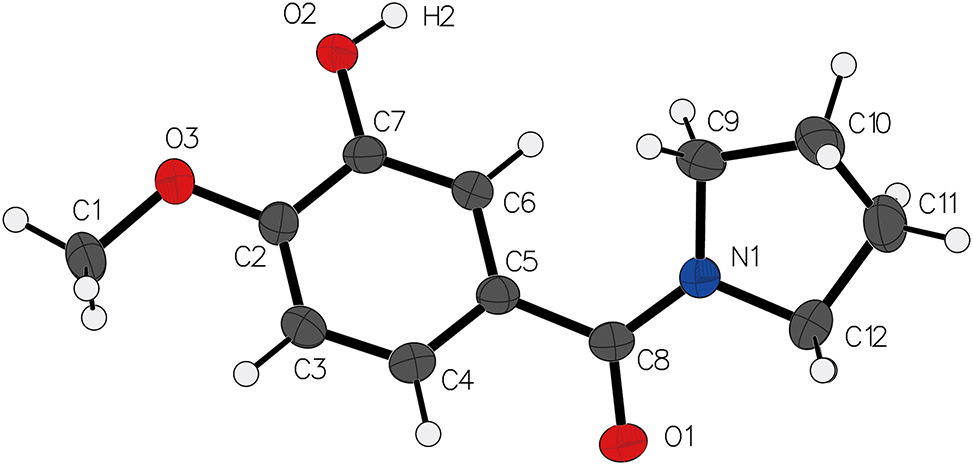
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.15 × 0.12 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture, φ and ω |
| θmax, completeness: | 27.5°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 11,456, 2464, 0.075 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 1826 |
| N(param)refined: | 147 |
| Programs: | Bruker [1], SHELX [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | −0.2490 (3) | 0.53479 (18) | 0.91431 (14) | 0.0388 (5) |
| H1A | −0.323975 | 0.559521 | 0.856501 | 0.058* |
| H1B | −0.168916 | 0.602558 | 0.939835 | 0.058* |
| H1C | −0.342298 | 0.509485 | 0.958918 | 0.058* |
| C2 | 0.0249 (3) | 0.45953 (15) | 0.83822 (12) | 0.0282 (4) |
| C3 | 0.0327 (3) | 0.56059 (15) | 0.78249 (12) | 0.0303 (4) |
| H3 | −0.069081 | 0.619808 | 0.782949 | 0.036* |
| C4 | 0.1873 (3) | 0.57615 (16) | 0.72614 (12) | 0.0298 (4) |
| H4 | 0.190937 | 0.646010 | 0.688599 | 0.036* |
| C5 | 0.3368 (3) | 0.49017 (15) | 0.72424 (11) | 0.0261 (4) |
| C6 | 0.3298 (3) | 0.38858 (15) | 0.78090 (12) | 0.0281 (4) |
| H6 | 0.432736 | 0.330018 | 0.781001 | 0.034* |
| C7 | 0.1752 (3) | 0.37228 (14) | 0.83674 (12) | 0.0273 (4) |
| C8 | 0.5060 (3) | 0.51677 (15) | 0.66691 (11) | 0.0269 (4) |
| C9 | 0.5491 (3) | 0.30011 (16) | 0.61949 (14) | 0.0393 (5) |
| H9A | 0.405120 | 0.289784 | 0.600635 | 0.047* |
| H9B | 0.581753 | 0.258411 | 0.679190 | 0.047* |
| C10 | 0.6761 (3) | 0.25258 (18) | 0.54536 (14) | 0.0417 (5) |
| H10A | 0.714406 | 0.167674 | 0.557383 | 0.050* |
| H10B | 0.604099 | 0.258747 | 0.483085 | 0.050* |
| C11 | 0.8579 (3) | 0.33404 (19) | 0.55372 (15) | 0.0436 (5) |
| H11A | 0.926715 | 0.333648 | 0.496003 | 0.052* |
| H11B | 0.953434 | 0.309333 | 0.606028 | 0.052* |
| C12 | 0.7694 (3) | 0.45682 (17) | 0.57096 (13) | 0.0337 (4) |
| H12A | 0.868337 | 0.509628 | 0.605437 | 0.040* |
| H12B | 0.721566 | 0.496397 | 0.512046 | 0.040* |
| N1 | 0.6027 (2) | 0.42863 (13) | 0.62650 (10) | 0.0287 (4) |
| O1 | 0.5551 (2) | 0.62399 (11) | 0.65738 (9) | 0.0368 (4) |
| O2 | 0.1632 (2) | 0.27443 (11) | 0.89271 (9) | 0.0360 (4) |
| H2 | 0.248337 | 0.223156 | 0.879859 | 0.054* |
| O3 | −0.1202 (2) | 0.43677 (11) | 0.89632 (10) | 0.0385 (4) |
1 Source of materials
A mixture of 3-hydroxy-4-methoxybenzoic acid (1.68 g, 10 mmol), pyrrolidine (2.13 g, 30 mmol), 2-(7-azabenzotriazol-1-yl)-N,N,
2 Experimental details
The intensity data were processed using the Bruker Saint software [1]. All hydrogen atoms were treated as riding atoms, with their positions idealized and their Uiso values set to 1.2 times the Ueq of their respective parent atoms. The crystal structure was solved using ShelXT [2] and refined with ShelXL [3]. The entire refinement process was conducted using the Olex2 software [4].
3 Comment
Phenyl(pyrrolidin-1-yl)methanone derivatives have exhibited notable bioactivity, thereby resulting in extensive investigation and reporting of numerous associated crystal structures [5], [6], [7], [8], [9]. In the present investigation, we report the elucidation of the crystal structure of a newly synthesized compound, namely (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, which belongs to the class of phenyl(pyrrolidin-1-yl)methanone derivatives.
In the context of this molecular configuration, the hydrogen moieties in the benzene ring have been substituted by hydroxyl and methoxy functional groups. Notably, the hydroxyl moiety reveals a carbon-oxygen bond (C7–O2) length measuring 1.364(3) Å, whereas the methoxy moiety exhibits a carbon-oxygen bond (C2–O3) length of 1.366(3) Å. Furthermore, the carbon–oxygen–carbon bond angle (C2–O3–C1) within the methoxy group is determined to be
In the crystalline structure, an intermolecular hydrogen bonding interaction between the hydroxyl and ketone groups (O2–H2⋯O1) has been identified. Notably, the O2–H2⋯O1 hydrogen bond exhibits a bond length of 1.8371(14) Å and a bond angle of
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
-
Research funding: Natural Science Foundation of Shannxi Province (2021JM-561), Doctoral research fund project of Xianyang Vocational and Technical College (2021BK01) and the Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang City (2021QXNL–PT-0008).
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Wang, Y., Wang, Z., Li, Y., Wu, G., Cao, Z., Zhang, L. A general ligand design for gold catalysis allowing ligand-directed anti-nucleophilic attack of alkynes. Nat. Commun. 2014, 5, 3470; https://doi.org/10.1038/ncomms4470.Search in Google Scholar PubMed PubMed Central
6. Zhu, J., Wang, J., Dong, G. Catalytic activation of unstrained C(aryl)–C(aryl) bonds in 2,2′-biphenols. Nat. Chem. 2019, 11, 45–51; https://doi.org/10.1038/s41557-018-0157-x.Search in Google Scholar PubMed PubMed Central
7. Kiren, S., Padwa, A. A benzannulation protocol to prepare substituted aryl amines using a Michael-aldol reaction of β-keto sulfones. J. Org. Chem. 2009, 74, 7781–7789; https://doi.org/10.1021/jo9017793.Search in Google Scholar PubMed
8. Tang, Z., Mo, K., Ma, X., Huang, J., Zhao, D. para-Selective radical trifluoromethylation of benzamide derivatives via iminium intermediates. Angew. Chem. Int. Ed. 2022, 61, e202208089; https://doi.org/10.1002/ange.202208089.Search in Google Scholar
9. Holtz, E., Albrecht, U., Langer, P. One-pot reactions of 2,4-(dioxobutylidene)phosphoranes. Efficient synthesis of 4-(2-hydroxybenzoyl)salicylic acid derivatives and buta-1,3-diene-1,4-dicarboxylates. Tetrahedron 2007, 63, 3293–3301; https://doi.org/10.1016/j.tet.2007.02.062.Search in Google Scholar
10. Garrec, J., Cordier, M., Frison, G., Prévost, S. Palladium-catalyzed C8-arylation of naphthalenes through C–H activation: a combined experimental and computational study. Chem. Eur. J. 2019, 25, 14441–14446; https://doi.org/10.1002/chem.201903500.Search in Google Scholar PubMed
11. Ning, X.-Q., Lou, S.-J., Mao, Y.-J., Xu, Z.-Y., Xu, D.-Q. Nitrate-promoted selective C–H fluorination of benzamides and benzeneacetamides. Org. Lett. 2018, 20, 2445–2448; https://doi.org/10.1021/acs.orglett.8b00793.Search in Google Scholar PubMed
12. Wu, X., Fan, W., Pan, Y., Zhai, Y., Niu, Y., Li, C., Mei, Q. Synthesis, crystal structure and anti-fatigue effects of some benzamide derivatives. Molecules 2014, 19, 1034–1046; https://doi.org/10.3390/molecules19011034.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

