Abstract
C10H11NO2, monoclinic, P21/n (no. 14), a = 3.7617(3) Å, b = 12.9267(10) Å, c = 23.4609(17) Å, β = 92.337(3)°, V = 1139.87(15) Å3, Z = 4, R gt (F) = 0.0413, wR ref (F 2) = 0.1071, T = 100 K.
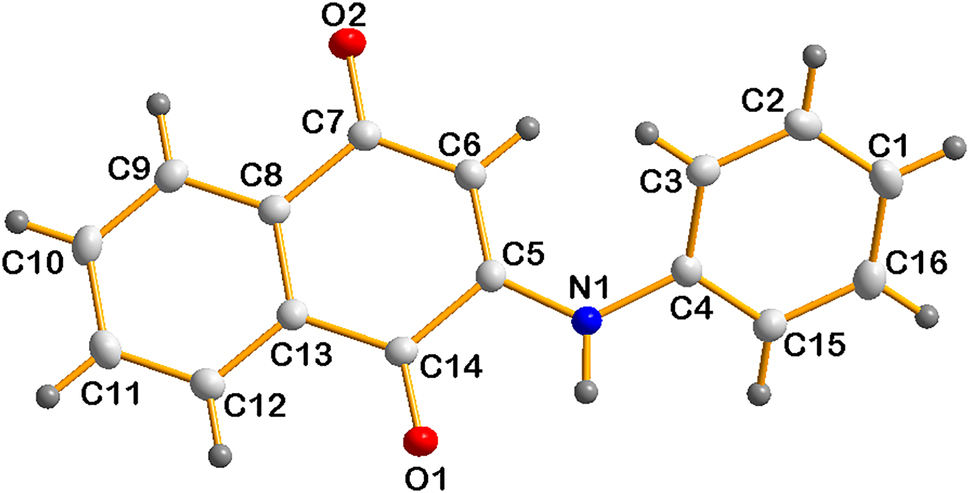
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red needle |
| Size: | 0.43 × 0.08 × 0.04 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture 5K Kappa Photon III |
| θ max, completeness: | 28.3°, >99 % |
| N(hkl)measured , N(hkl)unique, R int: | 19,336, 2811, 0.049 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2294 |
| N(param)refined: | 176 |
| Programs: | SHELX [1], Bruker [2], Olex2 [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| O1 | 0.0055 (3) | 0.58217 (7) | 0.54884 (4) | 0.0222 (2) |
| O2 | 0.7062 (3) | 0.53632 (7) | 0.75009 (4) | 0.0244 (2) |
| N1 | 0.2742 (3) | 0.39814 (8) | 0.57225 (5) | 0.0181 (2) |
| C1 | 0.5409 (4) | 0.08329 (10) | 0.58117 (6) | 0.0246 (3) |
| H1A | 0.601030 | 0.011946 | 0.582759 | 0.029* |
| C2 | 0.4078 (4) | 0.13178 (10) | 0.62849 (6) | 0.0238 (3) |
| H2 | 0.376798 | 0.093575 | 0.662524 | 0.029* |
| C3 | 0.3196 (4) | 0.23610 (10) | 0.62628 (5) | 0.0202 (3) |
| H3 | 0.225596 | 0.269034 | 0.658583 | 0.024* |
| C4 | 0.3695 (3) | 0.29224 (10) | 0.57662 (5) | 0.0176 (3) |
| C5 | 0.3273 (3) | 0.47199 (9) | 0.61246 (5) | 0.0165 (2) |
| C6 | 0.5037 (3) | 0.46219 (9) | 0.66401 (5) | 0.0181 (3) |
| H6 | 0.601033 | 0.396728 | 0.674412 | 0.022* |
| C7 | 0.5485 (3) | 0.54705 (9) | 0.70343 (5) | 0.0174 (3) |
| C8 | 0.4056 (3) | 0.65055 (9) | 0.68593 (5) | 0.0161 (2) |
| C9 | 0.4586 (3) | 0.73502 (10) | 0.72194 (5) | 0.0188 (3) |
| H9 | 0.579777 | 0.726369 | 0.757950 | 0.023* |
| C10 | 0.3345 (3) | 0.83196 (10) | 0.70533 (6) | 0.0211 (3) |
| H10 | 0.370949 | 0.889558 | 0.730028 | 0.025* |
| C11 | 0.1571 (3) | 0.84512 (10) | 0.65265 (6) | 0.0211 (3) |
| H11 | 0.071867 | 0.911634 | 0.641588 | 0.025* |
| C12 | 0.1041 (3) | 0.76178 (10) | 0.61629 (6) | 0.0188 (3) |
| H12 | −0.016143 | 0.770941 | 0.580245 | 0.023* |
| C13 | 0.2288 (3) | 0.66391 (9) | 0.63293 (5) | 0.0163 (2) |
| C14 | 0.1717 (3) | 0.57481 (9) | 0.59454 (5) | 0.0165 (3) |
| C15 | 0.5014 (3) | 0.24358 (10) | 0.52911 (5) | 0.0195 (3) |
| H15 | 0.533653 | 0.281620 | 0.495064 | 0.023* |
| C16 | 0.5859 (4) | 0.13895 (10) | 0.53164 (6) | 0.0235 (3) |
| H16 | 0.675241 | 0.105472 | 0.499134 | 0.028* |
| H1 | 0.182 (5) | 0.4211 (14) | 0.5380 (8) | 0.032 (5)* |
1 Source of materials
The 2-anilino-1,4-naphthoquinone was purchased from Sigma Aldrich and then dissolved in 3 mL acetone. The solution was kept to room temperature for crystallization. Red cuboid crystals were obtained.
2 Experimental details
The aromatic H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with fixed C–H distances for aromatic C–H of 0.93 Å (C–H) [U iso(H) = 1.2U eq]. The N1–H1 hydrogen was placed according to the Fourier electron difference density map. The graphics were obtained using the DIAMOND [4] program with 50 % probability ellipsoids. The highest peak is located 0.68 Å from C6 and the deepest hole is situated 0.61 Å from C14 respectively.
3 Comment
It is known that cancer is one of the diseases that have a great impact on our daily lives, and much scientific research is being conducted in order to limit and get rid of this incurable disease.
Epidermal growth factor receptor (EGFR) has been recognized as a very important aim for anticancer drug development. The 2-anilino-1,4-naphthoquinone and its derivatives have been shown to be related to be potential for their anticancer and EGFR inhibitor [5]. One of the most important naphthalene derivatives is naphthoquinone, with two carbonyl groups it is a privileged scaffold found in many classes of naturally occurring bioactive compounds and it has been used and its derivatives for cancer treatment [6, 7]. Different molecular mechanisms with regard to anticancer activities of the naphthoquinone-based compounds have been described [8], [9], [10]. In our laboratory, we have many results on the possibility of coordinating such compounds with the platinum group metals to increase the possibility to have an impact on cancer [11, 12].
There is one molecule in the asymmetric unit of the title structure. In the title structure, the O1–C14, O2–C7, N1–C4 and N1–C5 bond lengths were determined as 1.2219(17), 1.2312(17), 1.4176(17) and 1.3504(17) respectively. The C5–N1–C4, C3–C4–N1, C15–C4–N1, N1–C5–C6, N1–C5–C14, O2–C7–C6, O2–C7–C8, O1–C14–C5 and O1–C14–C13 bond angles were determined as 127.04(12), 121.45(12), 118.47(12), 127.41(12), 112.59(12), 121.66(12), 120.04(12) and 119.53(12) respectively. All parameters are in the expected ranges [13–15].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The authors acknowledge funding under the Swiss-South Africa joint research program (SSAJRP) from the SANRF (A. Roodt: UID: 107802) as well as from the Competitive Program for Rated Researchers of the SANRF (A. Roodt: UID: 111698), from the South African Department of Science Innovation (DSI) and the Department of Science and Technology (DST) respectively, “Department of Science and Innovation, Republic of South Africa” and “Department of Science and Technology, Republic of South Africa”.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
2. Bruker. SAINT-Plus (Version 7.12) and SADABS (Version 2004/1); Bruker AXS Inc.: Madison, WI, USA, 2004.Search in Google Scholar
3. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. 3.0c; Crystal Impact: Bonn, Germany, 2005.Search in Google Scholar
5. Mahalapbutr, P., Leechaisit, R., Thongnum, A., Todsaporn, D., Prachayasittikul, V., Rungrotmongkol, T., Prachayasittikul, S., Ruchirawat, S., Prachayasittikul, V., Pingaew, R. Discovery of anilino-1,4-naphthoquinones as potent EGFR tyrosine kinase inhibitors: synthesis, biological evaluation, and comprehensive molecular modeling. ACS Omega 2022, 7, 17881–17893; https://doi.org/10.1021/acsomega.2c01188.Search in Google Scholar PubMed PubMed Central
6. El-Najjar, N., Gali-Muhtasib, H., Ketola, R. A., Vuorela, P., Urtti, A., Vuorela, H. The chemical and biological activities of quinones: overview and implications in analytical detection. Photochem. Rev. 2011, 10, 353–370; https://doi.org/10.1007/s11101-011-9209-1.Search in Google Scholar
7. Sunassee, S. N., Davies-Coleman, M. T. Cytotoxic and antioxidant marine prenylated quinones and hydroquinones. Nat. Prod. Rep. 2012, 29, 513–535; https://doi.org/10.1039/c2np00086e.Search in Google Scholar PubMed
8. Pereyra, C. E., Dantas, R. F., Ferreira, S. B., Gomes, L. P., Silva, F. P.Jr. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207–226; https://doi.org/10.1186/s12935-019-0925-8.Search in Google Scholar PubMed PubMed Central
9. Salustiano, E. J. S., Netto, C. D., Fernandes, R. F., da Silva, A. J. M., Bacelar, T. S., Castro, C. P., Buarque, C. D., Maia, R. C., Rumjanek, V. M., Costa, P. R. R. Comparison of the cytotoxic effect of lapachol, alpha-lapachone and pentacyclic 1,4-naphthoquinones on human leukemic cells. Invest. New Drugs 2010, 28, 139–144; https://doi.org/10.1007/s10637-009-9231-y.Search in Google Scholar PubMed
10. Wellington, K. W. Understanding cancer and the anticancer activities of naphthoquinones? A review. RSC Adv. 2015, 5, 20309–20338; https://doi.org/10.1039/c4ra13547d.Search in Google Scholar
11. Elmakki, M. A. E., Alexander, O. T., Venter, G. J. S., Venter, J. A., Roodt, A. Structural study and iodomethane oxidative addition mechanistic investigation on model rhodium(I) carbonylation catalysts activated by indole-2-/indoline-2-carboxylate bidentate ligands. Inorganics 2022, 10, 1–24.10.3390/inorganics10120251Search in Google Scholar
12. Manicum, A. L., Visser, H. G., Engelbrecht, I., Roodt, A. Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl (cyclohexyldiphenylphosphine-κP)rhenium(I), C26H28O5Pre. Z. Kristallogr. N. Cryst. Struct. 2015, 230, 150–152; https://doi.org/10.1515/ncrs-2014-9013.Search in Google Scholar
13. Jha, R. K., Batabyal, M., Kumar, S. Blue light irradiated metal-oxidant-and base-free cross-dehydrogenative coupling of C(sp2)–H and N–H bonds: amination of naphthoquinones with amines. J. Org. Chem. 2023, 88, 7401–7424; https://doi.org/10.1021/acs.joc.3c00666.Search in Google Scholar PubMed
14. Singh, M. W., Karmakar, A., Barooah, N., Baruah, J. B. Variations in product in reactions of naphthoquinone with primary amines. Beilstein J. Org. Chem. 2007, 3, 10; https://doi.org/10.1186/1860-5397-3-10.Search in Google Scholar PubMed PubMed Central
15. Bayen, S., Barooah, N., Sarma, R. J., Sen, T. K., Karmakar, A., Baruah, J. B. Synthesis, structure and electrochemical properties of 2,5-bis(alkyl/arylamino)1,4-benzoquinones and 2-arylamino-1,4-naphthoquinones. Dyes Pigments 2007, 75, 770–775; https://doi.org/10.1016/j.dyepig.2006.07.033.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

