Abstract
C48H96B6N6O108Si48, trigonal,
Tables 1 and 2 contain details of the measurement method, a list of atomic coordinates, occupancy factors and displacement parameters, respectively.
Data collection and handling.
| Crystal: | Colorless powder |
| Size: | 0.07 × 0.07 × 0.07 mm |
| Wavelength: | Cu Kα radiation (1.54059 Å) |
| μ: | 5.6 mm−1 |
| Diffractometer, scan mode: | Siemens D5000, Debye-Scherrer Geometry |
| 2Θ range: | 8.04–97.98° |
| N(hkl)measured: | 466 |
| N(param)refined: | 26 |
| Programs: | Fullprof 2K [1], VESTA [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.0989 (2) | 0.1979 (2) | 0.07645 (12) | 0.02533* |
| O2 | 0.02965 (14) | 0.34557 (14) | 0.10906 (9) | 0.02533* |
| O3 | 0.87261 (10) | 0.12739 (10) | 0.08552 (16) | 0.02533* |
| O4 | 0.00000 | 0.27216 (17) | 0.00000 | 0.02533* |
| O5 | 0.11425 (10) | 0.88575 (10) | 0.49110 (17) | 0.02533* |
| Si1a | 0.00064 (10) | 0.23534 (11) | 0.06741 (4) | 0.01267* |
| B1b | 0.00064 (10) | 0.23534 (11) | 0.06741 (4) | 0.01267* |
| Si2c | 0.24315 (12) | 0.00000 | 0.50000 | 0.01267* |
| B2d | 0.24315 (12) | 0.00000 | 0.50000 | 0.01267* |
| C1 | 0.00000 | 0.00000 | 0.3685 (5) | 0.267 (3)* |
| C2 | 0.0636 (4) | 0.1271 (4) | 0.3393 (2) | 0.267 (3)* |
| C3 | 0.0650 (3) | 0.1300 (3) | 0.2670 (2) | 0.267 (3)* |
| N4 | 0.00000 | 0.00000 | 0.2531 (4) | 0.441 (6)* |
| C5 | 0.00000 | 0.00000 | 0.1904 (4) | 0.441 (6)* |
-
aOccupancy: 0.887(3), bOccupancy: 0.113(3), cOcupancy: 0.922(4), dOccupancy: 0.078(4).
1 Source of material
The crystals used in this study are taken from the original material as described in publication [3]. The crystals had been synthesized hydrothermally in sealed silica glass tubes at 453 K from a reaction mixture of tetramethoxysilane, boric acid, N-methyl-quinuclidinium hydroxide and water. The crystals were carefully separated from an impurity (SGT-type zeolite).
2 Experimental details
The Siemens D5000 diffractometer used for collecting the powder diffraction data was equipped with a Braun linear position-sensitive detector (2theta coverage = 6°) and a curved germanium (111) primary monochromator. The sample was sealed in a glass capillary (0.3 mm in diameter) to avoid a preferred orientation of the crystals. No absorption correction was necessary. Soft distance restraints were used for d(Si–O) = 1.58(1) Å, d(Si⋯Si) = 3.05(4) Å, d(O⋯O) = 2.59(3) Å, d(C–C) = 1.54(1) Å, d(C–N) = 1.48(1) Å, d(N⋯C, next-next neighbour) = 2.45(4) Å and d(C⋯C, next-next neighbour) = 2.50(4) Å. Isotropic displacement parameters B(iso) of the framework atoms were fixed at crystal chemically meaningful values to allow for a reliable refinement of the occupancy factors of Si and B. Isotropic displacement parameters of C and N atoms were refined. The scattering power of the hydrogen atoms which could not be located was included in the refinement by increasing the occupancy factor of the carbon atoms accordingly. This technique was used since the highly diffuse electron density at the position of the carbon atoms includes also the electrons of the hydrogen atoms.
3 Comment
Zeolite levyne, known as a mineral in its Ca dominated form with formula (Ca,Na2,K2)[Al2Si4O12]*6H2O since 1824 [4], has a microporous aluminiumsilicate framework with tetrahedrally coordinated Al and Si atoms. The first synthetic levyne has been reported in 1969 [5]. In 1959, the general structure of levyne was solved from a twinned single crystal [6] and later (1975) analyzed in detail by Merlino et al. [7]. Although Levyne-type zeolites are usually aluminiumsilicates, they can be synthesized with different framework compositions: in an all-silica form, as aluminophosphates, silico-aluminophosphates and metal-aluminophosphates and also as borosilicates. All these forms are typically synthesized with organic molecules or organic cations such as chinuclidine or aminoadamantane. The microporous framework of levyne consists of two polyhedral units, the small double-six-ring which is empty and the large [49 65 83]-cage which contains either an organic molecule/cation or inorganic cations and water (see Figure 1).
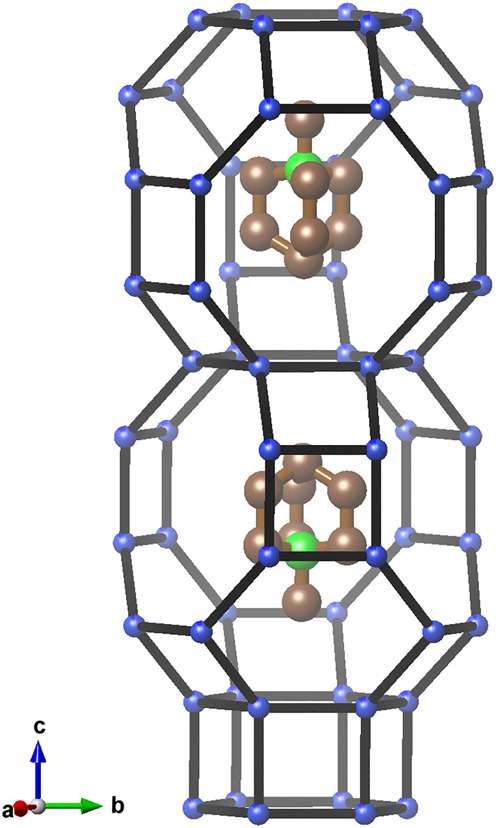
Characteristic section of the structure of the title compound. Oxygen atoms are omitted for clarity.
Boron containing levyne (B-levyne) has been described rarely. Three papers present the synthesis and characterization of the materials [3, 8, 9]. Only two structure analyses have been published: A detailed study on quinuclidine containing B-levyne at different temperatures [10] and a conference abstract describing briefly the structures of two B-levyne materials containing quinuclidinium and N-methyl-quinuclidinium, respectively; however, without reporting details or atomic coordinates, occupancies and displacement parameters [11]. To perform this study, the original material as described in Ref. [3] was used. The 11B MAS NMR spectrum showed two sharp and symmetric signals with chemical shifts of −3.60 and −4.55 ppm. This indicates that boron is exclusively tetrahedrally coordinated, replacing silicon atoms at T-positions of the silicate framework [3].
The Rietveld refinement of the title compound gave T–O distances of 1.577–1.589 Å. These values deviate from those of aluminium containing levyne (T–O distances in the range of 1.65–1.66 Å, [7]) because of the smaller boron atoms. The refinement of occupancy factors led to 5.4 boron atoms per unit cell. To compensate the charge of the six organic cations, six boron atoms at T sites are necessary. Since background correction and displacement parameters have an impact on the occupancy factors, it is assumed that, in fact, six boron atoms are present per unit cell. The boron atoms nearly randomly replace silicon to a small extend at T sites (11 % at T1 and 8 % at T2). This leads to a virtually even distribution of ≤6 negative charges about the 108 framework oxygen atoms. Table 2 lists atomic coordinates, occupancy factors and displacement parameters.
A difference Fourier map calculated by using only the framework atoms (Si, B, O) showed a remaining positive electron density clearly resembling the geometry of the N-methyl-quinuclidinium cation (see Figure 2). The [49 65 83]-cages are fully occupied by methyl-quinuclidinium cations. The distribution of electron density proved that the cation adopts only one particular orientation in the cage. This is in contrast to the boron-levyne occluding quinuclidine. Here, the quinuclidine occupies the cage in at least two different orientations [10]. Short distances between the carbon atom of the methyl group and the oxygen atoms of the framework (3.37 Å) show that the N-methyl-quinuclidinium cation fits just tightly into the cage. The c0 lattice parameter of B-levyne occluding the N-methyl-quinuclidinium cation is considerably larger (22.3058(2) Å, this study) than the c0 parameter of the B-levyne occluding the smaller quinuclidine molecule (21.9173(4) Å, [10]) showing some flexibility of the levyne cage. In contrast, the diameter of the N-methyl-quinuclidinium containing cage perpendicular to the c-axis is slightly smaller (a0 = 12.8892(1) Å) than the quinuclidine containing cage (a0 = 12.9475(2) Å [10]). While high displacement parameters of the carbon atoms (see Table 1) indicated some motional freedom of the cation, the N-methyl-quinuclidinium does not rotate freely about the 3-fold axis at room temperature – as one might expect. This is due to the weak interactions between the large cation carrying only one positive charge and the cage wall, which is only weakly negatively charged. Although there are two possible orientations of the N-methyl-quinuclidinium cation in a particular cage with respect to the c-axis (up and down), only one orientation is observed.
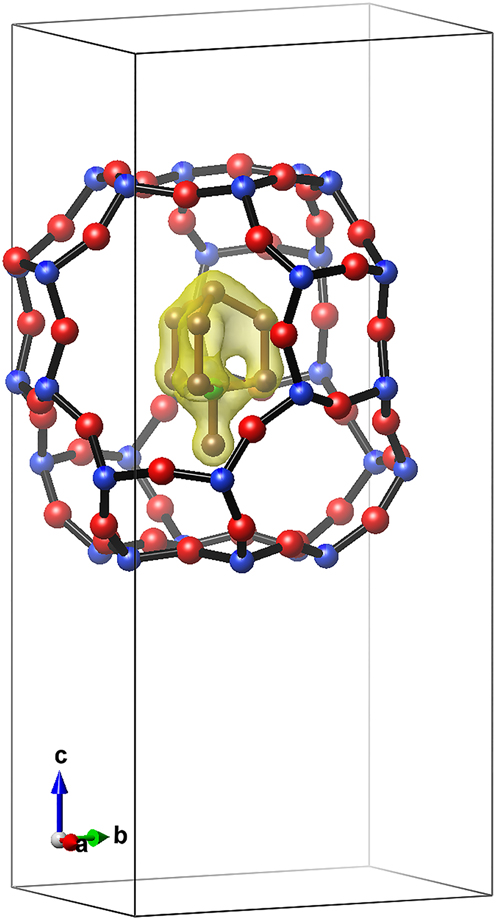
Difference Fourier map showing a remaining positive electron density (yellow) that clearly resembles the geometry of the N-methyl-quinuclidinium cation.
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: MA 6641/3-1
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The work was funded by the Deutsche Forschungsgemeinschaft with grant number MA 6641/3-1.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rodriguez-Carvajal, J. FullProf: A Program for Rietveld Refinement and Profile Matching Analysis of Complex Powder Diffraction Patterns Version 7.30; ILL: Grenoble, France, 2020. http://www.ill.eu/sites/fullprof/index.html.Search in Google Scholar
2. Momma, K., Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276; https://doi.org/10.1107/s0021889811038970.Search in Google Scholar
3. Gruenewald-Lueke, A., Marler, B., Hochgraefe, M., Gies, H. Quinuclidine derivatives as structure directing agents for the synthesis of boron containing zeolites. J. Mater. Chem. 1999, 9, 2529–2536; https://doi.org/10.1039/a903999f.Search in Google Scholar
4. Mindat.org, an open database of minerals; The Hudson Institute of Mineralogy: Keswick, USA. https://www.mindat.org/min-7154.html (accessed Apr 21, 2023).Search in Google Scholar
5. Kerr, G. T. Synthetic Zeolite and Method for Preparing the Same. U. S. Patent 3,459,676, 1969.Search in Google Scholar
6. Barrer, R. M., Kerr, I. S. Intracrystalline channels in levynite and some related zeolites. Trans. Faraday Soc. 1959, 55, 1915–1923; https://doi.org/10.1039/tf9595501915.Search in Google Scholar
7. Merlino, S., Galli, E., Alberti, A. The crystal structure of levyne. Tschermaks Mineral. Petrogr. Mittl. 1975, 22, 117–129; https://doi.org/10.1007/bf01089112.Search in Google Scholar
8. Millini, R., Carati, A., Bellussi, G. Synthesis of a new porous borosilicate with the levyne structure. Zeolites 1992, 12, 265–268; https://doi.org/10.1016/0144-2449(92)90099-b.Search in Google Scholar
9. De Luca, P., Violante, D., Vuono, D., Catanzaro, L., Nagy, J., Nastro, A. Synthesis and characterization of Al,B-levyne type crystals from gels containing methyl-quinuclidinium ions. Microporous Mesoporous Mater. 2004, 71, 39–49; https://doi.org/10.1016/j.micromeso.2004.03.010.Search in Google Scholar
10. Leardini, L., Martucci, A., Alberti, A., Cruciani, G. Template burning effects on stability and boron coordination in boron levyne studied by in situ time resolved synchrotron powder diffraction. Microporous Mesoporous Mater. 2013, 167, 117–126; https://doi.org/10.1016/j.micromeso.2012.02.013.Search in Google Scholar
11. Gruenewald-Lueke, A., Marler, B., Hochgraefe, M., Gies, H. Die Struktur von RUB-1, dem Boranalogon des Zeolithminerals Levyn. Z. Kristallogr. 1997, 12, 131.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

