Abstract
C38H62N6Zn2, triclinic,
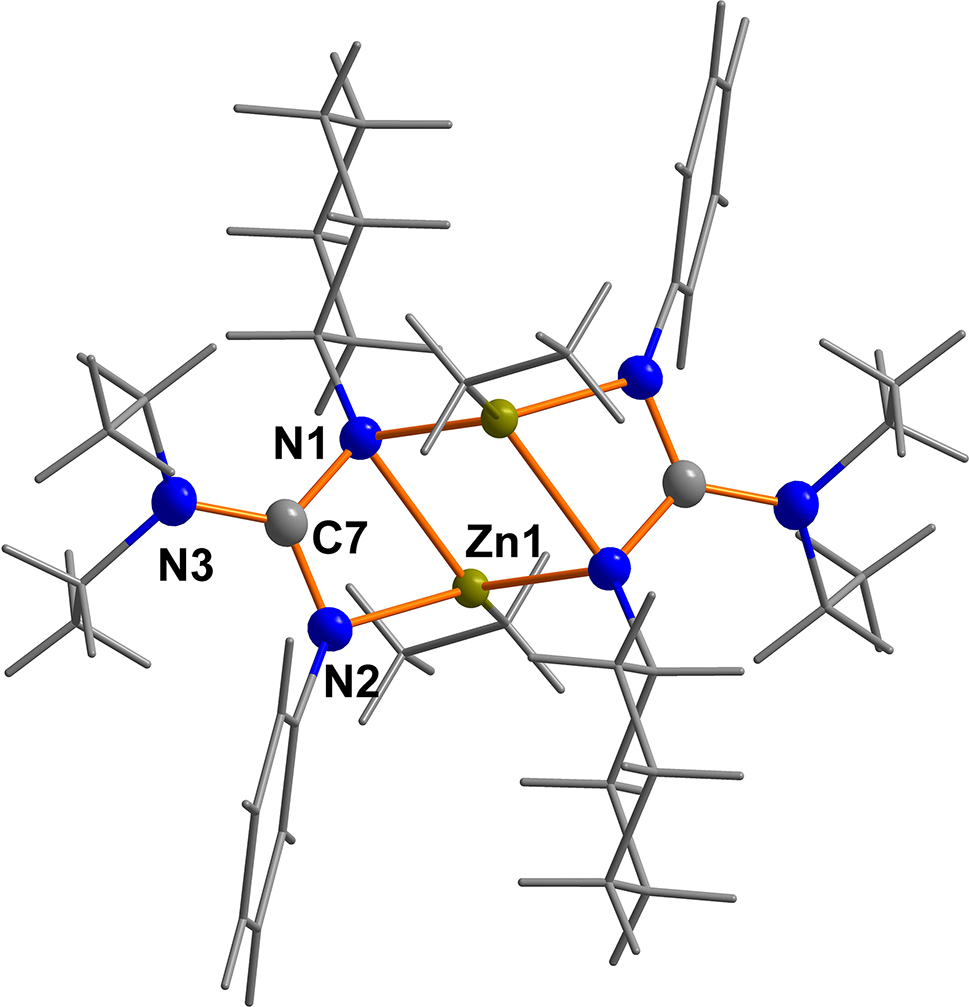
A part of the title coordination compound is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.35 × 0.33 × 0.30 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.28 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θmax, completeness: | 25.1°, 97 % |
| N(hkl)measured, N(hkl)unique, Rint: | 7251, 3331, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3149 |
| N(param)refined: | 211 |
| Programs: | Shelx [1], Bruker [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Zn1 | 0.60539 (2) | 0.40972 (2) | 0.51052 (2) | 0.01920 (7) |
| N1 | 0.44183 (15) | 0.46831 (13) | 0.33300 (13) | 0.0178 (3) |
| N2 | 0.39439 (15) | 0.26901 (13) | 0.35955 (13) | 0.0196 (3) |
| N3 | 0.23948 (16) | 0.27281 (14) | 0.13577 (14) | 0.0244 (3) |
| C1 | 0.48522 (18) | 0.53042 (16) | 0.24683 (16) | 0.0199 (3) |
| H1 | 0.4233 | 0.4662 | 0.1458 | 0.024* |
| C2 | 0.4477 (2) | 0.66790 (17) | 0.26216 (18) | 0.0254 (4) |
| H2A | 0.4998 | 0.7290 | 0.3631 | 0.031* |
| H2B | 0.3317 | 0.6526 | 0.2258 | 0.031* |
| C3 | 0.5038 (2) | 0.73836 (19) | 0.1827 (2) | 0.0331 (4) |
| H3A | 0.4438 | 0.6821 | 0.0804 | 0.040* |
| H3B | 0.4824 | 0.8288 | 0.1995 | 0.040* |
| C4 | 0.6803 (2) | 0.75754 (19) | 0.2307 (2) | 0.0365 (4) |
| H4A | 0.7409 | 0.8206 | 0.3310 | 0.044* |
| H4B | 0.7130 | 0.7993 | 0.1751 | 0.044* |
| C5 | 0.7179 (2) | 0.6208 (2) | 0.21221 (19) | 0.0327 (4) |
| H5A | 0.8337 | 0.6360 | 0.2481 | 0.039* |
| H5B | 0.6651 | 0.5608 | 0.1109 | 0.039* |
| C6 | 0.6616 (2) | 0.54984 (18) | 0.29112 (18) | 0.0266 (4) |
| H6A | 0.6825 | 0.4592 | 0.2731 | 0.032* |
| H6B | 0.7232 | 0.6053 | 0.3935 | 0.032* |
| C7 | 0.35327 (18) | 0.33511 (15) | 0.27347 (16) | 0.0182 (3) |
| C8 | 0.28979 (18) | 0.15661 (15) | 0.34987 (15) | 0.0195 (3) |
| C9 | 0.3447 (2) | 0.04614 (17) | 0.37047 (18) | 0.0261 (4) |
| H9 | 0.4483 | 0.0444 | 0.3831 | 0.031* |
| C10 | 0.2494 (2) | −0.06100 (18) | 0.3727 (2) | 0.0316 (4) |
| H10 | 0.2886 | −0.1354 | 0.3872 | 0.038* |
| C11 | 0.0981 (2) | −0.06112 (18) | 0.35419 (19) | 0.0310 (4) |
| H11 | 0.0330 | −0.1351 | 0.3553 | 0.037* |
| C12 | 0.0425 (2) | 0.04837 (17) | 0.33387 (17) | 0.0268 (4) |
| H12 | −0.0613 | 0.0494 | 0.3213 | 0.032* |
| C13 | 0.13690 (19) | 0.15602 (17) | 0.33178 (17) | 0.0230 (3) |
| H13 | 0.0973 | 0.2304 | 0.3179 | 0.028* |
| C14 | 0.1342 (2) | 0.34620 (19) | 0.06832 (19) | 0.0327 (4) |
| H14A | 0.1373 | 0.3382 | −0.0205 | 0.039* |
| H14B | 0.1743 | 0.4447 | 0.1309 | 0.039* |
| C15 | −0.0364 (2) | 0.2925 (2) | 0.0362 (2) | 0.0488 (5) |
| H15A | −0.0785 | 0.1961 | −0.0290 | 0.073* |
| H15B | −0.1003 | 0.3463 | −0.0068 | 0.073* |
| H15C | −0.0406 | 0.3004 | 0.1237 | 0.073* |
| C16 | 0.2077 (2) | 0.12696 (18) | 0.05327 (18) | 0.0330 (4) |
| H16A | 0.1322 | 0.1052 | −0.0460 | 0.040* |
| H16B | 0.1564 | 0.0714 | 0.0887 | 0.040* |
| C17 | 0.3547 (3) | 0.0867 (2) | 0.0590 (2) | 0.0462 (5) |
| H17A | 0.4088 | 0.1439 | 0.0279 | 0.069* |
| H17B | 0.3249 | −0.0099 | −0.0029 | 0.069* |
| H17C | 0.4260 | 0.0999 | 0.1558 | 0.069* |
| C18 | 0.8251 (2) | 0.40070 (19) | 0.5614 (2) | 0.0302 (4) |
| H18A | 0.8913 | 0.4925 | 0.5857 | 0.036* |
| H18B | 0.8652 | 0.3804 | 0.6472 | 0.036* |
| C19 | 0.8492 (3) | 0.2964 (2) | 0.4505 (2) | 0.0460 (5) |
| H19A | 0.7856 | 0.2044 | 0.4259 | 0.069* |
| H19B | 0.9616 | 0.2994 | 0.4875 | 0.069* |
| H19C | 0.8158 | 0.3179 | 0.3664 | 0.069* |
1 Source of materials
All manipulations were carried out under dry nitrogen using standard Schlenk and cannula techniques. Solvents were dried with appropriate drying agents, degassed, and stored over a potassium mirror or activated molecular sieves prior to use. ZnEt2 (1.0 M solution in hexane; Alfa Aesar) was obtained commercially and used as received. Et2NH (Aldrich) was dried over KOH and redistilled before use. Carbodiimine CyN=C=NC6H5 was prepared according to the literature procedures [5]. To a stirred solution of diethylamine (0.20 mL, 2.0 mmol) in hexane (15 mL), diethylzinc (2.00 mL of a 1.0 M solution in hexane, 2.0 mmol) was slowly dropped into the above solution at ambient temperature. The mixture was heated to 60
2 Experimental details
2.1 Comment
N,N-Bidentate ligands generated by the deprotonation of guanidines can provide a wide range of patterns by adjusting the type of the substituents on the conjugated N–C–N skeleton, and exhibit extensive utility in metal complexes [6]. Various types of metal guanidinate complexes and their properties have been reported, such as cobalt [7], titanium [8, 9], tungsten [10], cerium [11], platinum [12] and lutetium [13]. For zinc complex [14], several examples with guanidinate ligands have been studied [15], they exhibited good to excellent catalytic activity. With particular relevance to this work, the room temperature reaction between N-cyclohexyl-N-phenylmethanediimine and ZnMe2 yielded the dinuclear Zn alkyl complex [
The title compound crystallised as the dimeric complex [
Acknowledgment
This work was supported by the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (award No. 201802098) and Laboratory of Protein-based Functional Materials (award No. 2022P010).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (award No. 201802098) and Laboratory of Protein-based Functional Materials (award No. 2022P010).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
2. Bruker. Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2000.Search in Google Scholar
3. Bruker. Apex3 and Saint; Bruker AXS Inc.: Madison, WI, USA, 2016.Search in Google Scholar
4. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Version 4.2.2; Crystal Impact: Bonn, Germany, 2016.Search in Google Scholar
5. Ali, A. R., Ghosh, H., Patel, B. K. A greener synthetic protocol for the preparation of carbodiimide. Tetrahedron Lett. 2010, 51, 1019–1021; https://doi.org/10.1016/j.tetlet.2009.12.017.Search in Google Scholar
6. Forrest, S. J. K., Schluschass, B., Yuzik-Klimova, E. Y., Schneider, S. Nitrogen fixation via splitting into nitrido complexes. Chem. Rev. 2021, 121, 6522–6587; https://doi.org/10.1021/acs.chemrev.0c00958.Search in Google Scholar PubMed
7. Zhang, Y., Du, L., Liu, X., Ding, Y. Synthesis, characterization, and thermal properties of cobalt(II) compounds with guanidinate ligands. New J. Chem. 2018, 42, 9110–9115; https://doi.org/10.1039/c8nj01232f.Search in Google Scholar
8. Carmalt, C. J., Newport, A. C., O’Neill, S. A., Parkin, I. P., White, A. J., Williams, D. J. Synthesis of titanium(IV) guanidinate complexes and the formation of titanium carbonitride via low-pressure chemical vapor deposition. Inorg. Chem. 2005, 44, 615–619; https://doi.org/10.1021/ic049013u.Search in Google Scholar PubMed
9. Wu, B., Feng, R., Yin, Z.-B., Yan, H., Wang, X., Wang, G.-X., Wei, J., Xi, Z. Synthesis and structural analysis of titanium-μ-dinitrogen complex supported by di-anionic guanidinate ligands. Sci. China Chem. 2023, 66, 755–759; https://doi.org/10.1007/s11426-022-1490-8.Search in Google Scholar
10. Nolan, M. M., Touchton, A. J., Richey, N. E., Ghiviriga, I., Rocca, J. R., Abboud, K. A., McElwee-White, L. Synthesis and characterization of tungsten nitrido amido guanidinato complexes as precursors for chemical vapor deposition of WN(x)C(y) thin films. Eur. J. Inorg. Chem. 2018, 2018, 46–53; https://doi.org/10.1002/ejic.201701225.Search in Google Scholar PubMed PubMed Central
11. Kaur, P., Muriqi, A., Wree, J. L., Ghiyasi, R., Safdar, M., Nolan, M., Karppinen, M., Devi, A. Atomic/molecular layer deposition of cerium(III) hybrid thin films using rigid organic precursors. Dalton Trans. 2022, 51, 5603–5611; https://doi.org/10.1039/d2dt00353h.Search in Google Scholar PubMed
12. Sinha, N. K., Mishra, V., Thirupathi, N. Influence of the steric/electronic properties of N-aryl substituents in cycloplatinated guanidinate(1-) complexes on the formation of discrete Pt → Ag complexes and one-dimensional coordination polymer. Inorg. Chem. 2023, 62, 7644–7661; https://doi.org/10.1021/acs.inorgchem.2c04172.Search in Google Scholar PubMed
13. Jiang, W., Zhang, L., Zhang, L. Reactivity of a mixed methyl-aminobenzyl guanidinate lutetium complex towards iPrN=C=NiPr, CS2 and Ph2PH. Dalton Trans. 2022, 51, 12650–12660; https://doi.org/10.1039/d2dt02008d.Search in Google Scholar PubMed
14. Jones, C., Furness, L., Nembenna, S., Rose, R. P., Aldridge, S., Stasch, A. Bulky guanidinato and amidinato zinc complexes and their comparative stabilities. Dalton Trans. 2010, 39, 8788–8795; https://doi.org/10.1039/c0dt00589d.Search in Google Scholar PubMed
15. Li, J., Shi, J., Han, H., Guo, Z., Tong, H., Wei, X., Liu, D., Lappert, M. F. Synthesis, structures, and reactivities of guanidinatozinc complexes and their catalytic behavior in the Tishchenko reaction. Organometallics 2013, 32, 3721–3727; https://doi.org/10.1021/om400345f.Search in Google Scholar
16. Neuhäuser, C., Reinmuth, M., Kaifer, E., Himmel, H. J. Synthesis of oligomeric zinc complexes with bicyclic and acyclic guanidinate ligands. Eur. J. Inorg. Chem. 2012, 2012, 1250–1260; https://doi.org/10.1002/ejic.201101223.Search in Google Scholar
17. Barman, M. K., Baishya, A., Nembenna, S. Bulky guanidinate calcium and zinc complexes as catalysts for the intramolecular hydroamination. J. Organomet. Chem. 2019, 887, 40–47; https://doi.org/10.1016/j.jorganchem.2019.02.005.Search in Google Scholar
18. Börner, J., dos Santos Vieira, I., Jones, M. D., Doring, A., Kuckling, D., Flörke, U., Herres-Pawlis, S. Zinc complexes with guanidine-pyridine hybrid ligands – guanidine effect and catalytic activity. Eur. J. Inorg. Chem. 2011, 2011, 4441–4456; https://doi.org/10.1002/ejic.201100540.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

