Abstract
C16H12F3NO, orthorhombic, P212121 (no. 19), a = 6.9928(6) Å, b = 8.9764(8) Å, c = 21.216(2) Å, V = 1331.7(2) Å3, Z = 2, Rgt(F) = 0.0583, wRref(F2) = 0.1552, T = 298 K.
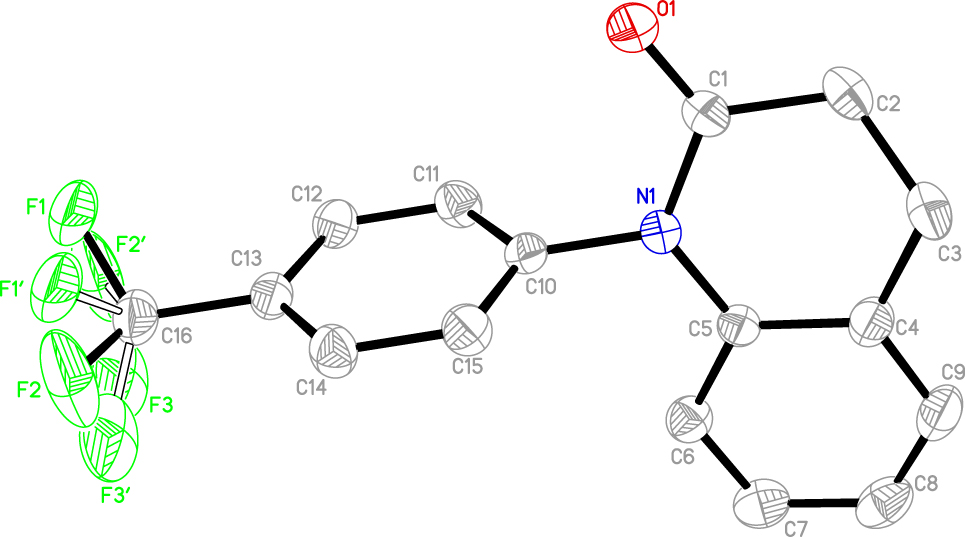
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.40 × 0.33 × 0.31 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.12 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX II, φ and ω |
| θmax, completeness: | 25.0°, 97 % |
| N(hkl)measured, N(hkl)unique, Rint: | 5991, 2329, 0.085 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1800 |
| N(param)refined: | 201 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| F1a | 0.5240 (11) | 0.4950 (7) | 0.2014 (4) | 0.100 (2) |
| F2a | 0.4364 (13) | 0.5906 (14) | 0.2861 (3) | 0.120 (3) |
| F3a | 0.6505 (11) | 0.6895 (9) | 0.2334 (6) | 0.117 (3) |
| F1′b | 0.4046 (19) | 0.5099 (11) | 0.2596 (8) | 0.100 (2) |
| F2′b | 0.625 (2) | 0.585 (2) | 0.2007 (5) | 0.120 (3) |
| F3′b | 0.5472 (18) | 0.6953 (15) | 0.2792 (9) | 0.117 (3) |
| N1 | −0.0638 (4) | 0.9572 (4) | 0.09622 (16) | 0.0388 (9) |
| O1 | −0.1864 (4) | 0.7488 (4) | 0.05416 (18) | 0.0568 (9) |
| C1 | −0.1877 (6) | 0.8832 (5) | 0.0578 (2) | 0.0434 (11) |
| C2 | −0.3150 (7) | 0.9794 (6) | 0.0192 (2) | 0.0570 (13) |
| H2A | −0.4252 | 0.9220 | 0.0058 | 0.068* |
| H2B | −0.2468 | 1.0113 | −0.0183 | 0.068* |
| C3 | −0.3814 (6) | 1.1136 (6) | 0.0548 (3) | 0.0596 (14) |
| H3A | −0.4534 | 1.1781 | 0.0268 | 0.071* |
| H3B | −0.4655 | 1.0827 | 0.0887 | 0.071* |
| C4 | −0.2166 (5) | 1.1969 (6) | 0.0813 (2) | 0.0468 (11) |
| C5 | −0.0591 (5) | 1.1130 (5) | 0.1010 (2) | 0.0374 (10) |
| C6 | 0.0974 (5) | 1.1859 (5) | 0.1264 (2) | 0.0459 (11) |
| H6 | 0.2034 | 1.1314 | 0.1394 | 0.055* |
| C7 | 0.0973 (7) | 1.3366 (6) | 0.1326 (2) | 0.0570 (13) |
| H7 | 0.2028 | 1.3843 | 0.1500 | 0.068* |
| C8 | −0.0563 (8) | 1.4181 (6) | 0.1134 (3) | 0.0605 (14) |
| H8 | −0.0567 | 1.5211 | 0.1180 | 0.073* |
| C9 | −0.2088 (7) | 1.3475 (6) | 0.0874 (2) | 0.0576 (14) |
| H9 | −0.3116 | 1.4042 | 0.0734 | 0.069* |
| C10 | 0.0735 (5) | 0.8710 (4) | 0.1303 (2) | 0.0384 (10) |
| C11 | 0.2299 (5) | 0.8155 (6) | 0.0997 (2) | 0.0456 (11) |
| H11 | 0.2465 | 0.8334 | 0.0569 | 0.055* |
| C12 | 0.3621 (5) | 0.7336 (5) | 0.1323 (2) | 0.0471 (12) |
| H12 | 0.4674 | 0.6939 | 0.1114 | 0.057* |
| C13 | 0.3396 (5) | 0.7101 (4) | 0.1952 (2) | 0.0410 (10) |
| C14 | 0.1831 (6) | 0.7657 (5) | 0.2256 (2) | 0.0481 (11) |
| H14 | 0.1670 | 0.7481 | 0.2685 | 0.058* |
| C15 | 0.0498 (6) | 0.8470 (5) | 0.1934 (2) | 0.0459 (11) |
| H15 | −0.0561 | 0.8857 | 0.2143 | 0.055* |
| C16 | 0.4824 (7) | 0.6224 (6) | 0.2297 (3) | 0.0571 (13) |
-
aOccupancy: 0.611(5), bOccupancy: 0.389(5).
1 Source of materials
1-(4-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydroquinoline (5.0 mmol, 1.05 g) and cesium carbonate (Cs2CO3, 7.5 mmol) were mixed in a 25 mL Schlenk tube containing a magnetic stirring bar, then we added dry DMF (50 mL) to the tube to dissolve the substrate and finally added (diacetoxyiodo)benzene (1.0 mmol). Oxygen atmosphere was incorporated through an O2 balloon. The resulting mixture was stirred at RT with the irradiation of a 20 W blue LED light for 36 h. After the reaction was completed, the reaction solution underwent an aqueous workup and was extracted three times with ethyl acetate. The combined organic layers were dried over sodium sulfate, and concentrated in vacuo. The title compound was purified by flash chromatography on silica gel using petroleum ether/ethyl acetate (10:1).
2 Experimental details
All hydrogen atomic positions were refined with variable isotropic displacement parameters. Hydrogen atoms were assigned with common isotropic displacement factors Uiso (H) = 1.2 times Uiso (C, phenyl ring and methylene carbon) and Uiso (H) = 1.5 times Ueq (C, methyl carbon). All the H atoms were refined as riding on their parent atom.
3 Comment
Nitrogen heterocyclic compounds are valuable and prevalent pharmacophores with diverse bioactivity [3], including anti-bacterial, anti-inflammatory, and anti-cancer activity, among others [4], [5], [6], [7]. N-aryl tetrahydroquinolinone derivatives can be conveniently prepared according to the one-step palladium-catalyzed N-arylation reaction. The construction of N-aryl tetrahydroisoquinolinones is regarded as a desirable and valuable synthetic goal [7], [8], [9], which would be of great significance to the synthetic and pharmaceutical fields [10, 11]. This electron-donor-acceptor complex-mediated oxidation process eliminates the need to use photo catalysts and allows for the effective preparation of a broad range of synthetically and biologically valuable quinolinones under very mild conditions. Mechanistic studies indicated a short radical chain reaction initiated by visible-light-induced electron transfer within the tertiary amine diacetoxyiodo benzene electron-donor-acceptor complex [12]. Although its molecular structure has been discovered, its crystal structure has not yet been reported.
The title compound contained one quinoline and one phenyl moiety. Owing to the carbons at positions C2 and C3 are saturated carbons containing two hydrogens, all the atoms of quinoline ring are not coplanar. And the torsion angles of C1–N1–C10–C15 and C5–N1–C10–C15 are −105.1° and 78.3°, respectively. The C1 position of the aromatic ring in the parent nucleus of 3,4-dihydroquinolin-2(1H)-one is oxidized. The trifluoromethyl group replaces the hydrogen in the benzene ring at position C13. All the bond lengths and angles are comparable with its analogues and in the expected ranges [13–16].
Funding source: Jinzhou Medical University Research Project
Award Identifier / Grant number: 6001/173211016, 6001/173220901
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was funded by Jinzhou Medical University Research Project (6001/173211016, 6001/173220901).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
3. Trost, B. M., Maulide, N., Livingston, R. C. A ruthenium-catalyzed, atom-economical synthesis of nitrogen heterocycles. J. Am. Chem. Soc. 2008, 130, 16502–16503. https://doi.org/10.1021/ja807696e.Search in Google Scholar PubMed PubMed Central
4. Liu, H., Quan, Y., Xie, L., Li, X., Xie, X. The cascade [1,5]-hydride shift/intramolecular C(sp3)—H activation: a powerful approach to the construction of spiro-tetrahydroquinoline skeleton. Front. Chem. 2022, 10, 840934. https://doi.org/10.3389/fchem.2022.840934.Search in Google Scholar PubMed PubMed Central
5. Lu, L. Q., Chen, J. R., Xiao, W. J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 2012, 45, 1278–1293. https://doi.org/10.1021/ar200338s.Search in Google Scholar PubMed
6. Yang, X., Wang, L., Hu, F., Xu, L., Li, S., Li, S.-S. Redox-triggered switchable synthesis of 3,4-dihydroquinolin-2(1H)-one derivatives via hydride transfer/N-dealkylation/N-acylation. Org. Lett. 2021, 23, 358–364. https://doi.org/10.1021/acs.orglett.0c03863.Search in Google Scholar PubMed
7. Wen, J., Wei, W., Xue, S., Yang, D., Lou, Y., Gao, C., Wang, H. Metal-free oxidative spirocyclization of alkynes with sulfonylhydrazides leading to 3-sulfonated azaspiro[4,5] trienones. J. Org. Chem. 2015, 80, 4966–4972. https://doi.org/10.1021/acs.joc.5b00361.Search in Google Scholar PubMed
8. Guo, M., Dong, F., Yin, X., Xu, L., Wang, L., Li, S.-S. Facile syntheses of tetrahydroquinolines and 1,2-dihydroquinolines via vinylogous cascade hydride transfer/cyclization. Org. Chem. Front. 2021, 8, 2224–2231. https://doi.org/10.1039/D0QO01622E.Search in Google Scholar
9. Gao, F., Yang, C., Gao, G. L., Zheng, L., Xia, W. Visible-light induced trifluoromethylation of N-arylcinnamamides for the synthesis of CF3-containing 3,4-disubstituted dihydroquinolinones and 1-azaspiro[4.5]decanes. Org. Lett. 2015, 17, 3478–3481. https://doi.org/10.1021/acs.orglett.5b01530.Search in Google Scholar PubMed
10. Meneyrol, J., Helissey, P., Tratrat, C., Giorgi-Renault, S., Husson, H. P. A facile route for the preparation of N-phenyl tetrahydroquinolines and tetrahydroisoquinolines. Synth. Commun. 2001, 31, 987–992. https://doi.org/10.1081/SCC-100103526.Search in Google Scholar
11. Wang, L.-X., Qiu, B., An, X.-D., Dong, P.-Z., Liu, R.-B., Xiao, J. Organocatalytic cascade aldimine condensation/[1,6]-hydride transfer/mannich-type cyclization: sustainable access to indole-2,3-fused diazocanes. Green Chem. 2021, 23, 8181–8186. https://doi.org/10.1039/D1GC02570H.Search in Google Scholar
12. Xie, X., Guo, X., Qiao, K., Shi, L. Visible-light promoted photocatalyst-free aerobic α-oxidation of tertiary amines to amides. Org. Biomol. Chem. 2022, 20, 8031–8036. https://doi.org/10.1039/d2ob01565j.Search in Google Scholar PubMed
13. Zhang, C.-Y., Nie, X.-L., Huang, G.-P., Xiong, Y.-Z., Huang, J.-P. Crystal structure of 1-cyclopropyl-7-ethoxy-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C15H13F2NO4. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 923–925. https://doi.org/10.1515/ncrs-2021–0151.10.1515/ncrs-2021-0151Search in Google Scholar
14. Li, C.-J. Crystal structure of 6-(quinolin-8-yl)benzo[a]phenanthridin-5(6H)-one, C26H16N2O. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 969–970. https://doi.org/10.1515/ncrs-2021–0170.10.1515/ncrs-2021-0170Search in Google Scholar
15. Shi, B.-C., Chen, H.-D., Zhu, X.-D., Zhang, M.-M., Zhang, Z.-F. Crystal structure of 6-oxo-4-phenyl-1-propyl-1,6-dihydropyridine-3-carbonitrile, C15H14N2O. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 977–978. https://doi.org/10.1515/ncrs-2021–0178.10.1515/ncrs-2021-0178Search in Google Scholar
16. Yang, F., Yao, L., Zeng, X.-L. The crystal structure of 4-chloro-2-(quinolin-8-yl) isoindoline-1,3-dione, C17H9ClN2O2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 999–1000. https://doi.org/10.1515/ncrs-2021–0188.10.1515/ncrs-2021-0188Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

