Abstract
C31H29N2BO4Cl2, triclinic,
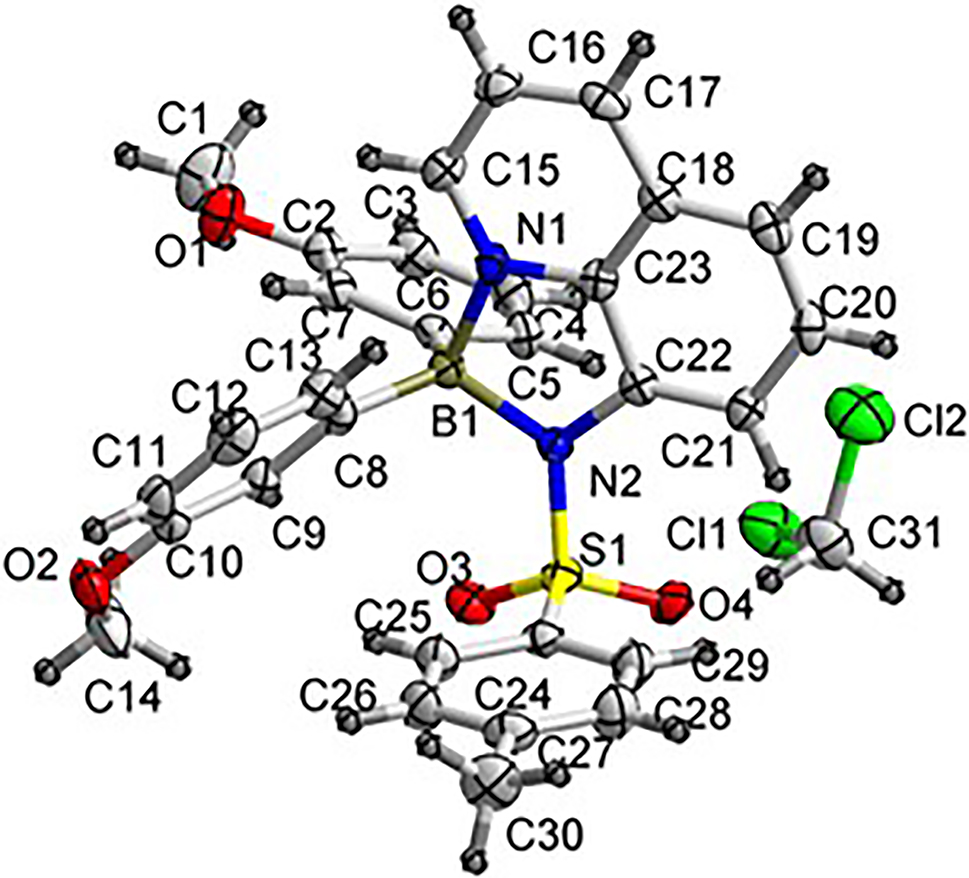
The crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block |
| Size: | 0.13 × 0.12 × 0.1 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.34 mm−1 |
| Diffractometer, scan mode: | Bruker D8 VENTURE, ω and φ scans |
| θ max, completeness: | 27.5°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 13,743, 6597, 0.043 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4883 |
| N(param)refined: | 373 |
| Programs: | Bruker programs [1], SHELX [2], [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | U iso*/U eq | |
|---|---|---|---|---|
| S1 | 0.21869 (6) | 0.72291 (4) | 0.87012 (4) | 0.02221 (13) |
| Cl1 | 0.05747 (9) | 0.31422 (7) | 0.83673 (5) | 0.0545 (2) |
| Cl2 | 0.37115 (9) | 0.33595 (7) | 0.86335 (6) | 0.0586 (2) |
| O3 | 0.09889 (18) | 0.75753 (12) | 0.82058 (11) | 0.0295 (4) |
| O4 | 0.19962 (19) | 0.62273 (12) | 0.93308 (10) | 0.0292 (4) |
| O1 | 0.2782 (2) | 0.70862 (13) | 0.37230 (11) | 0.0357 (4) |
| N1 | 0.6067 (2) | 0.73957 (14) | 0.65325 (12) | 0.0210 (4) |
| O2 | 0.0434 (2) | 1.10077 (13) | 0.64094 (13) | 0.0419 (4) |
| N2 | 0.3843 (2) | 0.70763 (14) | 0.78747 (11) | 0.0207 (4) |
| C22 | 0.5258 (2) | 0.66114 (16) | 0.80826 (14) | 0.0199 (4) |
| C6 | 0.3556 (2) | 0.68228 (16) | 0.61039 (14) | 0.0210 (4) |
| C7 | 0.3381 (3) | 0.72363 (17) | 0.51995 (14) | 0.0222 (4) |
| H7 | 0.356247 | 0.800099 | 0.500351 | 0.027* |
| C23 | 0.6538 (2) | 0.68270 (16) | 0.72791 (14) | 0.0203 (4) |
| C18 | 0.8137 (3) | 0.65087 (16) | 0.72405 (15) | 0.0230 (4) |
| C2 | 0.2947 (3) | 0.65559 (18) | 0.45774 (15) | 0.0252 (5) |
| C15 | 0.7149 (3) | 0.76705 (18) | 0.57275 (15) | 0.0265 (5) |
| H15 | 0.681992 | 0.806336 | 0.520531 | 0.032* |
| C5 | 0.3294 (3) | 0.56856 (17) | 0.63568 (16) | 0.0265 (5) |
| H5 | 0.339978 | 0.537432 | 0.696689 | 0.032* |
| C8 | 0.3718 (3) | 0.89408 (16) | 0.67044 (14) | 0.0230 (4) |
| C19 | 0.8428 (3) | 0.58887 (17) | 0.80669 (16) | 0.0284 (5) |
| H19 | 0.948297 | 0.564279 | 0.808479 | 0.034* |
| C24 | 0.2387 (3) | 0.83354 (17) | 0.93983 (14) | 0.0239 (4) |
| C21 | 0.5586 (3) | 0.60049 (17) | 0.88676 (15) | 0.0251 (5) |
| H21 | 0.475602 | 0.582799 | 0.942199 | 0.030* |
| C3 | 0.2700 (3) | 0.54352 (18) | 0.48349 (16) | 0.0298 (5) |
| H3 | 0.241301 | 0.496631 | 0.441061 | 0.036* |
| C20 | 0.7186 (3) | 0.56497 (17) | 0.88321 (16) | 0.0279 (5) |
| H20 | 0.740400 | 0.522093 | 0.937250 | 0.033* |
| C9 | 0.2250 (3) | 0.93712 (17) | 0.65596 (14) | 0.0247 (5) |
| H9 | 0.152011 | 0.886931 | 0.646894 | 0.030* |
| C17 | 0.9260 (3) | 0.68224 (18) | 0.63844 (16) | 0.0289 (5) |
| H17 | 1.036083 | 0.664207 | 0.632263 | 0.035* |
| C13 | 0.4758 (3) | 0.97138 (18) | 0.68232 (15) | 0.0291 (5) |
| H13 | 0.576428 | 0.944825 | 0.691825 | 0.035* |
| C25 | 0.2040 (3) | 0.94305 (18) | 0.90964 (16) | 0.0323 (5) |
| H25 | 0.167430 | 0.958167 | 0.852916 | 0.039* |
| C10 | 0.1846 (3) | 1.05202 (18) | 0.65468 (15) | 0.0288 (5) |
| C16 | 0.8761 (3) | 0.73907 (18) | 0.56372 (16) | 0.0298 (5) |
| H16 | 0.951891 | 0.759421 | 0.505560 | 0.036* |
| C4 | 0.2883 (3) | 0.50116 (18) | 0.57316 (17) | 0.0323 (5) |
| H4 | 0.272131 | 0.424226 | 0.591789 | 0.039* |
| C29 | 0.2921 (3) | 0.81138 (19) | 1.02264 (16) | 0.0332 (5) |
| H29 | 0.315142 | 0.736138 | 1.043778 | 0.040* |
| C27 | 0.2776 (3) | 1.01072 (19) | 1.04520 (16) | 0.0320 (5) |
| C11 | 0.2904 (3) | 1.12680 (18) | 0.66683 (16) | 0.0345 (6) |
| H11 | 0.262823 | 1.205381 | 0.665667 | 0.041* |
| C12 | 0.4350 (3) | 1.08630 (19) | 0.68054 (16) | 0.0349 (6) |
| H12 | 0.507781 | 1.137125 | 0.688870 | 0.042* |
| C26 | 0.2230 (3) | 1.03045 (19) | 0.96274 (17) | 0.0355 (6) |
| H26 | 0.198079 | 1.105600 | 0.942224 | 0.043* |
| C28 | 0.3114 (3) | 0.9006 (2) | 1.07421 (17) | 0.0373 (6) |
| H28 | 0.348617 | 0.885604 | 1.130708 | 0.045* |
| C1 | 0.2295 (4) | 0.6440 (2) | 0.30698 (18) | 0.0468 (7) |
| H1A | 0.218159 | 0.692070 | 0.250507 | 0.070* |
| H1B | 0.127320 | 0.613229 | 0.339329 | 0.070* |
| H1C | 0.309474 | 0.582200 | 0.286153 | 0.070* |
| C30 | 0.2996 (4) | 1.1075 (2) | 1.10112 (19) | 0.0440 (7) |
| H30A | 0.347426 | 1.168132 | 1.055929 | 0.066* |
| H30B | 0.369589 | 1.081913 | 1.142931 | 0.066* |
| H30C | 0.196168 | 1.135170 | 1.140848 | 0.066* |
| B1 | 0.4162 (3) | 0.76092 (19) | 0.67822 (16) | 0.0203 (5) |
| C31 | 0.1650 (3) | 0.3598 (2) | 0.91181 (19) | 0.0427 (6) |
| H31A | 0.133500 | 0.319552 | 0.976009 | 0.051* |
| H31B | 0.138899 | 0.441143 | 0.920738 | 0.051* |
| C14 | −0.0780 (4) | 1.0289 (2) | 0.6422 (3) | 0.0599 (9) |
| H14A | −0.041911 | 0.978445 | 0.588939 | 0.090* |
| H14B | −0.173387 | 1.074208 | 0.634887 | 0.090* |
| H14C | −0.102797 | 0.984454 | 0.703825 | 0.090* |
1 Source of materials
The title complex was synthesized from a mixture of quinolin-8-amine, 4-methylbenzenesulfonyl chloride and potassium trifluoro(3-methoxylphenyl)-λ 4-borane suspended in MeCN under air atomsphere at 130 ℃ for 24 h. Until the completion of this reaction, the resulting residue was adsorbed onto silica gel of a DCM solution, loaded directly onto a silica gel column, and purified by eluting first with EtOAc:PE 1:5 to get the target product as yellow solid.
2 Experimental details
Semi-empirical absorption corrections were applied using SADABS program. The structure was solved with SHELXT-2018, and refined using the SHELXL-2019 software package. All the hydrogen atoms were introduced at the calculated positions.
3 Comment
Four-coordinate organboron compounds, which contain π-conjugated chelated backbones, have been under constant investigation over the past decades [5]. They have been documented to express unique properties [6–11]. Therefore, their design [12] and synthesis have also attracted substantial interst.
Single-crystal X-ray diffraction analysis revealed that each asymmetric unit consisted of one B-containing molecule and one free solvent CH2Cl2 molecule. As exhibited in the Figure, the central boron atom B1 adopted a typical tetrahedral coordination geometry to be bound to two nitrogen atoms (N1, N2) from the quinoline unit and two carbon atoms (C6, C8) from two methoxybenzene units. The B–N and B–C bond lengths are in the range of 1.609(3)–1.613(3) Å and the C/N–B–C/N angles are in the range of 95.54(14)–117.14(14)°, which are in accordance with reported boron compounds [13–15]. The methoxybenzene units are closely perpendicular to the quinoline unit with the dihedral angles of 80.98 and 70.89°, respectively. Meanwhile, the C–H⃛π interactions makes some contribution to the formation of the crystal. The solvent CH2Cl2 molecules are entrapped in the crystal.
Funding source: the Natural Science Basic Re-search Plan in Shaanxi Province of China
Award Identifier / Grant number: No. 2022JQ-127
Funding source: Xijing University Special Fund for Talent Research in Special Zone .
Award Identifier / Grant number: (XJ22T03)
-
Author contributions: The author has accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This project was funded by Xijing University Special Fund for Talent Research in Special Zone (XJ22T03) and the Natural Science Basic Re-search Plan in Shaanxi Province of China (No. 2022JQ-127).
-
Conflict of interest statement: The author declares no conflicts of interest regarding this article.
References
1. Bruker. SAINT, Program for Data Extraction and Reduction; Bruker AXS, Inc: Madison, WI, 2001.Search in Google Scholar
2. Sheldrick, G. M. SADABS, Program for Empirical Adsorption Correction of Area Detector Data; University of Göttingen: Germany, 2003.Search in Google Scholar
3. Sheldrick, G. M. SHELXT-2018, Program for the Crystal Structure Solution; University of Göttingen: Germany, 2018.Search in Google Scholar
4. Sheldrick, G. M. SHELXL-2019, Program for the Crystal Structure Refinement; University of Göttingen: Germany, 2019.Search in Google Scholar
5. Mellerup, S.–K., Wang, S.–N. Boron–doped moleculaes for optoelectronics. Trends Chem. 2019, 1, 77–89; https://doi.org/10.1016/j.trechm.2019.01.003.Search in Google Scholar
6. Chen, P.–Z., Niu, L.–Y., Chen, Y.–Z., Yang, Q.–Z. Difluoroboron β-diketonate dyes: spectroscopic properties and applications. Coord. Chem. Rev. 2017, 350, 196–216; https://doi.org/10.1016/j.ccr.2017.06.026.Search in Google Scholar
7. Frath, D., Massue, J., Ulrich, G., Ziessel, R. Luminescent materials: locking π–conjugated and heterocyclic ligands with boron(III). Angew. Chem. Int. Ed. 2014, 53, 2290–2310; https://doi.org/10.1002/anie.201305554.Search in Google Scholar PubMed
8. Boens, N., Verbelen, B., Dehaen, W. Postfunctionalization of the BODIPY Core: synthesis and spectroscopy. Eur. J. Org. Chem. 2015, 2015, 6577–6595; https://doi.org/10.1002/ejoc.201500682.Search in Google Scholar
9. Liu, K., Lalancette, R.–A., Jakle, F. B–N Lewis pair functionalization of anthracene: structural dynamics, optoelectronic properties and O2 sensitization. J. Am. Chem. Soc. 2017, 139, 18170–18175; https://doi.org/10.1021/jacs.7b11062.Search in Google Scholar PubMed
10. Guan, C., Huang, L., Ren, C., Zou, G. Development of a telescoped process for preparation of N,O-chelated diarylborinates. Org. Process Res. Dev. 2018, 22, 824–828; https://doi.org/10.1021/acs.oprd.8b00109.Search in Google Scholar
11. Zhu, C., Ji, X., You, D., Chen, T.–L., Mu, A.–U., Barker, K.–P., Klivansky, L.–M., Liu, Y., Fang, L. B–N coordination promoted delocalization and hypercojugation lead to extraordinary redox activities in ladder-type conjugated molecules. J. Am. Chem. Soc. 2018, 140, 18173–18186; https://doi.org/10.1021/jacs.8b11337.Search in Google Scholar PubMed
12. Ding, S.–Y., Zu, W.–S., Miao, Z.–C., Xu, L. Synthetic and computational study of four-coordiante B,B-diaryl 8-aminoquinolate complexes. Chin. J. Org. Chem. 2022, 42, 812–818; https://doi.org/10.6023/cjoc202108040.Search in Google Scholar
13. Rodrigues, A. I., Figueira, C. A., Gomes, C. S. B., Suresh, D., Ferreira, B., Di Paolo, R. E., Pereira, D. S., Dias, F. B., Calhorda, M. J., Morgado, J., Maçanita, A. L., Gomes, P. T. Boron complexes of aromatic 5-substituted iminopyrrolyl ligands: synthesis, structure, and luminescence properties. Dalton Trans. 2019, 48, 13337–13352; https://doi.org/10.1039/c9dt02718a.Search in Google Scholar PubMed
14. Nagata, Y., Chujo, Y. Main-chain-type N,N-chelate organoboron aminoquinolate polymers: synthesis, luminescence, and energy transfer behavior. Macromolecules 2008, 41, 3488–3492; https://doi.org/10.1021/ma702873a.Search in Google Scholar
15. Más–Montoya, M., Usea, L., Espinosa Ferao, A., Montenegro, M. F., Arellano, C. R., Tárraga, A., Rodríguez–López, J. N., Curiel, D. Single heteroatom fine-tuning of the emissive properties in organoboron complexes with 7-(azaheteroaryl)indole systems. J. Org. Chem. 2016, 81, 3296–3302; https://doi.org/10.1021/acs.joc.6b00265.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2

