Abstract
C12H12O2S, monoclinic, P21/c (no. 14), a = 7.4771(1) Å, b = 8.6860(1) Å, c = 16.0445(2) Å, β =
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.10 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 2.57 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θ max, completeness: | 75.1°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 9298, 2062, 0.030 |
| Criterion for I obs, N(hkl)gt: |
I
obs
|
| N(param)refined: | 136 |
| Programs: | Bruker [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| S | 0.12584 (4) | 0.78885 (3) | 0.04374 (2) | 0.01739 (17) |
| O1 | 0.24912 (11) | 0.53637 (9) | 0.11665 (5) | 0.0151 (2) |
| C1 | 0.23558 (15) | 0.53453 (13) | −0.03427 (7) | 0.0145 (3) |
| O2 | 0.33932 (13) | 0.31246 (10) | −0.10041 (6) | 0.0192 (2) |
| C2 | 0.30957 (16) | 0.38017 (14) | −0.03539 (7) | 0.0142 (3) |
| C3 | 0.34613 (16) | 0.30782 (13) | 0.04760 (8) | 0.0141 (3) |
| C4 | 0.40987 (17) | 0.15659 (14) | 0.05521 (8) | 0.0166 (3) |
| H4A | 0.4292 | 0.1010 | 0.0073 | 0.020* |
| C5 | 0.44444 (17) | 0.08887 (14) | 0.13297 (8) | 0.0175 (3) |
| H5A | 0.4876 | −0.0115 | 0.1374 | 0.021* |
| C6 | 0.41409 (17) | 0.17241 (15) | 0.20532 (8) | 0.0174 (3) |
| H6A | 0.4382 | 0.1274 | 0.2578 | 0.021* |
| C7 | 0.34853 (16) | 0.32141 (14) | 0.19896 (8) | 0.0158 (3) |
| H7A | 0.3267 | 0.3765 | 0.2467 | 0.019* |
| C8 | 0.31579 (16) | 0.38761 (13) | 0.11982 (7) | 0.0136 (3) |
| C9 | 0.21087 (16) | 0.60210 (13) | 0.04035 (7) | 0.0140 (3) |
| C10 | 0.18689 (18) | 0.61597 (14) | −0.11580 (7) | 0.0178 (3) |
| H10A | 0.2137 | 0.5507 | −0.1613 | 0.027* |
| H10B | 0.0609 | 0.6397 | −0.1210 | 0.027* |
| H10C | 0.2549 | 0.7095 | −0.1172 | 0.027* |
| C11 | 0.08496 (19) | 0.81606 (14) | 0.15264 (8) | 0.0187 (3) |
| H11A | 0.0126 | 0.7325 | 0.1714 | 0.022* |
| H11B | 0.1976 | 0.8193 | 0.1879 | 0.022* |
| C12 | −0.01438 (18) | 0.96876 (14) | 0.15675 (8) | 0.0209 (3) |
| H12A | −0.0392 | 0.9884 | 0.2134 | 0.031* |
| H12B | 0.0587 | 1.0502 | 0.1379 | 0.031* |
| H12C | −0.1253 | 0.9639 | 0.1215 | 0.031* |
1 Source of material
The 100 mL round bottom flask was filled with 2-hydroxypropiophenone (3 mmol), carbon disulfide (4.5 mmol), potassium carbonate (9 mmol) and bromoethane (4.5 mmol), DMSO (15 mL), and heated to 35 °C under efficient stirring. The reaction was monitored by thin layer chromatography (TLC, 254 nm). After the reaction is complete, the mixture is cooled to room temperature, poured into ice water. White solids are precipitated, filtered, washed with water and petroleum ether for three times, and the crude product is recrystallized with methanol: water = 5:1 to obtain the title compound.
2 Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms, with constrained isotropic displacement parameters.
3 Comment
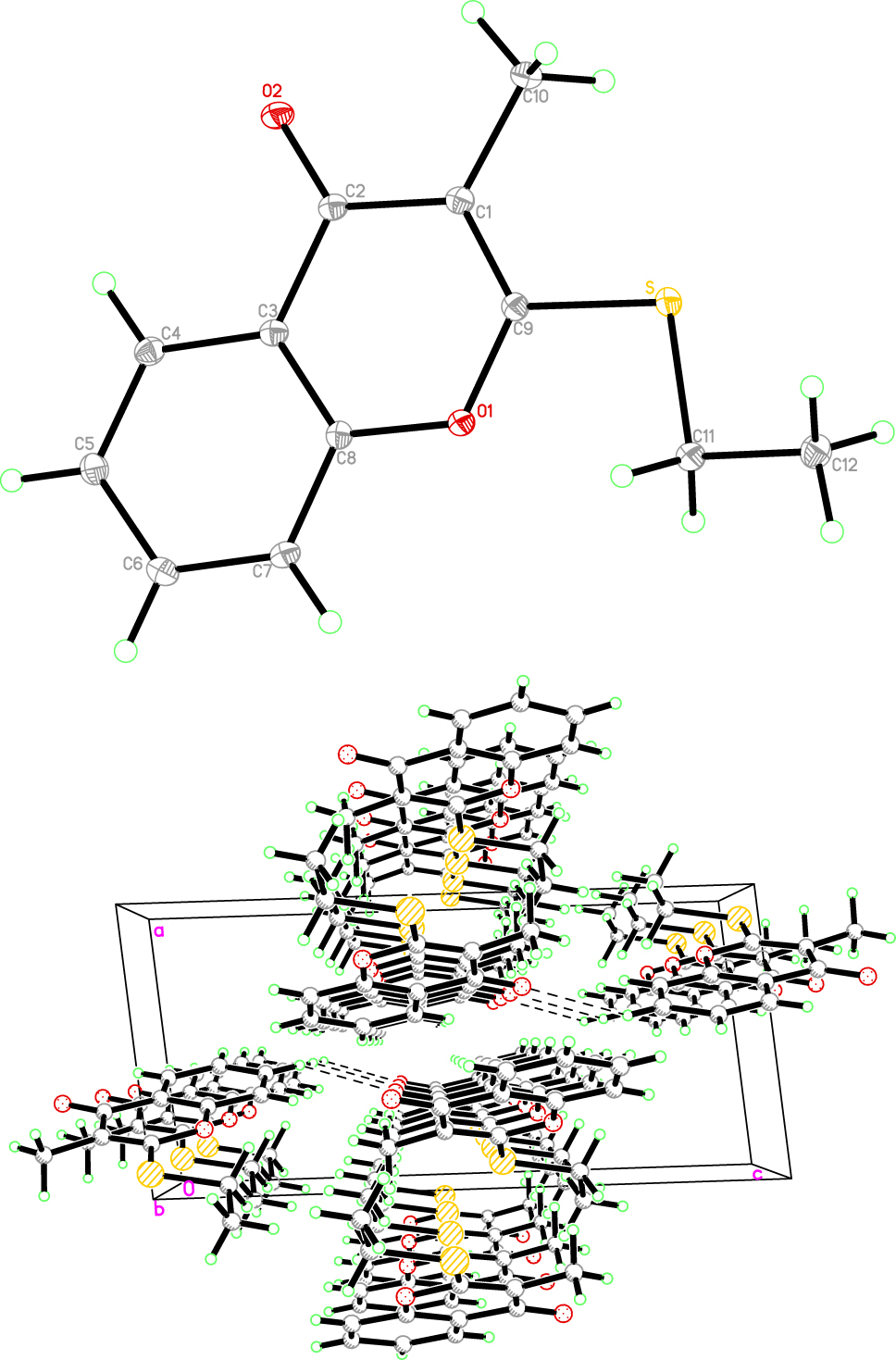
Chromone is an important oxygen-containing heterocyclic compound, and is a scaffold of natural or synthetic compounds, which are common in nature, especially in plants. The chromone skeleton is part of a variety of drugs showing biological activities [4]. In addition to their pharmacological significance, several chromones are used in other fields [5, 6]. As shown in the figure, the molecule consists of chromone skeleton, methyl and ethyl thio groups. The crystal structure is shown: as molecules (upper figure) and as a packing diagram (lower part of the figure). The dihedral angle between benzo fragment (C3/C4/C5/C6/C7/C8) and oxygen-containing heterocycle (C9/C1/C2/C3/C8/O1) is
Acknowledgements
We appreciate Wang huaqin in Nanjing University and Wu wenyuan in Nanjing Tech University for some data analysis.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Nanjing Senega Medical Technology Co. Ltd.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystall. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystall. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Khan, K. M., Ambreen, N., Mughal, U. R., Jalil, S., Perveen, S., Choudhary, M. I. 3-Formylchromones: potential antiinflammatory agents. Eur. J. Med. Chem. 2010, 45, 4058–4064; https://doi.org/10.1016/j.ejmech.2010.05.065.Search in Google Scholar PubMed
5. Teimouri, M. B. Serendipitous stereoselective synthesis of brand-new fluorescent dyes: (1Z)-3-(alkylimino)-1-[(chromone-3-yl)methylene]-1,3-dihydro-9H-furo[3,4-b]-chromen-9-one-type fluorophores with blue fluorescence emission properties. Tetrahedron 2011, 67, 1837–1843; https://doi.org/10.1016/j.tet.2011.01.033.Search in Google Scholar
6. Fan, L., Qin, J.-C., Li, T.-R., Wang, B.-D., Yang, Z.-Y. A novel rhodamine chromone-based “Off-On” chemosensor for the differential detection of Al(III) and Zn(II) in aqueous solutions. Sens. Actuators B 2014, 203, 550–556; https://doi.org/10.1016/j.snb.2014.07.017.Search in Google Scholar
7. Elagamy, A., Shaw, R., Shah, C., Pratap, R. Iodine-mediated synthesis of 2-(methylthio)-4H-chromen-4-ones and study of their halogenation reactions. J. Org. Chem. 2021, 86, 9478–9489; https://doi.org/10.1021/acs.joc.1c00788.Search in Google Scholar PubMed
8. Chai, G.-B., Qiu, Y.-A., Fu, C.-L., Ma, S.-M. Efficient assembly of chromone skeleton from 2,3-allenoic acids and benzynes. Org. Lett. 2011, 13, 5196–5199; https://doi.org/10.1021/ol202076c.Search in Google Scholar PubMed
9. Dziewulska-Kłaczkowska, A., Mazur, L. Structural studies and characterization of 3-formylchromone and products of its reactions with chosen primary aromatic amines. J. Mol. Struct. 2011, 985, 233–242; https://doi.org/10.1016/j.molstruc.2010.10.049.Search in Google Scholar
10. Gomes, L. R., Low, J. N., Cagide, F., Borges, F. The crystal structures of four N-(4-halophenyl)-4-oxo-4H-chromene-3-carboxamides. Acta Crystall. 2015, E71, 88–93; https://doi.org/10.1107/s2056989014027054.Search in Google Scholar
11. Savant, M. M., Gowda, N. S., Pansuriya, A. M., Bhuva, C. V., Kapuriya, N., Anandalwar, S. M., Prasad, S. J., Shah, A., Naliapara, Y. T. A concise synthetic strategy to functionalized chromenones via [5 + 1] heteroannulation and facile C–N/C–S/C–O bond formation with various nucleophiles. Tetrahedron Lett. 2011, 52, 254–257; https://doi.org/10.1016/j.tetlet.2010.11.020.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2

