Abstract
C13H18N2O4S, orthorhombic, P212121 (no. 19), a = 6.8619(2) Å, b = 12.0735(4) Å, c = 16.6591(5) Å, V = 1380.16(7) Å3, Z = 4, R gt (F) = 0.0236, wR ref (F 2) = 0.0604, T = 150 K.
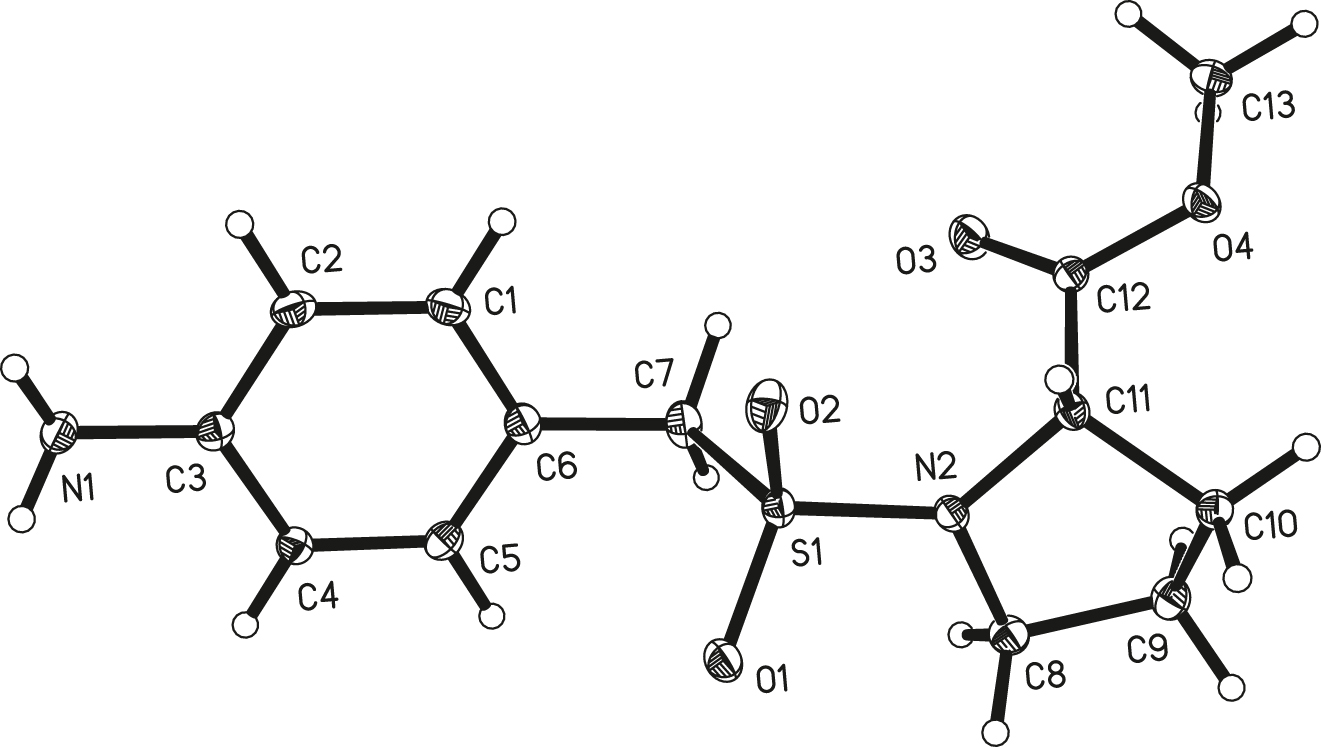
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.14 × 0.12 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 2.24 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θ max, completeness: | 79.0°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 20,828, 2946, 0.042 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2872 |
| N(param)refined: | 183 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.5972 (3) | 0.10403 (16) | 0.13039 (11) | 0.0262 (4) |
| H1 | 0.517115 | 0.168137 | 0.128250 | 0.031* |
| C2 | 0.5164 (3) | 0.00354 (16) | 0.15321 (10) | 0.0250 (4) |
| H2 | 0.381954 | −0.000393 | 0.166545 | 0.030* |
| C3 | 0.6311 (3) | −0.09186 (15) | 0.15674 (9) | 0.0198 (3) |
| C4 | 0.8288 (3) | −0.08351 (15) | 0.13699 (10) | 0.0201 (3) |
| H4 | 0.909299 | −0.147475 | 0.138953 | 0.024* |
| C5 | 0.9078 (3) | 0.01737 (15) | 0.11460 (10) | 0.0214 (4) |
| H5 | 1.042463 | 0.021778 | 0.101744 | 0.026* |
| C6 | 0.7935 (3) | 0.11253 (15) | 0.11059 (10) | 0.0224 (4) |
| C7 | 0.8846 (3) | 0.22168 (16) | 0.08884 (10) | 0.0261 (4) |
| H7A | 0.786612 | 0.269128 | 0.062011 | 0.031* |
| H7B | 0.993146 | 0.209151 | 0.050759 | 0.031* |
| C8 | 1.2917 (3) | 0.40152 (16) | 0.12293 (10) | 0.0227 (4) |
| H8A | 1.314161 | 0.356074 | 0.074345 | 0.027* |
| H8B | 1.375060 | 0.373634 | 0.167020 | 0.027* |
| C9 | 1.3305 (3) | 0.52418 (16) | 0.10640 (11) | 0.0252 (4) |
| H9A | 1.465720 | 0.544398 | 0.121083 | 0.030* |
| H9B | 1.308981 | 0.541944 | 0.049063 | 0.030* |
| C10 | 1.1842 (3) | 0.58433 (15) | 0.15935 (10) | 0.0207 (3) |
| H10A | 1.160560 | 0.660597 | 0.139818 | 0.025* |
| H10B | 1.229401 | 0.587452 | 0.215737 | 0.025* |
| C11 | 1.0013 (2) | 0.51285 (13) | 0.15131 (9) | 0.0177 (3) |
| H11 | 0.919980 | 0.519648 | 0.200867 | 0.021* |
| C12 | 0.8778 (2) | 0.53899 (14) | 0.07726 (10) | 0.0185 (3) |
| C13 | 0.7337 (3) | 0.68444 (15) | 0.00261 (10) | 0.0237 (4) |
| H13A | 0.611150 | 0.642766 | 0.001641 | 0.036* |
| H13B | 0.805258 | 0.671372 | −0.047409 | 0.036* |
| H13C | 0.705502 | 0.763671 | 0.007956 | 0.036* |
| N1 | 0.5498 (2) | −0.19452 (12) | 0.17535 (9) | 0.0238 (3) |
| H1A | 0.437345 | −0.187300 | 0.201944 | 0.025* |
| H1B | 0.631536 | −0.240553 | 0.194209 | 0.035* |
| N2 | 1.0839 (2) | 0.40101 (12) | 0.14615 (9) | 0.0191 (3) |
| O1 | 1.1177 (2) | 0.21852 (11) | 0.21203 (7) | 0.0263 (3) |
| O2 | 0.8100 (2) | 0.32514 (11) | 0.22329 (8) | 0.0276 (3) |
| O3 | 0.8086 (2) | 0.47123 (11) | 0.03267 (8) | 0.0301 (3) |
| O4 | 0.8508 (2) | 0.64820 (11) | 0.07014 (7) | 0.0244 (3) |
| S1 | 0.97480 (6) | 0.29069 (3) | 0.17654 (2) | 0.01842 (13) |
1 Source of material
(4–Nitrophenyl)methanesulfonyl chloride (2.81 g, 12.0 mmol), methyl pyrrolidine-2-carboxylate hydrochloride (2.39 g, 14.4 mmol) and 4-dimethylaminopyridine (3.52 g, 28.8 mmol) were dissolved in 48 mL of dichloromethane and reacted at room temperature for 3.0 h. The response endpoint was detected by thin layer chromatography (TLC). When the reaction was stopped, an appropriate amount of ethyl acetate was added to the reaction system, stirred and allowed to stand for 10 min. The filtrate obtained after filtering off the precipitate was concentrated under vacuum. The crude product was purified by silica-gel column chromatography (petroleum ether:ethyl acetate = 3:1, v:v). The white solid (1.62 g, 4.94 mmol) obtained after the reaction was dissolved in 20 mL of super-dry methanol with stannous chloride (5.57 g, 24.7 mmol) and refluxed at a controlled temperature of 343 K for 2.0 h. The response endpoint was detected by thin layer chromatography (TLC). When the reaction was stopped, an appropriate amount of saturated aqueous Na2CO3 solution was added to the reaction system, the pH was adjusted to 7–8, an appropriate amount of ethyl acetate was added, and stirred for 1.0 h. The filtrate was obtained by filtration of diatomaceous earth. The filtrate was successively washed with deionized water and brine, dried over anhydrous sodium sulfate and condensed under vacuum. The crude product was purified by silica-gel column chromatography (petroleum ether:ethyl acetate = 2:1, v:v). The title compound was recrystallized from dichloromethane and methanol (1:1, v:v) system to attain suitable crystals.
2 Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms. The H atoms at N1 were placed in difference maps and treated as riding.
3 Comment
Sulfonamides are important compounds with a wide range of applications [3–5]. The title compound was obtained from the raw material (4-nitrophenyl)methanesulfonyl chloride by substitution and reduction reactions. We further obtained the title compound, methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, as a single crystal.
The ORTEP diagram is presented in the Figure. Bond lengths and angles are all in the expected ranges [6]. The whole molecule contains two parts, p-aminobenzenesulfonyl and methyl L-prolinate, linked by N(2)–S(1). The bond length of N(2)–S(1) is 1.6096(15) Å. The bond angle of C(7)–S(1)–N(2) is 106.80(8)° [7]. For the p-aminobenzenesulfonyl side, the amino group at the C(3) position is obtained by reduction of the nitro group, and can act as a proton donor and form hydrogen bonds with other atoms [8, 9]. For the methyl L-prolinate, the C(11) is the chiral center and the molecule adopts the L stereoselective configuration. The carboxyl methyl ester at the C(11) can improve the molecular lipid solubility.
Funding source: Science and Technology Innovation Development Plan of Yantai
Award Identifier / Grant number: 2020XDRH105
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81473104
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Science and Technology Innovation Development Plan of Yantai (No. 2020XDRH105) and the National Natural Science Foundation of China (No. 81473104).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2 and SAINT Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Premakumari, C., Muralikrishna, A., Padmaja, A., Padmavathi, V., Park, S. J., Kim, T. J., Reddy, G. D. Synthesis, antimicrobial and anticancer activities of amido sulfonamido methane linked bis heterocycles. Arab. J. Chem. 2014, 7, 385–395; https://doi.org/10.1016/j.arabjc.2013.10.024.Search in Google Scholar
4. Rubab, L., Afroz, S., Ahmad, S., Hussain, S., Nawaz, I., Irfan, A., Batool, F., Kotwica-Mojzych, K., Mojzych, M. An update on synthesis of coumarin sulfonamides as enzyme inhibitors and anticancer agents. Molecules 2022, 27, 1604; https://doi.org/10.3390/molecules27051604.Search in Google Scholar PubMed PubMed Central
5. Naicker, T., Brasil, E., Samipillai, M., Govender, T., Baijnath, S. Crystal structure of 2-(4-fluorophenyl)-N-phenyl-2-(phenylamino) ethanesulfonamide-toluene (1/0.5), C23.5H23FN2O2S. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 169–171.10.1515/ncrs-2018-0253Search in Google Scholar
6. Gong, Y. X., Geng, Y. D., Zhou, S. J., Wang, G. Y. The crystal structure of 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N- methylmethanaminium 3-carboxyprop-2-enoate, C21H20FN3O6S. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 911–913.Search in Google Scholar
7. Xu, Y. R., Tang, H. J., Xu, Y. J., Guo, J. L., Zhao, X., Meng, Q. G., Xiao, J. H. Design, synthesis, bioactivity evaluation, crystal structures, and in silico studies of new α-amino amide derivatives as potential histone deacetylase 6 inhibitors. Molecules 2022, 27, 3335; https://doi.org/10.3390/molecules27103335.Search in Google Scholar PubMed PubMed Central
8. Krishnan, S., Kaliyaperumal, T., Marimuthu, R., Velusamy, S. Crystal structure, Hirshfeld surface, and DFT studies of 4-((pyrrolidin-1-ylsulfonyl)methyl)aniline. Eur. J. Chem. 2021, 12, 419–431; https://doi.org/10.5155/eurjchem.12.4.419-431.2177.Search in Google Scholar
9. Sridhar, B., Ravikumar, K., Krishnan, H., Singh, A. N. Molecular association of almotriptan with oxalate and terephthalate anions. J. Chem. Crystallogr. 2011, 41, 291–296; https://doi.org/10.1007/s10870-010-9875-7.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2

