Abstract
C23H34N2, monoclinic, C2/c (no. 15), a = 15.2448(11) Å, b = 8.2189(5) Å, c = 16.7350(16) Å, β = 103.133(7)°, V = 2042.0(3) Å3, Z = 4, R gt (F) = 0.0438, wR ref (F 2) = 0.1277, T = 150 K.
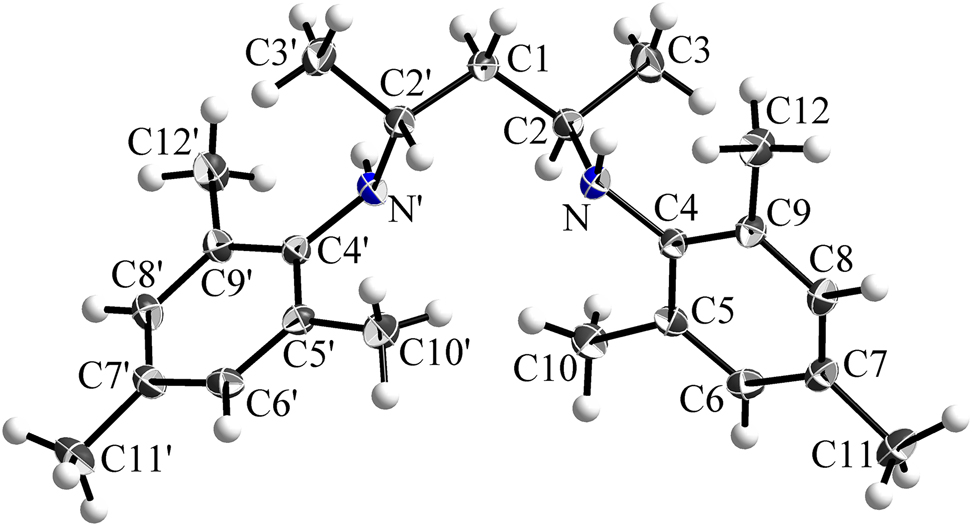
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.20 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.06 mm−1 |
| Diffractometer, scan mode: | STOE StadiVari, ω |
| θ max, completeness: | 30.0°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 34,500, 2983, 0.043 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2179 |
| N(param)refined: | 122 |
| Programs: | X-Area [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.5000 | 0.64420 (18) | 0.2500 | 0.0228 (3) |
| H1Aa | 0.4481 | 0.7154 | 0.2266 | 0.027* |
| H1Ba | 0.5519 | 0.7154 | 0.2734 | 0.027* |
| C2 | 0.52311 (7) | 0.54381 (13) | 0.18010 (7) | 0.0238 (2) |
| H2 | 0.4696 | 0.4754 | 0.1552 | 0.029* |
| C3 | 0.54380 (9) | 0.65475 (16) | 0.11294 (8) | 0.0356 (3) |
| H3A | 0.5923 | 0.7302 | 0.1372 | 0.053* |
| H3B | 0.4897 | 0.7167 | 0.0875 | 0.053* |
| H3C | 0.5626 | 0.5883 | 0.0711 | 0.053* |
| N | 0.59849 (6) | 0.43451 (11) | 0.21606 (6) | 0.0235 (2) |
| H1 | 0.6455 (11) | 0.499 (2) | 0.2479 (11) | 0.044 (4)* |
| C4 | 0.63402 (7) | 0.32711 (12) | 0.16458 (6) | 0.0202 (2) |
| C5 | 0.58201 (7) | 0.19841 (13) | 0.12242 (7) | 0.0223 (2) |
| C6 | 0.62219 (8) | 0.08763 (13) | 0.07811 (7) | 0.0246 (2) |
| H6 | 0.5864 | 0.0026 | 0.0490 | 0.030* |
| C7 | 0.71248 (8) | 0.09742 (13) | 0.07514 (7) | 0.0251 (2) |
| C8 | 0.76200 (7) | 0.22660 (14) | 0.11607 (7) | 0.0253 (2) |
| H8 | 0.8235 | 0.2370 | 0.1139 | 0.030* |
| C9 | 0.72504 (7) | 0.34183 (13) | 0.16031 (7) | 0.0223 (2) |
| C10 | 0.48513 (8) | 0.17106 (15) | 0.12607 (8) | 0.0311 (3) |
| H10A | 0.4457 | 0.2356 | 0.0833 | 0.047* |
| H10B | 0.4765 | 0.2044 | 0.1800 | 0.047* |
| H10C | 0.4703 | 0.0554 | 0.1173 | 0.047* |
| C11 | 0.75574 (10) | −0.03149 (16) | 0.03248 (8) | 0.0360 (3) |
| H11A | 0.8068 | 0.0158 | 0.0141 | 0.054* |
| H11B | 0.7115 | −0.0729 | −0.0151 | 0.054* |
| H11C | 0.7770 | −0.1210 | 0.0707 | 0.054* |
| C12 | 0.78280 (8) | 0.47986 (16) | 0.20280 (8) | 0.0322 (3) |
| H12A | 0.8428 | 0.4731 | 0.1910 | 0.048* |
| H12B | 0.7882 | 0.4721 | 0.2621 | 0.048* |
| H12C | 0.7549 | 0.5840 | 0.1828 | 0.048* |
-
aOccupancy: 0.5.
Source of material
All reactions were performed under ambient conditions. N 2,N 4-dimesitylpentane-2,4-diamine (1; mesityl=2,4,6-trimethylphenyl) was obtained as a by-product during the preparation and crystallization of NacNacMesH [N,N′-di(2,4,6-trimethylphenyl)-β-diketiminate] [5]. The NacNacMesH ligand was prepared according to a modified literature procedure [6]. Acetylacetone (Merck), 2,4,6-trimethylaniline (Alfa Aesar), and p-toluenesulfonic acid (Merck) were used without further purification. Acetylacetone (5 g, 50 mmol) and 2,4,6-trimethylaniline (13.5 g, 100 mmol) were dissolved in toluene (200 mL) and cooled in an ice bath. Subsequently, p-toluenesulfonic acid (8.6 g, 50 mmol) was added to the flask. After stirring the reaction mixture for 15 h at 110 °C, the solvent was removed under reduced pressure. The solid precipitate was dissolved in dichloromethane (100 mL) and the organic phase was extracted with a saturated potassium carbonate solution. The aqueous phase was re-extracted twice with dichloromethane and the solvent was removed from the combined organic phases under vacuum. The crude product was recrystallized from methanol. Few colorless crystals of 1 were obtained as a by-product besides the main product NacNacMesH.
Experimental details
The single crystal of 1 was selected under a microscope equipped with a light source in an Ar-filled glove box. Subsequently, the crystal was transferred under Ar to the diffractometer (STOE StadiVari) equipped with a PILATUS 300K detector (DECTRIS) and a Mo Kα radiation source (λ = 0.71073 Å). For the data collection the crystal was cooled in a 150 K cold stream of dry nitrogen. The single crystal structure was solved by direct methods using the program SHELXS-97 [2]. Structure refinements were performed by full-matrix least-squares calculations against F 2 (SHELXL-2014) [3]. A riding model was used to calculate and refine the positions of hydrogen atoms, except for the hydrogen atom bound to nitrogen which was located from the difference Fourier map and was refined with free positional and isotropic displacement parameters. Other elements than hydrogen were refined with anisotropic displacement parameters.
Comment
N 2,N 4-dimesitylpentane-2,4-diamine (1) crystallizes in the monoclinic space group C2/c with four molecules per unit cell and all atoms at general 8f Wyckoff positions except for C1 at a 4e site which lies on a twofold axis, defining one molecule half as asymmetric unit. It represents the fully reduced congener to the organic compound NacNacMesH (2), which serves as a ligand in e.g. magnesium organyls [7, 8]. However, due to the absence of any reducing agent in the reaction mixture the formation of compound 1 cannot be explained in a straightforward way. Structurally similar compounds like N 2,N 4-di(iso-propylphenyl)-3,3-dimethylpentane-2,4-diamine (3) have been reported before [9, 10], some of which were prepared by the reduction of the corresponding aryl-diimines with LiAlH4 [11]. Diamines obtained via this route have been applied as ligands for the generation of nickel complexes, which are used as catalytic species in the polymerization of polyethylene [11].
The presence of amine moieties was corroborated not only by evaluating the difference Fourier electron density map, revealing hydrogen atoms adjacent to the nitrogen atoms, but also by comparing the C–C and C–N bond lengths of 1 to the compounds 2 and 3 [8, 9]. The backbone of compound 1 comprises solely C–C and N–C single bonds [C1–C2 = 1.5364(13) Å, N–C2 = 1.4749(14) Å, N–C4 = 1.4237(13) Å] in analogy to molecule 3. In contrast, the backbone of the unsaturated compound 2 reveals shortened C=C and N=C double bonds of 1.397 and 1.324 Å (mean values), respectively [8]. The NH groups form neither intramolecular nor intermolecular hydrogen bonds of relevant strength, also no π–π interactions between the mesityl residues indicated by parallel orientation of the aryl rings are present. Only weak C–H⃛π interactions are found in the crystal of compound 1 (C12–H12b⃛C6(π)′, symmetry operation is 1.5-x,0.5+y,0.5-z; H⃛C(π) = 2.87 Å, C–H⃛C(π) = 147°).
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: 245845833
Acknowledgments
CW thanks the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, project number 245845833) within the International Research Training Group IRTG 2022 (ATUMS) for funding. Furthermore, CW thanks the Studienstiftung des Deutschen Volkes for granting a PhD scholarship.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, project number 245845833) within the International Research Training Group IRTG 2022 (ATUMS).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Stoe & Cie GmbH. X-Area, Version 1.76; Stoe & Cie GmbH: Darmstadt, Germany, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122, https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K., Putz, H. DIAMOND. Visual Crystal Structure Information System. Version 3.2k; Crystal Impact: Bonn, Germany, 2014.Search in Google Scholar
5. Wallach, C., Klein, W., Fässler, T. F. Nonagermanide zintl clusters with Mg2+ counter ions. Z. Anorg. Allg. Chem. 2022, 648, e202200065.Search in Google Scholar
6. Rossetto, E., Caovilla, M., Thiele, D., de Souza, R. F., Bernardo-Gusmão, K. Ethylene oligomerization using nickel-β-diimine hybrid xerogels produced by the sol–gel process. Appl. Catal., A 2013, 454, 152–159; https://doi.org/10.1016/j.apcata.2012.09.024.Search in Google Scholar
7. Green, S. P., Jones, C., Stasch, A. Stable magnesium (I) compounds with Mg–Mg bonds. Science 2007, 318, 1754–1757; https://doi.org/10.1126/science.1150856.Search in Google Scholar
8. Prust, J., Most, K., Müller, I., Alexopoulos, E., Stasch, A., Usón, I., Roesky, H. W. Synthesis and structures of β-diketoiminate complexes of magnesium. Z. Anorg. Allg. Chem. 2001, 627, 2032–2037; https://doi.org/10.1002/1521-3749(200108)627:8<2032::aid-zaac2032>3.0.co;2-6.10.1002/1521-3749(200108)627:8<2032::AID-ZAAC2032>3.0.CO;2-6Search in Google Scholar
9. Carey, D. T., Mair, F. S., Pritchard, R. G., Warren, J. E., Woods, R. J. Borane and alane reductions of bulky N, N′-diaryl-1, 3-diimines: structural characterization of products and intermediates in the diastereoselective synthesis of 1, 3-diamines. Dalton Trans. 2003, 2003, 3792–3798; https://doi.org/10.1039/b306401h.Search in Google Scholar
10. Magull, J., Simon, A. Zur Reaktion von Makrozyklen mit Lanthanoiden. II. Die Strukturen von [K(thf)3]2[(C22H28N4)2Sm2]⋅4̇THF und [(C22H22N4)Co]⋅DME. Z. Anorg. Allg. Chem. 1993, 615, 81–85.10.1002/zaac.19926150916Search in Google Scholar
11. Rong, G., Zifang, G., Junling, Z., Dongbing, L., Yan, L., Xiaofan, Z., Jie, F., Junhui, Z., Jingjing, L. Beijing Research Institute of Chemical Industry, China Petroleum & Chemical Corporation. Patent No. CN111116787, May 8, 2020. https://scifinder-n.cas.org/searchDetail/reference/622cbae55709576d8a282d95/referenceDetails (accessed Mar 12, 2022).Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

