Abstract
C19H16N2OS, triclinic, P1̄ (no. 2), a = 8.1510(3) Å, b = 8.8021(3) Å, c = 11.3953(5) Å, α = 72.546(2)°, β = 84.568(2)°, γ = 80.760(2)°, V = 768.86(5) Å3, Z = 2, Rgt(F) = 0.0491, wRref(F2) = 0.1494, T = 100 K.
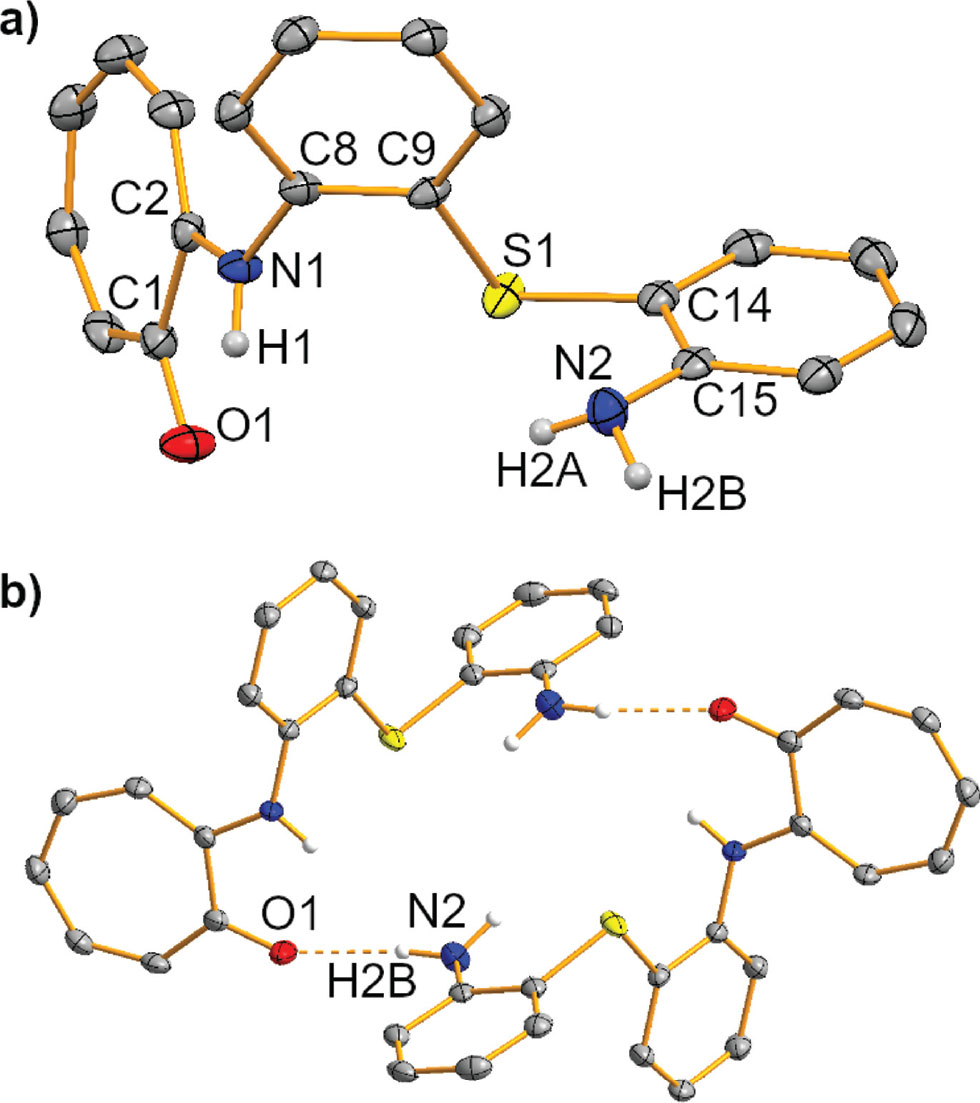
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.21 × 0.16 × 0.04 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.22 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX, φ and ω |
| θmax, completeness: | 28.0°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9366, 3619, 0.040 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2625 |
| N(param)refined: | 220 |
| Programs: | Bruker [1], [2], SHELX [3], [4], Mercury [5], Olex2 [6] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2649(3) | 0.9263(2) | 0.59895(19) | 0.0194(5) |
| C2 | 0.4020(3) | 0.8573(2) | 0.68386(19) | 0.0170(4) |
| C3 | 0.5422(3) | 0.7529(3) | 0.66942(19) | 0.0214(5) |
| H3 | 0.612709 | 0.721509 | 0.734592 | 0.026* |
| C4 | 0.5961(3) | 0.6864(3) | 0.5733(2) | 0.0262(5) |
| H4 | 0.696426 | 0.617702 | 0.584103 | 0.031* |
| C5 | 0.5225(3) | 0.7078(3) | 0.4652(2) | 0.0254(5) |
| H5 | 0.580316 | 0.654797 | 0.411164 | 0.031* |
| C6 | 0.3712(3) | 0.7996(3) | 0.4269(2) | 0.0232(5) |
| H6 | 0.340482 | 0.797943 | 0.350771 | 0.028* |
| C7 | 0.2605(3) | 0.8920(3) | 0.48436(19) | 0.0223(5) |
| H7 | 0.165653 | 0.941274 | 0.441153 | 0.027* |
| C8 | 0.4611(3) | 0.8454(2) | 0.89677(19) | 0.0173(4) |
| C9 | 0.3829(2) | 0.7484(2) | 1.00027(19) | 0.0172(4) |
| C10 | 0.4672(3) | 0.6839(3) | 1.10903(19) | 0.0205(5) |
| H10 | 0.416279 | 0.618802 | 1.178156 | 0.025* |
| C11 | 0.6259(3) | 0.7165(3) | 1.1144(2) | 0.0210(5) |
| H11 | 0.682462 | 0.671832 | 1.186693 | 0.025* |
| C12 | 0.7015(3) | 0.8159(3) | 1.0120(2) | 0.0218(5) |
| H12 | 0.807463 | 0.839519 | 1.016403 | 0.026* |
| C13 | 0.6186(3) | 0.8802(3) | 0.9025(2) | 0.0198(5) |
| H13 | 0.669291 | 0.946235 | 0.833736 | 0.024* |
| C14 | 0.1056(3) | 0.6179(3) | 1.13132(19) | 0.0193(4) |
| C15 | 0.0507(2) | 0.7064(3) | 1.21541(19) | 0.0184(4) |
| C16 | −0.0269(3) | 0.6290(3) | 1.3276(2) | 0.0219(5) |
| H16 | −0.062511 | 0.684984 | 1.384912 | 0.026* |
| C17 | −0.0509(3) | 0.4700(3) | 1.3539(2) | 0.0247(5) |
| H17 | −0.104983 | 0.421133 | 1.428000 | 0.030* |
| C18 | 0.0044(3) | 0.3819(3) | 1.2714(2) | 0.0263(5) |
| H18 | −0.009798 | 0.274248 | 1.290426 | 0.032* |
| C19 | 0.0811(3) | 0.4578(3) | 1.1605(2) | 0.0229(5) |
| H19 | 0.117034 | 0.400415 | 1.104136 | 0.027* |
| H1 | 0.282(3) | 0.972(3) | 0.788(2) | 0.034(7)* |
| H2A | 0.100(3) | 0.914(3) | 1.109(3) | 0.041(8)* |
| H2B | 0.013(4) | 0.921(3) | 1.240(3) | 0.046(8)* |
| N1 | 0.3736(2) | 0.9092(2) | 0.78604(16) | 0.0194(4) |
| N2 | 0.0760(3) | 0.8639(2) | 1.1906(2) | 0.0247(4) |
| O1 | 0.14534(19) | 1.01392(19) | 0.63363(14) | 0.0271(4) |
| S1 | 0.18083(7) | 0.71275(7) | 0.98081(5) | 0.02337(18) |
Comment
Aminotroponiminates (ATIs) are monoanionic ligands with applications in fields such as hydroamination and polymerization catalysis and the stabilization of low-valent main group species [7], [8], [9], [10], [11], [12]. Their potential to act as redox-active ligands has recently been demonstrated [13], [14], [15]. In the coordination chemistry of ATIs, it has been shown that not only their N,N′-binding pocket, but also their C7-ligand backbone can undergo directed bonding interactions with metal centers [16], [17], [18], [19]. Thus, ATIs can effectively act as ditopic, tridentate ligands. A strategy to further increase the denticity of this class of ligands is to connect two ATI ligands via linkers, generating so-called tropocoronands. These macrocyclic ligands have been employed for the chelation of metal atoms including Co, Ni, Cu, and Rh [20], [21], [22], [23], [24], [25], [26], [27], [28]. We became interested in tropocoronands containing unsaturated linker units. Reaction of 2,2′-thio-dianiline (1) with O-tosyltropone (2) in a 1.0:2.5 stoichiometry gave the title compound 2-((2-((2-aminophenyl)thio)phenyl)amino)-cyclohepta-2,4,6-trien-1-one (3) as the main product, which was isolated and fully characterized. The isolation of compound 3 shows that functionalization of the first N atom in 1 hampers functionalization of the second nitrogen atom in this substrate in the protocol that was employed. The asymmetric unit of the title compounds contains one formula unit of 3 (triclinic, P1̄, Z = 2, see the Figure). The three planar ring systems in 3 are twisted towards each other. The angle between the mean planes of the tropolone and the adjacent phenylene ring amounts to 68.3°. The angle between the mean planes of the two phenylene units is 76.3°. The C—N bond lengths in compound 3 suggest partial double bond character for C2—N1 [1.361(3) Å] and C15—N2 [1.374(3) Å], but not for C8—N1 [1.426(3) Å]. This demonstrates the stronger electron withdrawing character of the tropolon-2-yl unit compared to the phenylene unit in 3. The C—S bond lengths are identical within limits of error and virtually identical to those in the free 2,2′-thiodianiline substituent [1.772(2) Å] [7], [8], [9], [10], [11], [12], [29] In the solid state, the title compound is linked via two N—H⋯O hydrogen bonds, forming dimers with Ci symmetry (see the Figure). In this scenario, O1 acts as a H-bond acceptor, while H2B represents the H-bond donor. Overall, this leads to a so-called R22(22) motif, i.e. a ring strucutre formed by 22 atoms including two hydrogen bond donors and two hydrogen bond acceptors [30]. Using the C7H5ONH fragment, 15 structures of 2-(amino)tropones can be found in the Cambride Structural Database [31]. The majority of these compounds form dimers in the solid state through N—H⋯O hydrogen bonding [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]. In comparison to the title compound, all of these dimers form R22(10) motifs, i.e. the rings generated through hydrogen bonding are significantly smaller. In addition, two examples of hydrogen-bonded coordination polymers and two monomeric species have been reported in the literature [14], [43], [44], [45].
Source of material
Ethanol (30 mL) was added to a mixture of 2,2′-thio-dianiline (1) (157 mg, 0.726 mmol) and O-tosyltropone (2) (500 mg, 1.81 mmol). The reaction mixture was heated under reflux for 3 d. Aqueous sodium hydroxide (2 M, 20 mL) and CH2Cl2 (20 mL) were added, the aqueous phase was separated and extracted with CH2Cl2 (2 × 10 mL). The combined organic phases were dried over Na2CO3 and all volatiles were removed in vacuo. The crude reaction product was purified by column chromatography (Hexan/Ethyl acetate 5:1). The product was obtained as colourless crystals. Yield: 70 mg, 0.220 mmol, 30%.
The atom labeling used for the NMR spectroscopic characterization is the same as the atom labeling in the single crystal X-ray structure analysis.
1H-NMR (500 MHz, CDCl3): δ = 6.72 (td, 1H, 3JHH = 7.50 Hz, 4JHH = 1.29 Hz, 18-H), 6.72 (dd, 1H, 3JHH = 8.10 Hz, 4JHH = 1.35 Hz, 16-H), 6.78 (m, 1H, 5-H), 6.85 (dd, 1H, 3JHH = 10.3 Hz, 4JHH = 0.52 Hz, 3-H), 6.96 (dd, 1H, 3JHH = 7.95 Hz, 4JHH = 1.42 Hz, 10-H), 7.11 (m, 1H, 4-H), 7.14 (m, 1H, 11-H), 7.20 (m, 1H, 17-H), 7.22 (m, 1H, 12-H), 7.32 (m, 3H, 6-H, 7-H, 13-H) 7.36 (dd, 3JHH = 7.7 Hz, 4JHH = 1.5 Hz, 19-H), 8.69 (br. s, 1H, NH) ppm.
13C-NMR (125 MHz, CDCl3): δ = 110.83 (s, 3-C), 113.31 (s, 14-C), 115.66 (s, 16-C), 119.15 (s, 18-C), 124.84 (s, 5-C), 126.33 (s, 13-C), 126.63 (s, 12-C), 127.46 (s, 11-C), 128.19 (s, 10-C), 131.09 (s, 7-C), 131.38 (s, 17-C), 134.45 (s, 9-C), 135.58 (s, 8-C), 136.05 (s, 4-C), 137.42 (s, 19-C), 137.60 (s, 6-C), 149.09 (s, 15-C), 153.96 (s, 2-C), 177.17 (s, 1-C) ppm.
Anal. calc. for C19H16N2OS (320.41 g/mol): C, 71.22; H, 5.03; N, 8.74; found: C, 70.99; H, 4.95; N, 8.61.
m. p.: 175 °C.
Experimental details
The Uiso values of H atoms were set to 1.2 * Ueq of the parent atoms. Coordinates of hydrogen atoms bound to N were refined without any constraints or restraints. All other hydrogen atoms were refined with riding coordinates.
Acknowledgements
The authors thank Prof. Holger Braunschweig for constant support and the Fonds der Chemischen Industrie (Liebig scholarship to C. L.), the DFG, and the University of Würzburg for generous financial support. This publication was supported by the Open Access Publication Fund of the University of Würzburg.
References
1. Bruker. APEX2 ver. 2014.9. Bruker AXS GmbH, Karlsruhe, Germany (2014).Suche in Google Scholar
2. Bruker. SAINT+ ver. 8.38A. Bruker AXS GmbH, Karlsruhe, Germany (2019).Suche in Google Scholar
3. Sheldrick, G.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Suche in Google Scholar
4. Sheldrick, G.: Crystal structure refinement with SHELXL. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar
5. Macrae, C. F.; Sovago, I.; Cottrell, S. J.; Galek, P. T. A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G. P.; Stevens, J. S.; Towler, M.; Wood, P. A.: Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 53 (2020) 226–235.10.1107/S1600576719014092Suche in Google Scholar
6. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structrue solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–241.10.1107/S0021889808042726Suche in Google Scholar
7. Hanft, A.; Lichtenberg, C.: New perspectives for aminotroponiminates: coordination chemistry, redox behavior, cooperativity, and catalysis. Eur. J. Inorg. Chem. 2018 (2018) 3361–3373.10.1002/ejic.201800465Suche in Google Scholar
8. Roesky, P. W.: The coordination chemistry of aminotroponiminates. Chem. Soc. Rev. 29 (2000) 335–345.10.1039/a906763iSuche in Google Scholar
9. Roesky, P. W.: Bulky amido ligands in rare earth chemistry – syntheses, structures, and catalysis. Z. Anorg. Allg. Chem. 629 (2003) 1881–1894.10.1002/zaac.200300240Suche in Google Scholar
10. Dias, H. V. R.; Wang, Z.; Jin, W.: Aminotroponiminato complexes of silicon, germanium, tin and lead. Coord. Chem. Rev. 176 (1998) 67–86.10.1016/S0010-8545(98)00112-XSuche in Google Scholar
11. Kuehl, O.: N-heterocyclic germylenes and related compounds. Coord. Chem. Rev. 248 (2004) 411–427.10.1016/j.ccr.2003.12.004Suche in Google Scholar
12. Jenter, J.; Luhl, A.; Roesky, P. W.; Blechert, S.: Aminotroponiminate zinc complexes as catalysts for the intramolecular hydroamination. J. Organomet. Chem. 696 (2011) 406–418.10.1016/j.jorganchem.2010.10.017Suche in Google Scholar
13. Lichtenberg, C.; Krummenacher, I.: Aminotroponiminates as tunable, redox-active ligands: reversible single electron transfer and reductive dimerisation. Chem. Commun. 52 (2016) 10044–10046.10.1039/C6CC05762DSuche in Google Scholar PubMed
14. Hanft, A.; Lichtenberg, C.: Aminotroponiminates: ligand-centred, reversible redox events under oxidative conditions in sodium and bismuth complexes: Dalton Trans. 47 (2018) 10578–10589.10.1039/C8DT01019FSuche in Google Scholar
15. Hanft, A.; Krummenacher, I.; Lichtenberg, C.: Alkali-metal aminotroponiminates: selectivities and equilibria in reversible radical coupling of delocalized π-electron systems. Chem. Eur. J. 25 (2019) 11883–11891.10.1002/chem.201901962Suche in Google Scholar PubMed
16. Lichtenberg, C.: Aminotroponiminates: alkali metal compounds reveal unprecedented coordination modes. Organometallics 35 (2016) 874–902.10.1021/acs.organomet.6b00042Suche in Google Scholar
17. Hanft, A.; Lichtenberg, C.: Rationalizing the effect of ligand substitution patterns on coordination and reactivity of alkali metal aminotroponiminates. Organometallics 37 (2018) 1781–1787.10.1021/acs.organomet.8b00208Suche in Google Scholar
18. Hanft, A.; Jürgensen, M.; Bertermann, R.; Lichtenberg, C.: Sodium aminotroponiminates: ligand-induced disproportionation, mixed-metal compounds, and exceptional activity in polymerization catalysis. ChemCatChem 10 (2018) 4018–4027.10.1002/cctc.201800580Suche in Google Scholar
19. Pittracher, M.; Frisch, U.; Kopacka, H.; Wurst, K.; Müller, T.; Oehninger, L.; Ott, I.; Wuttke, E.; Scheerer, S.; Winter, R. F.; Bildstein, B.: π-Complexes of tropolone and its N-derivatives: ambidentate [O,O]/[N,O]/[N,N]-cycloheptatrienyl pentamethylcyclopentadienyl ruthenium sandwich complexes. Organometallics 33 (2014) 1630–1643.10.1021/om401200tSuche in Google Scholar
20. Jaynes, S.; Doerrer, L. H.; Liu, S.; Lippard, S. J.: Synthesis, tuning of the stereochemistry, and physical properties of cobalt(II) tropocoronand complexes. Inorg. Chem. 34 (1995) 5735–5744.10.1021/ic00127a010Suche in Google Scholar
21. Davis, M.; Roberts, M. M.; Zask, A.; Nakanishi, K.; Nozoe, T.; Lippard, S. J.: Stereochemical and electronic spin state tuning of the metal center in the nickel(II)tropocoronands. J. Am. Chem. Soc. 107 (1985) 3864–3870.10.1021/ja00299a018Suche in Google Scholar
22. Davis, M.; Zask, A.; Nakanishi, K.; Lippard, S. J.: Copper(II) tropocoronands: synthesis, structure, and properties of mononuclear complexes. Inorg. Chem. 24 (1985) 3737–3743.10.1021/ic00217a009Suche in Google Scholar
23. Villacorte, M.; Gibson, D.; Williams, I. D.; Lippard, S. J.: Dicopper(I)tropocoronands: synthesis, X-ray crystal structure, and spectral properties of neutral binuclear copper(I) complexes bridged by symmetrically substituted alkynes. J. Am. Chem. Soc. 107 (1985) 6732–6734.10.1021/ja00309a064Suche in Google Scholar
24. Kozhukh, J.; Minier, M. A.; Lippard, S. J.: Synthesis and characterization of mononuclear, pseudotetrahedral cobalt(III) compounds. Inorg. Chem. 54 (2015) 418–424.10.1021/ic5009279Suche in Google Scholar PubMed PubMed Central
25. Hopmann, K. H.; Conradie, J.; Zangen, E.; Tonzetich, Z. J.; Lippard, S. J.: Singlet-triplet gaps of cobalt nitrosyls: insights from tropocoronand complexes. Inorg. Chem. 54 (2015) 7362–7367.10.1021/acs.inorgchem.5b00901Suche in Google Scholar PubMed
26. Davis, M.; Lippard, S. J.: Tropocoronands as binucleating ligands. synthesis, structure, and properties of a (μ-acetato)(μ-methoxo)dicopper(II) derivative. Inorg. Chem. 24 (1985) 3688–3691.10.1021/ic00216a043Suche in Google Scholar
27. Jaynes, S.; Ren, T.; Masschelein, A.; Lippard, S. J.: Stereochemical control of reactivity in Co(III) alkyl complexes of the tropocoronand ligand system. J. Am. Chem. Soc. 115 (1993) 5589–5599.10.1021/ja00066a027Suche in Google Scholar
28. Shindo, K.; Wakabayashi, H.; Kurihara, T.; Zhang, L.-C.; Ebata, K.; Sakurai, H.; Nozoe, T.: Synthesis and crystal structure of tristropocryptands. J. Chin. Chem. Soc. 50 (2003) 47–50.10.1002/jccs.200300006Suche in Google Scholar
29. Yuan, Y.-Q.; Guo, S.-R.; Wang, L.-J.: Crystal structure of 2,2′-diaminodiphenyl sulfide, C12H12N2S. Z. Kristallogr. NCS 223 (2008) 507–508.10.1524/ncrs.2008.0223Suche in Google Scholar
30. Etter, M. C.; MacDonald, J. C.; Bernstein, J.: Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. B46 (1990) 256–262.10.1107/S0108768189012929Suche in Google Scholar PubMed
31. Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C.: The Cambridge Structural Database. Acta Crystallogr. B72 (2016) 171–179.10.1016/B978-0-12-409547-2.02529-4Suche in Google Scholar
32. Roesky, P. W.; Bürgstein, M. R.: Bridged aminotroponiminate complexes of the lanthanides. Inorg. Chem. 38 (1999) 5629–5632. (LIGVOM).10.1021/ic990533cSuche in Google Scholar PubMed
33. Dwivedi, A. D.; Binnani, C.; Tyagi, D.; Rawat, K. S.; Li, P.-Z.; Zhao, Y.; Mobin, S. M.; Pathak, B.; Singh, S. K.: Troponate/aminotroponate ruthenium-arene complexes: synthesis, structure, and ligand-tuned mechanistic pathway for direct C-H bond arylation with aryl chlorides in water. Inorg. Chem. 55 (2016) 6739–6749. (OTIMUB).10.1021/acs.inorgchem.6b01028Suche in Google Scholar PubMed
34. Barret, M. C.; Bhatia, P. H.; Kociok-Köhn, G.; Molloy, K. C.: New copper(II) 2-(alkylamino)troponates. Transition Met. Chem. 39 (2014) 543–551. (NOPRUH).10.1007/s11243-014-9830-0Suche in Google Scholar
35. Nishinaga, T.; Aono, T.; Isomura, E.; Watanabe, S.; Miyake, Y.; Miyazaki, A.; Enoki, T.; Miyasaka, H.; Otani, H.; Iyoda, M.: Structural, electronic and magnetic properties of Cu(II) complexes of 2-substituted tropones bearing a ferrocenyl group at 5-position. Dalton Trans. 39 (2010) 2293–2300. (FULHIE).10.1039/b912255aSuche in Google Scholar
36. Hicks, F. A.; Brookhart, M.: A highly active anilinotropone-based neutral nickel(II) catalyst for ethylene polymerization. Organometallics 20 (2001) 3217–3219. (MIXLOU).10.1021/om010211sSuche in Google Scholar
37. Steyl, G.: 2-(4-Flouroanilino)tropone. Acta Crystallogr. E63 (2007) o4353. (SIMQAH).10.1107/S1600536807050271Suche in Google Scholar
38. Ito, Y.; Amimoto, K.; Kawato, T.: Prototropic tautomerism and solid-state photochromism of N-phenyl-2-aminotropones. Dyes Pigments 89 (2011) 319–323. (SAPLOM).10.1016/j.dyepig.2010.06.001Suche in Google Scholar
39. Kubo, K.; Matsumoto, T.; Ideta, K.; Mori, A.: Crystal structures of 3-methylpyrrolo[2,3-b]tropone and its copper(II) complex. Heterocycles 90 (2015) 104–107. (KUHSIR).10.3987/COM-14-S(K)17Suche in Google Scholar
40. Kubo, K.; Tsujimoto, T.; Mori, A.: 3-Phenylpyrrolo[2,3-b]tropone. Acta Crystallogr. E57 (2001) o225–o227. (HUFTUX).10.1107/S1600536801002021Suche in Google Scholar
41. Kubo, K.; Tsujimoto, T.; Kato, N.; Mori, A.: 2,3-Cyclohexanopyrrolo[2,3-b]tropone. Acta Crystallogr. E57 (2001) o370–o371. (QIBMUJ).10.1107/S1600536801005001Suche in Google Scholar
42. Wahlström, N.; Stensland, B.; Bergman, J.: Synthesis of the marine alkaloid caulersin. Tetrahedron 60 (2004) 2147–2153. (ITIJIE).10.1016/j.tet.2003.12.046Suche in Google Scholar
43. Jansen van Vuuren, L.; Visser, H. G.; Schutte-Smith, M.: Crystal structure of 2-(methylamino)tropone. Acta Crystallogr. E75 (2019) 1128–1132. (WOLQUM).10.1107/S2056989019009502Suche in Google Scholar PubMed PubMed Central
44. Huczyński, A.; Majcher, U.; Maj, E.; Wietrzyk, J.; Janczak, J.; Moshari, M.; Tuszynski, J. A.; Bartl, F.: Synthesis, antiproliferative activity and molecular docking of Colchicine derivatives. Bioorg. Chem. 64 (2016) 103–112. (XAJCOD).10.1016/j.bioorg.2016.01.002Suche in Google Scholar PubMed
45. Siwatch, R. K.; Kundu, S.; Kumar, D.; Nagendran, S.: Bulky aminotroponiminate-stabilized germylene monochloride and its alkyne derivatives. Organometallics 30 (2011) 1998–2005. (OZINUH).10.1021/om200035zSuche in Google Scholar
©2020 Anna Hanft et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4
Artikel in diesem Heft
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4

