Abstract
C14H12N2O2, monoclinic, P21, a = 8.3844(1) Å, b = 20.0465(3) Å, c = 14.0511(2) Å, β = 94.786(1)°, V = 2353.44(6) Å3, Z = 4, Rgt(F) = 0.0356, wRref(F2) = 0.1022, T = 150(2) K.
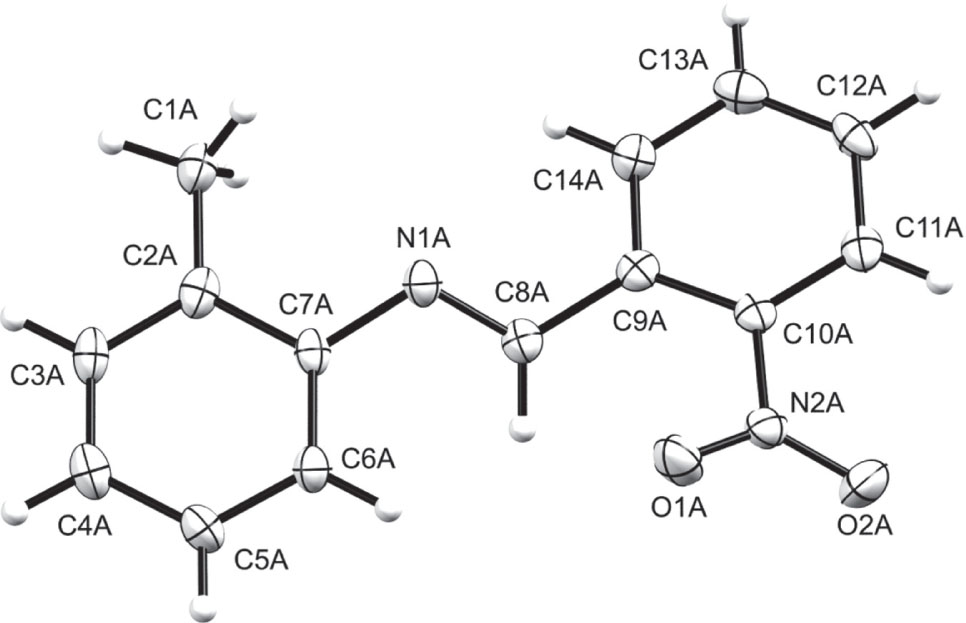
The crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.25 × 0.21 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 27°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 14543, 9464, 0.013 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 8295 |
| N(param)refined: | 653 |
| Programs: | Bruker programs [1], SHELX [2], [3], Mercury [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1A | 0.4015(2) | 0.55500(12) | 0.37669(13) | 0.0412(5) |
| O2A | 0.5065(3) | 0.62258(15) | 0.2793(2) | 0.0463(7) |
| N1A | 0.1639(3) | 0.40462(12) | 0.32621(16) | 0.0268(5) |
| N2A | 0.4102(3) | 0.57940(14) | 0.2971(2) | 0.0295(6) |
| C1A | −0.0121(4) | 0.2835(2) | 0.3225(3) | 0.0354(8) |
| H1AA | 0.035361 | 0.278918 | 0.261464 | 0.053* |
| H1AB | −0.089541 | 0.320229 | 0.318349 | 0.053* |
| H1AC | −0.066653 | 0.241933 | 0.337057 | 0.053* |
| C2A | 0.1163(4) | 0.29782(18) | 0.3997(3) | 0.0284(7) |
| C3A | 0.1524(5) | 0.25363(18) | 0.4753(2) | 0.0319(8) |
| H3A | 0.090519 | 0.214086 | 0.478350 | 0.038* |
| C4A | 0.2731(5) | 0.26488(18) | 0.5454(3) | 0.0334(8) |
| H4A | 0.294676 | 0.233351 | 0.595261 | 0.040* |
| C5A | 0.3639(5) | 0.32295(18) | 0.5428(3) | 0.0326(8) |
| H5A | 0.448144 | 0.331089 | 0.590860 | 0.039* |
| C6A | 0.3313(4) | 0.36882(16) | 0.4700(2) | 0.0274(7) |
| H6A | 0.392737 | 0.408581 | 0.468839 | 0.033* |
| C7A | 0.2092(4) | 0.35717(15) | 0.3985(2) | 0.0236(7) |
| C8A | 0.2696(4) | 0.44361(15) | 0.29730(18) | 0.0257(6) |
| H8A | 0.377908 | 0.439977 | 0.322627 | 0.031* |
| C10A | 0.2942(4) | 0.55774(17) | 0.2196(2) | 0.0243(7) |
| C11A | 0.2541(4) | 0.60282(17) | 0.1475(3) | 0.0291(7) |
| H11A | 0.305097 | 0.645116 | 0.147011 | 0.035* |
| C12A | 0.1381(4) | 0.5855(2) | 0.0758(2) | 0.0318(7) |
| H12A | 0.108913 | 0.615832 | 0.025413 | 0.038* |
| C13A | 0.0652(5) | 0.5239(2) | 0.0778(3) | 0.0347(8) |
| H13A | −0.014547 | 0.512061 | 0.028691 | 0.042* |
| C14A | 0.1070(4) | 0.47888(19) | 0.1511(2) | 0.0293(7) |
| H14A | 0.055429 | 0.436694 | 0.151183 | 0.035* |
| O1B | 0.3488(2) | 0.44289(13) | −0.12691(13) | 0.0408(5) |
| O2B | 0.2402(3) | 0.37674(14) | −0.0293(2) | 0.0439(7) |
| N1B | 0.5896(3) | 0.59253(12) | −0.07785(16) | 0.0266(5) |
| N2B | 0.3388(3) | 0.41924(15) | −0.0475(2) | 0.0307(6) |
| C1B | 0.7740(5) | 0.7122(2) | −0.0734(3) | 0.0412(9) |
| H1BA | 0.737180 | 0.745292 | −0.028744 | 0.062* |
| H1BB | 0.868060 | 0.729522 | −0.102284 | 0.062* |
| H1BC | 0.802447 | 0.670832 | −0.038929 | 0.062* |
| C2B | 0.6411(4) | 0.69831(18) | −0.1512(3) | 0.0281(7) |
| C3B | 0.6077(5) | 0.74462(19) | −0.2248(3) | 0.0358(9) |
| H3B | 0.669126 | 0.784399 | −0.225711 | 0.043* |
| C4B | 0.4865(5) | 0.7335(2) | −0.2964(3) | 0.0404(10) |
| H4B | 0.465900 | 0.765203 | −0.346112 | 0.048* |
| C5B | 0.3961(5) | 0.67606(19) | −0.2951(3) | 0.0362(8) |
| H5B | 0.312687 | 0.668318 | −0.343823 | 0.043* |
| C6B | 0.4266(4) | 0.62982(18) | −0.2230(2) | 0.0299(7) |
| H6B | 0.364533 | 0.590206 | −0.222557 | 0.036* |
| C7B | 0.5493(4) | 0.64117(17) | −0.1504(2) | 0.0261(7) |
| C8B | 0.4833(3) | 0.55495(15) | −0.04935(19) | 0.0253(6) |
| H8B | 0.375409 | 0.559104 | −0.075268 | 0.030* |
| C9B | 0.5273(4) | 0.50392(16) | 0.0252(2) | 0.0227(7) |
| C10B | 0.4553(4) | 0.44168(17) | 0.0298(2) | 0.0239(7) |
| C11B | 0.4945(4) | 0.39617(16) | 0.1030(2) | 0.0277(7) |
| H11B | 0.442460 | 0.354114 | 0.103916 | 0.033* |
| C12B | 0.6108(4) | 0.4135(2) | 0.1743(2) | 0.0317(7) |
| H12B | 0.639189 | 0.383319 | 0.225068 | 0.038* |
| C13B | 0.6859(4) | 0.4750(2) | 0.1716(2) | 0.0313(8) |
| H13B | 0.766594 | 0.486544 | 0.220334 | 0.038* |
| C14B | 0.6446(4) | 0.51962(18) | 0.0987(2) | 0.0294(7) |
| H14B | 0.696743 | 0.561692 | 0.098405 | 0.035* |
| O1C | 0.0060(2) | 0.52358(13) | −0.14939(13) | 0.0410(5) |
| O2C | 0.0113(4) | 0.62417(15) | −0.2026(3) | 0.0552(8) |
| N1C | −0.2910(3) | 0.38935(12) | −0.18932(16) | 0.0254(5) |
| N2C | −0.0470(3) | 0.56852(14) | −0.20288(19) | 0.0308(6) |
| C1C | −0.5187(4) | 0.2845(2) | −0.1814(3) | 0.0369(8) |
| H1CA | −0.561627 | 0.239262 | −0.177192 | 0.055* |
| H1CB | −0.474432 | 0.290408 | −0.243272 | 0.055* |
| H1CC | −0.604727 | 0.316997 | −0.175393 | 0.055* |
| C2C | −0.3904(4) | 0.29512(17) | −0.1032(3) | 0.0283(7) |
| C3C | −0.3739(5) | 0.25360(18) | −0.0231(3) | 0.0328(8) |
| H3C | −0.448291 | 0.218263 | −0.018322 | 0.039* |
| C4C | −0.2548(5) | 0.26174(18) | 0.0490(3) | 0.0348(8) |
| H4C | −0.247465 | 0.232460 | 0.102397 | 0.042* |
| C5C | −0.1451(5) | 0.31315(19) | 0.0433(3) | 0.0341(8) |
| H5C | −0.061836 | 0.318885 | 0.092781 | 0.041* |
| C6C | −0.1566(4) | 0.35630(17) | −0.0344(2) | 0.0272(7) |
| H6C | −0.080465 | 0.391092 | −0.038181 | 0.033* |
| C7C | −0.2790(4) | 0.34877(15) | −0.1067(2) | 0.0225(6) |
| C8C | −0.2624(3) | 0.45148(15) | −0.1785(2) | 0.0240(6) |
| H8C | −0.232616 | 0.468866 | −0.116658 | 0.029* |
| C9C | −0.2756(4) | 0.49630(16) | −0.2618(2) | 0.0238(7) |
| C10C | −0.1826(4) | 0.55382(17) | −0.2721(2) | 0.0251(7) |
| C11C | - 0.2082(4) | 0.59679(18) | −0.3480(3) | 0.0323(8) |
| H11C | −0.144153 | 0.635648 | −0.351797 | 0.039* |
| C12C | −0.3283(4) | 0.5829(2) | −0.4190(2) | 0.0327(8) |
| H12C | −0.347264 | 0.612118 | −0.471925 | 0.039* |
| C13C | −0.4200(4) | 0.5265(2) | −0.4123(2) | 0.0327(8) |
| H13C | −0.501370 | 0.516528 | −0.461431 | 0.039* |
| C14C | −0.3950(4) | 0.48389(17) | −0.3348(2) | 0.0266(7) |
| H14C | −0.460608 | 0.445476 | −0.331263 | 0.032* |
| O1D | −0.2547(2) | 0.46948(12) | −0.59930(13) | 0.0396(5) |
| O2D | −0.2554(4) | 0.36818(14) | −0.5488(2) | 0.0505(7) |
| N1D | 0.0371(3) | 0.60515(12) | −0.55713(16) | 0.0248(5) |
| N2D | −0.1996(3) | 0.42452(15) | −0.5473(2) | 0.0304(6) |
| C1D | 0.2644(4) | 0.7109(2) | −0.5656(3) | 0.0354(8) |
| H1DA | 0.223232 | 0.738723 | −0.515688 | 0.053* |
| H1DB | 0.354379 | 0.733681 | −0.591855 | 0.053* |
| H1DC | 0.300545 | 0.668050 | −0.538146 | 0.053* |
| C2D | 0.1324(4) | 0.69913(17) | −0.6447(2) | 0.0252(7) |
| C3D | 0.1152(5) | 0.74168(18) | −0.7234(3) | 0.0317(8) |
| H3D | 0.187856 | 0.777729 | −0.727599 | 0.038* |
| C4D | −0.0060(5) | 0.73235(19) | −0.7958(3) | 0.0352(9) |
| H4D | −0.014867 | 0.761420 | - 0.849382 | 0.042* |
| C5D | −0.1135(5) | 0.68070(18) | −0.7896(3) | 0.0321(8) |
| H5D | −0.197030 | 0.674270 | −0.838694 | 0.039* |
| C6D | −0.0993(4) | 0.63846(17) | −0.7119(2) | 0.0281(7) |
| H6D | −0.173984 | 0.603204 | −0.707312 | 0.034* |
| C7D | 0.0247(4) | 0.64717(16) | −0.6395(2) | 0.0243(6) |
| C8D | 0.0110(3) | 0.54362(14) | −0.56873(19) | 0.0226(6) |
| H8D | −0.017620 | 0.526500 | −0.630852 | 0.027* |
| C9D | 0.0252(4) | 0.49789(16) | −0.4853(2) | 0.0217(6) |
| C10D | −0.0647(4) | 0.44009(17) | −0.4770(2) | 0.0238(7) |
| C11D | −0.0384(4) | 0.39611(17) | −0.4002(3) | 0.0299(7) |
| H11D | −0.101405 | 0.356934 | −0.396863 | 0.036* |
| C12D | 0.0807(4) | 0.4105(2) | −0.3292(3) | 0.0338(8) |
| H12D | 0.100686 | 0.380960 | −0.276681 | 0.041* |
| C13D | 0.1713(4) | 0.4682(2) | −0.3349(2) | 0.0316(8) |
| H13D | 0.252138 | 0.478449 | −0.285662 | 0.038* |
| C14D | 0.1445(4) | 0.51060(18) | −0.4116(2) | 0.0291(7) |
| H14D | 0.208492 | 0.549517 | −0.414633 | 0.035* |
Source of material
The title compound was synthesized via a solvent free approach by stirring equimolar o-toluidine (0.11 g, 1.0 mmol) and 2-nitrobenzaldehyde (0.1 g, 1.0 mmol) in a poly top vial for 5 minutes using a glass rod to yield a yellow compound. Rectangular crystals suitable for X-ray diffraction were obtained by slow evaporation from diethylether within 48 h. Yield = 0.23 g (96%), 1H NMR (400 MHz, Chloroform-d) δ 8.83 (s, 1H), 8.32 (dd, J = 7.8 Hz, 1H), 8.06 (dd, J = 8.2 Hz, 1H), 7.74 (t, J = 7.6 Hz, 1H), 7.62 (td, J = 7.8 Hz, 1H), 7.23 (d, J = 6.6 Hz, 3H), 7.21–7.13 (m, 1H), 7.05–6.99 (m, 1H), 2.40 (s, 3H). 13C NMR (400 MHz, Chloroform-d) δ = 155.04, 150.18, 149.35, 133.51, 132.27, 131.29, 131.08, 130.44, 129.89, 126.90, 126.62, 124.50, 117.84, 17.87. C14H12N2O2 Calcd: C = 69.99%, H = 5.03%, N = 11.66%; Found: C = 70.01%, H = 5.10%, N = 11.30%.
Experimental details
The structure was solved by the intrinsic phasing using the SHELXT [2] program and refined using SHELXL [3]. The visual crystal structure information was performed using Mercury [4]. All C—Haromatic and O—H bond distances were restrained to 0.95 Å, 0.98 Å and 0.99 Å with Uiso(Haromatic) = 1.2Ueq and Uiso(Hhydroxyl) = 1.5Ueq of parent atom, respectively.
Discussion
Schiff bases are organic compounds with an azomethine or imine (—C=N—) functional group, where the imine nitrogen atom can be linked to different groups such as alkyl, cycloalkyl, aryl or heterocyclic groups, but not hydrogen atom [5]. They are well known for their significant biological activities such as antioxidant [6], [7], antibacterial [5], [8], antifungal [9], [10], and anticancer agents [11], [12]. Other applications also include, as sensors [13], [14], corrosion inhibitors [15], [16] and chelating ligands in coordination chemistry [17], [18]. The condensation reaction between primary amines (R-NH2), with active carbonyl compounds (aldehyde or ketone), affords Schiff bases [19], [20], [21]. Schiff bases with aryl substituents are relatively stable and easy to prepare when compared with those that have alkyl substituents and during condensation reactions, aldehydes react faster than ketones [22]. The title compound has been synthesized by Zhou et al. [23] using the conventional method. We now report a solvent-free approach via mechanochemistry techniques. Aside from being a greener approach, the mechanochemistry method (grinding) also afforded the product within 5 minutes and even higher yield when compared to the one reported in the literature [23].
The crystal structure of the title compound has four symmetrically non-equivalent molecules in the asymmetric unit. The molecular units have similar conformation with dihedral angle between the anilyl and phenyl rings ranging from 5.2(1)° to 6.4(1)°, which is slightly wider than that observed in (E)-1-(2-nitrophenyl)-N-(p-tolyl)methanimine (3.09(8)°) [24]. All intramolecular bond parameters are comparable with closely related compounds in literature [25], [26], [27], [28], [29], [30], [31]. Non-classical intermolecular C—H⋯O hydrogen bonding exist between the aromatic hydrogens (H13A-D) and the nitro group’s oxygen atom (O1A-D). This forms chains which extends diagonally with respect to the crystallographic a and c axes. Intermolecular π⋯π interactions were also observed in the crystal packing of the title compound.
Acknowledgements
We thank Ekiti state university and TETFUND for research grant and University of KwaZulu-Natal for the providing the research facilities.
References
1. Bruker, APEXII, Bruker AXS Inc, Madison, WI, USA (2009).Search in Google Scholar
2. Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A64 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A.: Mercury CSD 2.0 − new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 41 (2008) 466–470.10.1107/S0021889807067908Search in Google Scholar
5. da Silva, C. M.; da Silva, D. L.; Modolo, L. V.; Alves, R. B.; de Resende, M. A.; Martins, C. V. B.; de Fátima, A.: Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2 (2011) 1–8.10.1016/j.jare.2010.05.004Search in Google Scholar
6. Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P.: The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg. Med. Chem. Lett. 15 (2005) 4600–4603.10.1016/j.bmcl.2005.06.095Search in Google Scholar PubMed
7. Cheng, L.-X.; Tang, J.-J.; Luo, H.; Jin, X.-L.; Dai, F.; Yang, J.; Qian, Y.-P.; Li, X.-Z.; Zhou, B.: Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett. 20 (2010) 2417–2420.10.1016/j.bmcl.2010.03.039Search in Google Scholar PubMed
8. Jarrahpour, A.; Motamedifar, M.; Pakshir, K.; Hadi, N.; Zarei, M.: Synthesis of novel azo Schiff bases and their antibacterial and antifungal activities. Molecules. 9 (2004) 815–824.10.3390/91000815Search in Google Scholar PubMed PubMed Central
9. Chen, Y.; Mi, Y.; Sun, X.; Zhang, J.; Li, Q.; Ji, N.; Guo, Z.: Novel Inulin Derivatives Modified with Schiff Bases: Synthesis, Characterization, and Antifungal Activity. Polymers. 11 (2019) 998.10.3390/polym11060998Search in Google Scholar PubMed PubMed Central
10. Jarrahpour, A.; Khalili, D.; De Clercq, E.; Salmi, C.; Brunel, J.: Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules. 12 (2007) 1720–1730.10.3390/12081720Search in Google Scholar PubMed PubMed Central
11. Mahal, A.; Wu, P.; Jiang, Z. H.; Wei, X.: Schiff Bases of Tetrahydrocurcumin as Potential Anticancer Agents. ChemistrySelect. 4 (2019) 366–369.10.1002/slct.201803159Search in Google Scholar
12. Desai, S. B.; Desai, P.; Desai, K.: Synthesis of some Schiff bases, thiazolidinones and azetidinones derived from 2, 6-diaminobenzo [1, 2-d: 4, 5-d′] bisthiazole and their anticancer activities. Heterocycl. Commun. 7 (2001) 83–90.10.1515/HC.2001.7.1.83Search in Google Scholar
13. Berhanu, A. L.; Gaurav;Mohiuddin, I.; Malik, A. K.; Aulakh, J. S.; Kumar, V.; Kim, K.-H.: A review of the applications of Schiff bases as optical chemical sensors. Trends Anal. Chem. 116 (2019) 74–91.10.1016/j.trac.2019.04.025Search in Google Scholar
14. Aksuner, N.; Henden, E.; Yilmaz, I.; Cukurovali, A.: Selective optical sensing of copper(II) ions based on a novel cyclobutane-substituted Schiff base ligand embedded in polymer films. Sensor. Actuat. B-Chem. 134 (2008) 510–515.10.1016/j.snb.2008.05.041Search in Google Scholar
15. Shetty, P.: Schiff bases: An overview of their corrosion inhibition activity in acid media against mild steel. Chem. Eng. Comm. 206 (2019) 1–45.10.1080/00986445.2019.1630387Search in Google Scholar
16. Talebian, M.; Raeissi, K.; Atapour, M.; Fernández-Pérez, B.; Betancor-Abreu, A.; Llorente, I.; Fajardo, S.; Salarvand, Z.; Meghdadi, S.; Amirnasr, M.: Pitting corrosion inhibition of 304 stainless steel in NaCl solution by three newly synthesized carboxylic Schiff bases. Corros. Sci. 160 (2019) 108130.10.1016/j.corsci.2019.108130Search in Google Scholar
17. Pervaiz, M.; Ahmad, I.; Yousaf, M.; Kirn, S.; Munawar, A.; Saeed, Z.; Adnan, A.; Gulzar, T.; Kamal, T.; Ahmad, A.; Rashid, A.: Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochim. Acta A. 206 (2019) 642–649.10.1016/j.saa.2018.05.057Search in Google Scholar PubMed
18. Bernadette Amali, I.; Kesavan, M. P.; Vijayakumar, V.; Indra Gandhi, N.; Rajesh, J.; Rajagopal, G.: Structural analysis, antimicrobial and cytotoxic studies on new metal(II) complexes containing N2O2 donor Schiff base ligand. J. Mol. Struct. 1183 (2019) 342–350.10.1016/j.molstruc.2019.02.005Search in Google Scholar
19. Tai, X.; Yin, X.; Chen, Q.; Tan, M.: Synthesis of some transition metal complexes of a novel Schiff base ligand derived from 2, 2′-bis (p-methoxyphenylamine) and salicylicaldehyde. Molecules. 8 (2003) 439–443.10.3390/80500439Search in Google Scholar
20. Naeimi, H.; Safari, J.; Heidarnezhad, A.: Synthesis of Schiff base ligands derived from condensation of salicylaldehyde derivatives and synthetic diamine. Dyes Pigm. 73 (2007) 251–253.10.1016/j.dyepig.2005.12.009Search in Google Scholar
21. Pillai, R. R.; Karrouchi, K.; Fettach, S.; Armaković, S.; Armaković, S. J.; Brik, Y.; Taoufik, J.; Radi, S.; El Abbes Faouzi, M.; Ansar, M. H.: Synthesis, spectroscopic characterization, reactive properties by DFT calculations, molecular dynamics simulations and biological evaluation of Schiff bases tethered 1,2,4-triazole and pyrazole rings. J. Mol. Struct. 1177 (2019) 47–54.10.1016/j.molstruc.2018.09.037Search in Google Scholar
22. Hine, J.; Yeh, C. Y.: Equilibrium in formation and conformational isomerization of imines derived from isobutyraldehyde and saturated aliphatic primary amines. J. Am. Chem. Soc. 89 (1967) 2669–2676.10.1021/ja00987a030Search in Google Scholar
23. Zhou, Y.; Liu, Q.; Lv, W.; Pang, Q.; Ben, R.; Qian, Y.; Zhao, J.: Indazolin-s-ylidene–N-Heterocyclic Carbene complexes of Rhodium, Palladium, and Gold: Synthesis, characterization, and catalytic hydration of alkynes. Organometallics. 32 (2013) 3753–3759.10.1021/om4002928Search in Google Scholar
24. Yeap, G.-Y.; Fun, H.-K.; Teo, S.-B.; Teoh, S.-G.: Structure of 2-1(4-Methylphenylimino)-methyl]-1-nitrobenzene. Acta Crystallogr. C48 (1992) 1898–1900.10.1107/S0108270192005997Search in Google Scholar
25. Umadevi, M.; Devaraj, S.; Kandaswamy, M.; Chakkaravarthi, G.; Manivannan, V.: 1-[2-(2,4-Dinitrobenzylideneamino)phenyl]-3-phenylthiourea. Acta Crystallogr. E65 (2009) o2447.10.1107/S1600536809035880Search in Google Scholar
26. Dias, L. C.; de Lima, G. M.; Pinheiro, C. B.; Rodrigues, B. L.; Donnici, C. L.; Fujiwara, R. T.; Bartholomeu, D. C.; Ferreira, R. A.; Ferreira, S. R.; Mendes, T. A. O.; da Silva, J. G.; Alves, M. R. A.: Design, structural and spectroscopic elucidation of new nitroaromatic carboxylic acids and semicarbazones for the in vitro screening of anti-leishmanial activity. J. Mol. Struct. 1079 (2015) 298–306.10.1016/j.molstruc.2014.08.047Search in Google Scholar
27. Kawamoto, T.; Kushi, Y.: The effect of aromatic-aromatic interactions on the crystallization of helical nickel(II) complexes. Inorganica Chim. Acta. 282 (1998) 71–75.10.1016/S0020-1693(98)00195-9Search in Google Scholar
28. Aldoshin, S. M.; Chuev, I. I.; Kozina, O. A.: A study of the possibility of constructing a required crystal structure of nitrobenzilidene derivatives of o-amidoanilines by modifying their molecular structure. Mol. Cryst. Liq. Crys. 264 (1995) 215–226.10.1080/10587259508037315Search in Google Scholar
29. Glidewell, C.; Howie, R. A.; Low, J. N.; Skakle, J. M. S.; Wardell, S. M. S. V.; Wardell, J. L.: Nine isomeric nitrobenzylidene-iodoanilines: interplay of C-H⋯O hydrogen bonds, iodo⋯nitro interactions and aromatic [pi]⋯[pi] stacking interactions. Acta Crystallogr. B58 (2002) 864–876.10.1107/S0108768102009941Search in Google Scholar
30. Ferguson, G.; Glidewell, C.; Low, J. N.; Skakle, J. M. S.; Wardell, J. L.: Solvent-dependent polymorphism in isomeric N-(nitrobenzylidene)iodoanilines. Acta Crystallogr. C61 (2005) o445–o449.10.1107/S0108270105016239Search in Google Scholar PubMed
31. Diko, N.; Zamisa, S. J.; Friedrich, H. B.; Shozi, M. L.: Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2. Z. Kristallogr. NCS 234 (2019) 1059–1061.10.1515/ncrs-2019-0260Search in Google Scholar
©2020 Sulaiman A. Olagboye et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4
Articles in the same Issue
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4

