Abstract
C28H26Cl6N4NiO8, monoclinic, I2/a (no. 15), a = 15.7577(10) Å, b = 12.6174(7) Å, c = 16.7333(10) Å, β = 99.609(6)Å, V = 3280.3(3) Å3, Z = 4, Rgt(F) = 0.0674, wRref(F2) = 0.1665, T = 291.2(3) K.
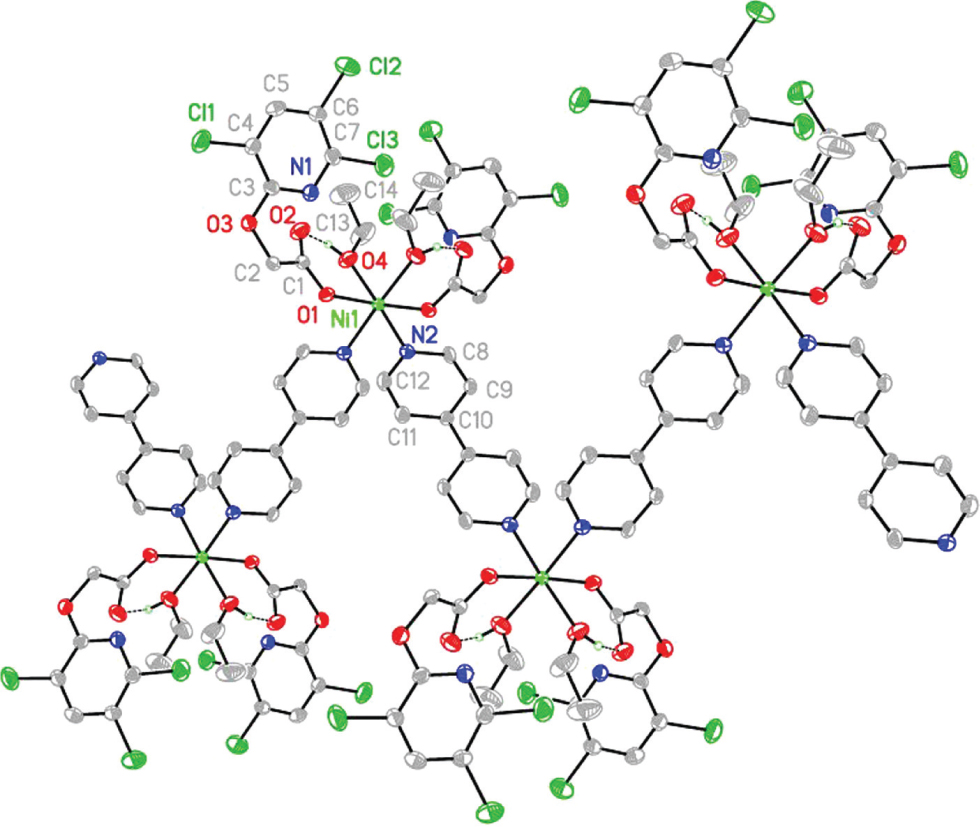
A part of the molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green block |
| Size: | 0.29 × 0.25 × 0.22 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.14 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 28.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 7139, 3421, 0.051 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2154 |
| N(param)refined: | 217 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.7488(3) | 0.4078(4) | 0.3258(3) | 0.0379(12) |
| C2 | 0.7127(4) | 0.3887(4) | 0.2373(3) | 0.0414(13) |
| H2A | 0.7416 | 0.3282 | 0.2182 | 0.050* |
| H2B | 0.6520 | 0.3717 | 0.2321 | 0.050* |
| C3 | 0.6715(3) | 0.5633(4) | 0.1934(3) | 0.0408(13) |
| C4 | 0.6951(4) | 0.6599(4) | 0.1645(4) | 0.0517(15) |
| C5 | 0.6454(4) | 0.7480(5) | 0.1724(4) | 0.0599(17) |
| H5 | 0.6608 | 0.8144 | 0.1554 | 0.072* |
| C6 | 0.5715(4) | 0.7347(4) | 0.2068(4) | 0.0543(16) |
| C7 | 0.5525(3) | 0.6362(5) | 0.2317(3) | 0.0466(14) |
| C8 | 0.5976(3) | 0.2057(4) | 0.5490(3) | 0.0442(13) |
| H8 | 0.5999 | 0.2613 | 0.5858 | 0.053* |
| C9 | 0.5386(3) | 0.1276(4) | 0.5532(3) | 0.0435(13) |
| H9 | 0.5032 | 0.1307 | 0.5924 | 0.052* |
| C10 | 0.5312(3) | 0.0435(4) | 0.4989(3) | 0.0325(11) |
| C11 | 0.5867(4) | 0.0459(4) | 0.4435(3) | 0.0547(16) |
| H11 | 0.5848 | −0.0081 | 0.4055 | 0.066* |
| C12 | 0.6452(4) | 0.1277(5) | 0.4439(4) | 0.0557(16) |
| H12 | 0.6816 | 0.1266 | 0.4056 | 0.067* |
| C13 | 0.8826(5) | 0.5237(6) | 0.5548(4) | 0.097(3) |
| H13A | 0.9334 | 0.4930 | 0.5872 | 0.117* |
| H13B | 0.8415 | 0.5370 | 0.5908 | 0.117* |
| C14 | 0.9061(6) | 0.6224(6) | 0.5252(5) | 0.120(3) |
| H14A | 0.8573 | 0.6531 | 0.4910 | 0.181* |
| H14B | 0.9518 | 0.6121 | 0.4945 | 0.181* |
| H14C | 0.9253 | 0.6690 | 0.5699 | 0.181* |
| Cl1 | 0.78527(12) | 0.67082(15) | 0.11876(13) | 0.0864(7) |
| Cl2 | 0.50957(14) | 0.84462(14) | 0.21820(14) | 0.0975(7) |
| Cl3 | 0.46002(10) | 0.61493(14) | 0.27188(10) | 0.0668(5) |
| N1 | 0.6018(3) | 0.5516(3) | 0.2259(2) | 0.0412(11) |
| N2 | 0.6521(2) | 0.2079(3) | 0.4962(2) | 0.0343(9) |
| Ni1 | 0.7500 | 0.32936(6) | 0.5000 | 0.0262(2) |
| O1 | 0.7342(2) | 0.3337(2) | 0.3724(2) | 0.0397(8) |
| O2 | 0.7901(3) | 0.4905(3) | 0.3453(2) | 0.0568(11) |
| O3 | 0.7229(2) | 0.4792(3) | 0.1871(2) | 0.0499(10) |
| O4 | 0.8467(3) | 0.4474(3) | 0.4975(2) | 0.0553(11) |
| H4 | 0.846(3) | 0.477(3) | 0.4507(8) | 0.083* |
Source of material
NiCl2 ⋅ 6H2O (0.3 mmol, 0.074 g). 4,4′-dipyridine (4,4′-dipy, 0.3 mmol, 0.047 g) and ligand 3,5,6-trichloropyridine-2-oxyacetic acid (3,5,6-Htcpa, 0.3 mmol, 0.078 g) were dissolved in a mixture of 20 mL of water/ ethanol (V:V = 1:1). Then the pH of the mixture was neutralized with KOH (0.1 mol⋅L−1) to pH = 6.5. After that, the mixed solution was sealed in a 25 mL Teflon reactor and kept under autogeneous pressure at 393 K for 3 days. After cooling to room temperature at a rate of 279 K⋅h−1, green block crystals of the title compound were obtained and collected. Yield: 20 mg (16%, based on ligand 3,5,6-Htcpa). Anal. Calcd for C28H26Cl6N4NiO8(%): C, 41.08; H, 3.20; N, 6.85. Found: C, 41.01; H, 3.29; N, 6.92.
Experimental details
CrysAlisPro 1.171.39.46 (Rigaku Oxford Diffraction, 2018) [1] was used for empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. Using Olex2 [2], the structure was solved with the ShelXT [3] structure solution program using Intrinsic Phasing and refined with the ShelXL [4] refinement package. The H atoms bonded to C atoms were fixed, with C—H distance of 0.93 Å; and/or positioned geometrically in the riding-model approximation, with C—H distance of 0.97 Å; Uiso(H) = 1.2Ueq(C).
Comment
At the beginning stage of the coordination chemistry, aromatic carboxylate ligands have been frequently selected to construct metal-organic frameworks due to their rich structural features and valuable potential applications [5], [6], [7], [8], [9]. Subsequently, modification of these carboxylate ligands by various electron donating and withdrawing substituents groups such as Me- [10], [11], tBu- [12], [13], MeO- [10], [14], [15], —OH and —SO3H [16], [17], [18], —SH [19], [20], —NH2 [21], [22], —NO2 [23], [24], —CF3 [25], —CN [26], —F [27], [28], [29], [30], —Cl [31], [32], [33], —Br [34], [35], [36], [37], —I [38], [39], [40], has been employed. Because of the steric and/or electronic effects of the substituent groups, these decorated ligands may afford new supramolecular assemblies owing to their special linking types and ligand-metal interactions. Among them, the halogen containing carboxylate ligands are attractive as they may form halogen bonds, which played an important role in the fields of molecular recognition and supramolecular assemblies.
Lately, a trichloro substituent aromatic carboxylate ligand, namely, 3,5,6-trichloropyridine-2-oxyacetic acid (3,5,6-Htcpa) has aroused our interests. A series of compounds, including co-crystal ZnII [41] and NiII complexes [42], binuclear CdII cluster [43], 1D NiII [44] , CoII [45] and MnII [46] polymers containing this ligand, have been reported by us recently.
Single-crystal X-ray diffraction analysis shows that the asymmetric unit of I includes one half of a NiII ion, one 3,5,6-tcpa ligands, one half of a 4,4′-dipy together with one ethanol molecule.
The six-coordinated NiII ion is bonded to two carboxy oxygen of two unidentate 3,5,6-tcpa anions [Ni1-O1 = 2.109(3) Å], two pyridyl nitrogen atoms of two 4,4′-dipy [Ni1-N2 = 2.168(4) Å] as well as two hydroxy oxygen atoms of two ethanol molecules [Ni1-O4 = 2.136(4) Å] (see the figure).
The trans O1-Ni1-O1A and O4-Ni1-N2 bond angles are 177.05(17)° and 177.13(14)° respectively, and the cis ones lie in the range of 85.75(13) to 92.19(13)°.
The μ2-4,4′-dipy molecules bridge the neighbouring NiII ions with their nitrogen atoms to form zigzag chain structure along the a axis. The Ni⋯Ni separation across 4,4′-dipy is 11.452 Å. The adjacent Ni⋯Ni distance parallel to the a axis is 15.758 Å. The 3D crystal packing is constructed by O—H⋯O hydrogen bonding and weak Cl⋯Cl halogen bonding.
The hydroxy atom (O4) of ethanol as donor involves in intra-molecular hydrogen bond with uncomplexed carboxy oxygen (O2) of 3,5,6-tcpa as acceptor.
The halogen⋯halogen interactions are seen between C6-Cl2 and C7G-Cl3G (symmetry code for G: x, 3/2 − y, 1/2 + z) of adjacent 1D chain with a Cl2⋯Cl3G separation of 3.444 Å. In I, the geometries of these halogen bonds is characterized by angles of C6-C12⋯Cl3G (119.20°) and Cl2⋯Cl3G-C7G (93.02°), nearly corresponding to halogen bond of Type II [47], [48].
Comparing I with [Ni(3,5,6-tcpa)2(4,4′-dipy) 1/2]n (II) [44], both of which prepared from the same starting reactants, some important similarities and differences can be found as follows: (i) The structure. They are both 1D polymer. The title structure is a zigzag chain, while II is a linear structure. (ii) The μ2-function of 4,4′-dipy. Both 4,4′-dipy join NiII centers to form the corresponding polymer. (iii) The composition. There are complexed ethonal molecules in I, but absence in II. This is because of the use of the mixed H2O-ethanol solvent for synthesis of I, while pure H2O solvent for that of II. (iv) The coordination mode of 3,5,6-tcpa. It is monodentate in I, but bidentate in II. (v) The coordination geometry of NiII ion. It is in octahedron for I, while in tetragonal pyramid for II.
From the above careful disscussion, it was confirmed again that the different synthesis conditions, such as by changing the solvent, greatly affected the composition and structure of complexes.
Now, many further attempts on developing new complexes with 3,5,6-Htcpa as the first ligand, for instance by the use of other solvents, introducing other N/O-donor bridging or chelate auxiliary ligands will be gradually implemented.
Acknowledgements
This work was supported by the Key scientific research projects in Colleges and Universities of Henan province (No. 17A150040).
References
1. Oxford Diffraction Ltd: CrysAlisPRO. Rigaku Oxford Diffraction, Version 1.171.39.6a, England (2018).Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2 : a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
3. Sheldrick, G. M.: SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
5. Mehrotra, R. C.; Bohra, R.: Metal carboxylates. Academic Press, London (1983).Suche in Google Scholar
6. Li, J.-X.; Du, Z.-X.: Crystal structure of catena-poly[(μ2-4,4′-bipyridine-κ2N:N′)-tetraquamanganese(II)]-(3-(carboxylatomethyl) benzoic acid)-water (1/2/2), C28H34MnN2O14. Z. Kristallogr. NCS 230 (2015) 339–340.10.1515/ncrs-2014-9130Suche in Google Scholar
7. Du, Z.-X.; Li, J.-X.: Crystal structure of catena-poly[(μ2-4,4′-bipyridine-κ2N:N′)-tetraqua-cobalt(II)]-(3-(carboxylatomethyl) benzoic acid)-water (1/2/2), C28H34CoN2O14. Z. Kristallogr. NCS 230 (2015) 321–322.10.1515/ncrs-2014-9082Suche in Google Scholar
8. Li, J.-X.; Du, Z.-X.: Syntheses, structures and magnetic properties of two mononuclear nickel(II) complexes based on bicarboxylate ligands. Z. Naturforsch. 70b (2015) 505–511.10.1515/znb-2015-0010Suche in Google Scholar
9. Li, J.-X.; Du, Z.-X.: Zinc and cobalt complexes with (2-carboxyphenoxy) acetic acid ligand: syntheses, structures, fluorescent and magnetic properties. J. Coord. Chem. 69 (2016) 2563–2572.10.1080/00958972.2016.1216106Suche in Google Scholar
10. Han, M.-L.; Wang, J.-G.; Ma, L.-F.; Guo, H.; Wang, L.-Y.: Construction of Cd(II) coordination polymers based on R-isophthalate (R = -CH3 or -OCH3) and flexible N-donor co-ligands: syntheses, structures and photoluminescence. CrystEngComm 14 (2012) 2691–2701.10.1039/c2ce06511hSuche in Google Scholar
11. Alturk, S.; Avci, D.; Basoglu, A.; Tamer, O.; Atalay, Y.; Dege, N.: Copper(II) complex with 6-methylpyridine-2-carboxyclic acid: experimental and computational study on the XRD, FT-IR and UV-Vis spectra, refractive index, band gap and NLO parameters. Spectrochim. Acta, Part A 190 (2018) 220–230.10.1016/j.saa.2017.09.041Suche in Google Scholar PubMed
12. Wang, J.-G.; Chai, N.; Wang, S.-C.; Ma, L.-F.; Wang, L.-Y.: Two new 3-D coordination polymers with 5-tert-butyl isophthalic acid and flexible N-donor co-ligands bearing linear trinuclear secondary building blocks. Inorg. Chem. Commun. 30 (2013) 143–146.10.1016/j.inoche.2013.02.005Suche in Google Scholar
13. Chang, X.-H.: Crystal structure of poly[(μ4-5-tertbutylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2- imidazolidinone-κO)zinc(II)] C17H22N2O5Zn. Z. Kristallogr. NCS 233 (2018) 1043–1045.10.1515/ncrs-2018-0161Suche in Google Scholar
14. Qin, J.-H.; Ma, L.-F.; Hu, Y.; Wang, L.-Y.: Syntheses, structures and photoluminescence of five zinc(ii) coordination polymers based on 5-methoxyisophthalate and flexible N-donor ancillary ligands. CrystEngComm 14 (2012) 2891–2898.10.1039/c2ce06581aSuche in Google Scholar
15. Ma, L.-F.; Zhao, J.-W.; Han, M.-L.; Wang, L.-Y.; Du, M.: Two novel 3-D coordination polymers with 5-methoxyisophthalate and flexible N-donor co-ligands showing pentanuclear or alternate mono/binuclear Cu(II) units. Dalton Trans. 41 (2012) 2078–2083.10.1039/C1DT11206FSuche in Google Scholar
16. Du, Z.-X.; Li, J.-X.; Han, R.-Q.: Syntheses, characterizations and crystal structures of two copper coordination polymers both having Cu2Cl2 bridging subunit [CuI(bipy) 1/2Cl]n (1) and {[(CuII)4(phen)4(SSA)2Cl2](H2O)2(DMF)2}n (2). J. Chem. Crystallogr. 41 (2011) 34–38.10.1007/s10870-010-9831-6Suche in Google Scholar
17. Li, J.-X.; Du, Z.-X.: Syntheses, structures and fluorescent properties of copper(II) and manganese(II) helical complexes bridged by 4,4′-dipyridylsulfide. Chin. J. Struct. Chem. 31 (2012) 877–883.Suche in Google Scholar
18. Xu, T.-Y.; Wang, H.; Li, J.-M.; Zhao, Y.-L.; Han, Y.-H.; Wang, X.-L.; He, K.-H.; Wang, A.-R.; Shi, Z.-F.: A water-stable luminescent Zn(II) coordination polymer based on 5-sulfosalicylic acid and 1,4-bis(1H-imidazol-1-yl)benzene for highly sensitive and selective sensing of Fe3+ ion. Inorg. Chim. Acta 493 (2019) 72–80.10.1016/j.ica.2019.05.002Suche in Google Scholar
19. Li, J.-X.; Du, Z.-X.: Crystal structure of catena-5-sulfosalicylato-κ2O,O′)-bis(μ2-4- thiolatopyridinium-κ2S:S) cadmium(II)] hydrate, Cd(C7H4O6S)(C5H5NS)2⋅2.5 H2O. Z. Kristallogr. NCS 226 (2011) 331–332.10.1524/ncrs.2011.0148Suche in Google Scholar
20. Du, Z.-X.; Li, J.-X.: The synthesis, structure and magnetic properties of a mononuclear cobalt compound with dipyrimidine sulfane ligand derived from 2-thiobarbituric acid. Inorg. Chim. Acta 436 (2015) 159–162.10.1016/j.ica.2015.07.036Suche in Google Scholar
21. Chang, X.-H.; Zhai, Z.-M.; Lu, X.-M.: Crystal structure of tetraaqua-bis (μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′- dipyridylsulfide-κ1N) dizinc(II), C36H34N6O12S2Ni2. Z. Kristallogr. NCS 235 (2020) 73–75.10.1515/ncrs-2019-0492Suche in Google Scholar
22. Shao, Z.-C.; Meng, X.-R.; Hou, H.-W.: Two new Cd-II and Zn-II coordination polymers incorporating 1-aminobenzene-3,4,5-tricarboxylic acid: synthesis, crystal structure and characterization. Acta Crystallogr. C75 (2019) 1065–1072.10.1107/S2053229619009227Suche in Google Scholar
23. Han, M.-L.; Ling, X.-L.: Crystal structure of diaqua-bis (5-nitrobenzene-3-carboxy-1,2-dicarboxylato)- bis(1-(3-(1H-benzimidazol-1-yl)propyl)-benzimidazole)manganese(II), [Mn(H2O)2(O2NC9H3O6)2(C17H17N4)2], C52H44MnN10O18. Z. Kristallogr. NCS 227 (2012) 574–576.10.1524/ncrs.2012.0255Suche in Google Scholar
24. Li, G.-L.; Liu, G.-Z.; Ma, L.-F.; Xin, L.-Y.; Li, X.-L.; Wang, L.-Y.: Crystallographic determination of solid-state structural transformations in a dynamic metal-organic framework. Chem. Commun. 50 (2014) 2615–2617.10.1039/C3CC49106DSuche in Google Scholar PubMed
25. Chai, J.; Liu, Y.; Liu, B.; Yang, B.: Effect of substituent groups R = -CH3, -Br and -CF3) on the structure, stability and redox property of [Cr(R-pic)2(H2O)2]NO3⋅H2O complexes. J. Mol. Struct. 1150 (2017) 307–315.10.1016/j.molstruc.2017.08.099Suche in Google Scholar
26. Li, J.-X.; Du, Z.-X.; Wang, J.-G.; Wang, T.; Lv, J.-N.: Zinc and manganese coordination polymers constructed by a new coordination mode of 4,5-dicyanoimidazolate ligand: syntheses, crystal structures, fluorescent and magnetic properties. Inorg. Chem. Commun. 15 (2012) 243–247.10.1016/j.inoche.2011.10.036Suche in Google Scholar
27. Feng, X.; Sun, Y. L.; Li, R.-F.; Zhang, T.; Guo, N.; Wang, L. Y.: Two novel europium coordination polymers based on fluorine substituted and similar carboxylate ligands: syntheses, structures and luminescence. Inorg. Chem. Commun. 73 (2016) 190–195.10.1016/j.inoche.2016.10.003Suche in Google Scholar
28. Zhang, J.; Liu, Y.-Y.; Ying, K.: Crystal structure of 2,2′-bipyridino-tetrafluorophthalato-copper(II) [Cu(C8HF4O4)(C10H8N2)2](C8H2F4O4)(C8HF4O4), C44H20CuF12N4O12. Z. Kristallogr. NCS 227 (2012) 410–412.10.1524/ncrs.2012.0172Suche in Google Scholar
29. Zhang, J.; Liu, Y.-Y.; Ying, K.: Crystal structure of (1,10-phenanthroline)(tetrafluorophtahlato)copper(II), [Cu(C8F4O4)(C12H8N2)2](C8H2F4O4), C40H18CuF8N4O8. Z. Kristallogr. NCS 227 (2012) 568–570.10.1524/ncrs.2012.0249Suche in Google Scholar
30. Li, J.-X.; Du, Z.-X.; Feng, X.: A new binuclear NiII complex with tetrafluorophthalate and 2,2′-bipyridine ligands: synthesis, crystal structure and magnetic properties. Z. Naturforsch. 74b (2019) 833–838.10.1515/znb-2019-0128Suche in Google Scholar
31. Zhang, J.; Li, J.-X.: Synthesis, structure and magnetic properties of a binuclear copper(II) complex constructed by a new coordination mode of the tetrachlorophthalate ligand. Z. Naturforsch. 71b (2016) 45–49.10.1515/znb-2015-0135Suche in Google Scholar
32. Sharma, R.-P.; Saini, A.; Kumar, J.; Kumar, S.; Venugopalan, P.; Ferretti, V.: Coordination complexes of copper(II) with herbicide-trichlorophenoxyacetate: syntheses, characterization, single crystal X-ray structure and packing analyses of monomeric [Cu(γ-pic)3(2,4,5-trichlorophenoxyacetate)]⋅H2O, [trans-Cu(en)2(2,4,5-trichlorophenoxyacetate)2]⋅2 H2O and dimeric [Cu2(H2tea)2(2,4,5-trichlorophenoxyacetate)2]⋅2 (H2O). Inorg. Chim. Acta 457 (2017) 59–68.10.1016/j.ica.2016.12.008Suche in Google Scholar
33. Xu, X.; Hu, F.; Ma, Y.; Gao, J.; Shuai, Q.: Facile microwave synthesis, structural diversity and herbicidal activity of six novel alkaline-earth metal complexes (AECs) based on skeletal isomerization chlorophenoxyacetic acids. New J. Chem. 42 (2018) 4155–4166.10.1039/C8NJ00107CSuche in Google Scholar
34. Li, J.-X.; Du, Z.-X.; Bai, R.-F.: Crystal structure of aqua-bis(5-bromo-6-methylpicolinato-κ2N,O) zinc(II) dihydrate, C14H16Br2N2O7Zn. Z. Kristallogr. NCS 235 (2020) 63–65.10.1515/ncrs-2019-0486Suche in Google Scholar
35. Li, S.-H.; Wang, J.-G.: Crystal structure of (μ2-5-bromoisophthalate-κ 2O:O′)bis(2-methyl-4H-imidazole-κN)cobalt(II), C16H15BrCoN4O4. Z. Kristallogr. NCS 229 (2014) 421–422.10.1515/ncrs-2014-9057Suche in Google Scholar
36. Chang, X.-H.: The crystal structure of poly[(m4-4-bromoisophthalato -κ4O:O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn. Z. Kristallogr. NCS 235 (2020) 3–4.10.1515/ncrs-2019-0397Suche in Google Scholar
37. Miroslaw, B.; Mahmoudi, G.; Ferenc, W.; Cristovao, B.; Osypiuk, D.; Sarzynski, J.; Gluchowska, H.; Franconetti, A.; Frontera, A.: Halogen interactions in dinuclear copper(II) 2,4-dibromophenoxyacetate – crystal structure and quantum chemical calculations. J. Mol. Struct. 1202 (2020) 127227.10.1016/j.molstruc.2019.127227Suche in Google Scholar
38. Ridenour, J. A.; Carter, K. P.; Cahill, C. L.: RE-p-halobenzoic acid–terpyridine complexes, part III: structural and supramolecular trends in a series of p-iodobenzoic acid rare-earth hybrid materials. CrystEngComm 19 (2017) 1190–1203.10.1039/C6CE02356HSuche in Google Scholar
39. Carter, K. P.; Kalaj, M.; Cahill, C. L.: Harnessing uranyl oxo atoms via halogen bonding interactions in molecular uranyl materials featuring 2,5-diiodobenzoic acid and N-donor capping ligands. Inorg. Chem. Front. 4 (2017) 65–78.10.1039/C6QI00352DSuche in Google Scholar
40. Li, B.; Dong, M.-M.; Fan, H.-T.; Feng, C.-Q.; Zang, S.-Q.; Wang, L.-Y.: Halogen⋯halogen interactions in the assembly of high-dimensional supramolecular coordination polymers based on 3,5-diiodobenzoic acid. Cryst. Growth Des. 14 (2014) 6325–6336.10.1021/cg501073eSuche in Google Scholar
41. Li, J.-X.; Du, Z.-X.; Wang, J.; Feng, X.: Two mononuclear zinc(II) complexes constructed by two types of phenoxyacetic acid ligands: syntheses, crystal structures and fluorescence properties. Z. Naturforsch. 74b (2019) 839–845.10.1515/znb-2019-0147Suche in Google Scholar
42. Du, Z.-X.; Li, J.-X.: Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18. Z. Kristallogr. NCS 235 (2020) 881–883.10.1515/ncrs-2020-0075Suche in Google Scholar
43. Li, J.-X.; Du, Z.-X.: A binuclear cadmium(II) cluster based on π⋯π stacking and halogen⋯halogen interactions: synthesis, crystal analysis and fluorescent properties. J. Cluster Sci. 31 (2020) 507–511.10.1007/s10876-019-01666-wSuche in Google Scholar
44. Du, Z.-X.; Li, J.-X.; Bai, R.-F.: The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2- ((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′) dinickel(II)], C19H10Cl6N3NiO6. Z. Kristallogr. NCS 235 (2020) 55–56.10.1515/ncrs-2020-0254Suche in Google Scholar
45. Du, Z.-X.; Li, J.-X.; Bai, R.-F.: Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2- ((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′) dicobalt(II)], C19H10Cl6CoN3O6. Z. Kristallogr. NCS 235 (2020) 15–17.10.1515/ncrs-2019-0434Suche in Google Scholar
46. Li, J.-X.; Du, Z.-X.; Pan, Q.-Y.; Zhang, L.-L.; Liu, D.-L.: The first 3,5,6-trichloropyridine-2-oxyacetate bridged manganese coordination polymer with features of π⋯π stacking and halogen⋯halogen interactions: synthesis, crystal analysis and magnetic properties. (2020) https://doi.org/10.1016/j.ica.2020.119677.10.1016/j.ica.2020.119677Suche in Google Scholar
47. Biju, S.; Gopakumar, N.; Bunzli, J.-C. G.; Scopelliti, R.; Kim, H. K.; Reddy, M. L. P.: Brilliant photoluminescence and triboluminescence from ternary complexes of DyIII and TbIII with 3-phenyl-4-propanoyl-5-isoxazolonate and a bidentate phosphine oxide coligand. Inorg. Chem. 52 (2013) 8750–8758.10.1021/ic400913fSuche in Google Scholar PubMed
48. Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G.: The halogen bond. Chem. Rev. 116 (2016) 2478–2601.10.1021/acs.chemrev.5b00484Suche in Google Scholar PubMed PubMed Central
©2020 Jun-Xia Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4
Artikel in diesem Heft
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4

