Abstract
C9H13N3O2S, monoclinic, P21/c (no. 14), a = 8.0984(4) Å, b = 17.3203(10) Å, c = 9.6802(4) Å, β = 124.031(3)°, V = 1125.26(10) Å3, Z = 4, Rgt(F) = 0.0504, wRref(F2) = 0.1275, T = 293(2) K.
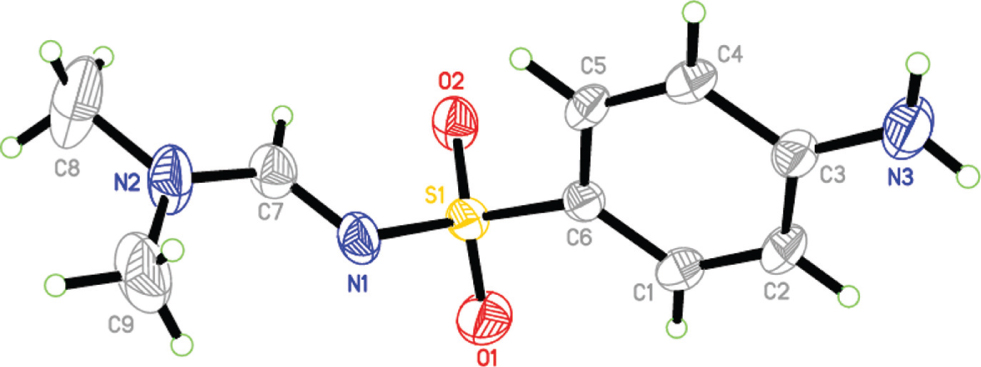
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.59 × 0.13 × 0.09 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.27 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 27.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 16215, 2581, 0.066 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1884 |
| N(param)refined: | 138 |
| Programs: | SHELX [1], Bruker [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.31663(8) | −0.59517(3) | −0.17899(7) | 0.03732(19) |
| O1 | 0.4058(3) | −0.64624(11) | −0.0384(2) | 0.0635(5) |
| O2 | 0.2455(2) | −0.52250(9) | −0.1591(2) | 0.0490(5) |
| N1 | 0.4747(3) | −0.58306(11) | −0.2299(3) | 0.0449(5) |
| N2 | 0.6689(4) | −0.49539(14) | −0.2558(3) | 0.0610(6) |
| N3 | −0.3860(3) | −0.74972(13) | −0.7473(3) | 0.0613(7) |
| H2 | −0.4202 | −0.7928 | −0.7311 | 0.074* |
| H3 | −0.4502 | −0.7290 | −0.8401 | 0.074* |
| C1 | 0.0513(4) | −0.71410(13) | −0.3266(3) | 0.0438(6) |
| H1A | 0.1191 | −0.7371 | −0.2219 | 0.053* |
| C2 | −0.1152(4) | −0.74905(13) | −0.4584(3) | 0.0472(6) |
| H2A | −0.1592 | −0.7955 | −0.4417 | 0.057* |
| C3 | −0.2195(3) | −0.71587(12) | −0.6171(3) | 0.0388(5) |
| C4 | −0.1487(3) | −0.64599(12) | −0.6387(3) | 0.0363(5) |
| H4A | −0.2143 | −0.6232 | −0.7436 | 0.044* |
| C5 | 0.0161(3) | −0.61130(12) | −0.5066(3) | 0.0344(5) |
| H5A | 0.0602 | −0.5646 | −0.5224 | 0.041* |
| C6 | 0.1189(3) | −0.64450(12) | −0.3492(3) | 0.0329(5) |
| C7 | 0.5336(4) | −0.51297(15) | −0.2254(3) | 0.0471(6) |
| H1 | 0.4779 | −0.4732 | −0.1997 | 0.057* |
| C8 | 0.7321(7) | −0.4158(2) | −0.2474(7) | 0.1030(14) |
| H8A | 0.6694 | −0.3832 | −0.2094 | 0.154* |
| H8B | 0.6943 | −0.3993 | −0.3561 | 0.154* |
| H8C | 0.8742 | −0.4124 | −0.1713 | 0.154* |
| C9 | 0.7652(5) | −0.5543(2) | −0.2925(5) | 0.0894(12) |
| H9A | 0.7915 | −0.5985 | −0.2234 | 0.134* |
| H9B | 0.8883 | −0.5345 | −0.2708 | 0.134* |
| H9C | 0.6797 | −0.5688 | −0.4076 | 0.134* |
Source of material
A mixture of benzenesulfonamide (344 mg, 2 mmol) and N,N-dimethylformamide dimethyl acetal (476 mg, 4 mmol) in ethanol (10 mL) was heated under reflux for 10 h. The reaction mixture was cooled and the separated solid was filtered, dried and recrystallized from ethanol. Yield 96%; 1H NMR (DMSO-d6; 500 MHz): δ 8.10 (s, 1H, N—C(H)=N), 7.39–7.37 (d, 2H, J = 8.5 Hz, Ar—H), 6.58–6.56 (d, 2H, J = 9.0 Hz, Ar—H), 5.82 (s, 2H, NH2), 3.10 (s, 3H, CH3), 2.87 (s, 3H, CH3) ppm; 13C NMR (DMSO-d6; 125 MHz): δ 35.30 (CH3), 41.14 (CH3), 113.01, 128.20, 129.03, 152.41, 159.43 (N—CH=N) ppm; MS: m/z = 227.1.
Experimental details
Cell refinement and data reduction were carried out by Bruker SAINT, SHELXT [1], [2].
Comment
Benzenesulfonamides (ArSO2NH2) incorporating aromatic and heterocyclic ring systems are an important group of compounds, which have a wide range of biological activity such as anti-inflammatory, COX-1/2 inhibiting activity, inhibitors of the metalloenzyme carbonic anhydrase, and antitumor activities [3], [4], [5], [6], [7], [8]. Recently, the synthesis of N-sulfonylformamidines has been reported through condensation of formamides and versatile sulfonamides using thionylchloride in the presence of chloroform as solvent [9]. The title compound was synthesized and reported according to method described by Silva, et al. [10]. Detailed synthetic studies indicate that this procedure gives the title compound.
The asymmetric unit of the title compound contains one independent molecule. The bond length between N1—C7 is 1.296(3) Å which indicates that it is a double bond, compared to 1.320(3) Å between N2—C7. Bond lengths and angles of the title molecule are in the expected ranges [11]. The molecules pack in the crystal structure via two strong classical intermolecular hydrogen bonds, N3—H2⋯N1i and N3—H3⋯O1ii in addition to one non-classical hydrogen bond C9—H9B⋯O2iii. The H⋯A distances are 2.31, 2.14 and 2.47 Å, respectively and the angles are 170, 164 and 161°, respectively. Symmetry codes: (i) x − 1, −y − 3/2, z − 1/2; (ii) x − 1, y, z − 1; (iii) x + 1, y, z.
Acknowledgements
The authors acknowledge financial support from the Researchers Supporting Project number (RSP-2019/40), King Saud University, Riyadh Saudi Arabia.
References
1. Sheldrick, G. M.: SHELXT–integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
2. Bruker: APEX S, SADABS and SHELXT. Bruker-AXS, Madison, WI, USA (2014).Search in Google Scholar
3. El-Azab, A. S.; Abdel-Aziz, A. A.-M.; Ayyad, R. R.; Ceruso, M.; Supuran, C. T.: Inhibition of carbonic anhydrase isoforms I, II, IV, VII and XII with carboxylates and sulfonamides incorporating phthalimide/phthalic anhydride scaffolds. Bioorg. Med. Chem. 24 (2016) 20–25.10.1016/j.bmc.2015.11.034Search in Google Scholar PubMed
4. Abdel-Aziz, A. A.-M.; El-Azab, A. S.; Ceruso, M.; Supuran, C. T.: Carbonic anhydrase inhibitory activity of sulfonamides and carboxylic acids incorporating cyclic imide scaffolds. Bioorg. Med. Chem. Lett. 24 (2014) 5185–5189.10.1016/j.bmcl.2014.09.076Search in Google Scholar PubMed
5. Abdel-Aziz, A. A.-M.; El-Azab, A. S.; Ekinci, D.; Sentürk, M.; Supuran, C. T.: Investigation of arenesulfonyl-2-imidazolidinones as potent carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 30 (2015) 81–84.10.3109/14756366.2014.880696Search in Google Scholar PubMed
6. Alanazi, A. M.; El-Azab, A. S.; Al-Suwaidan, I. A.; ElTahir, K. E. H.; Asiri, Y. A.; Abdel-Aziz, N. I.; Abdel-Aziz, A. A.-M.: Structure-based design of phthalimide derivatives as potential cyclooxygenase-2 (COX-2) inhibitors: anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 92 (2015) 115–123.10.1016/j.ejmech.2014.12.039Search in Google Scholar PubMed
7. Abdel-Aziz, A. A.-M.; Angeli, A.; El-Azab, A. S.; Hammouda, M. E. A.; El-Sherbeny, M. A.; Supuranc, C. T.: Synthesis and anti-inflammatory activity of sulfonamides and carboxylates incorporating trimellitimides: dual cyclooxygenase/carbonic anhydrase inhibitory actions. Bioorg. Chem. 84 (2019) 260–268.10.1016/j.bioorg.2018.11.033Search in Google Scholar PubMed
8. Abdel-Aziz, A. A.-M.; El-Azab, A. S.; El-Subbagh, H. I.; Al-Obaid, A. M.; Alanazi, A. M.; Al-Omar, M. A.: Design, synthesis, single-crystal and preliminary antitumor activity of novel arenesulfonylimidazolidin-2-ones. Bioorg. Med. Chem. Lett. 22 (2012) 2008–2014.10.1016/j.bmcl.2012.01.036Search in Google Scholar PubMed
9. Hudabaierdi, R.; Wusiman, A.; Mulati, A.: Improved synthesis of N-sulfonylformamidine derivatives promoted by thionyl chloride. Phosphorus, Sulfur Silicon Relat. Elem. 192 (2017) 485–489.10.1080/10426507.2017.1284843Search in Google Scholar
10. Silva, A. L.; Covarrubias-Zúñiga, A.; Maldonado, L. A.: A simple preparation of N,N-dimethyl-N′-alkyl (aryl) sulfonylformamidines. Org. Prep. Proced. Int. 34 (2002) 545–549.10.1080/00304940209355779Search in Google Scholar
11. Zhang, X.; Cao, Y.; Wang, Y.; Wang, S.; Xu, Z.: The crystal structure of catena-poly[bis((4-aminophenyl)sulfonyl)(pyrimidin-2-yl)amido-κ2N,N′)-bis(μ2-4,4′-bipyridine-κ2N:N′)zinc(II) – methanol (1/2), C32H34N10O6S2Zn. Z. Kristallogr. NCS 233 (2018) 413–415.10.1515/ncrs-2017-0319Search in Google Scholar
©2019 Alaa A.-M. Abdel-Aziz et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2
Articles in the same Issue
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2

