Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
-
Mohammed F. Alotibi
Abstract
C27H21N5O, triclinic, P1̄ (no. 2), a = 8.1464(7) Å, b = 10.3861(8) Å, c = 13.2507(9) Å, α = 84.898(6)°, β = 89.413(6)°, γ = 80.351(7)°, V = 1100.88(15) Å3, Z = 2, Rgt(F) = 0.0648, wRref(F2) = 0.1726, T = 296(2) K.
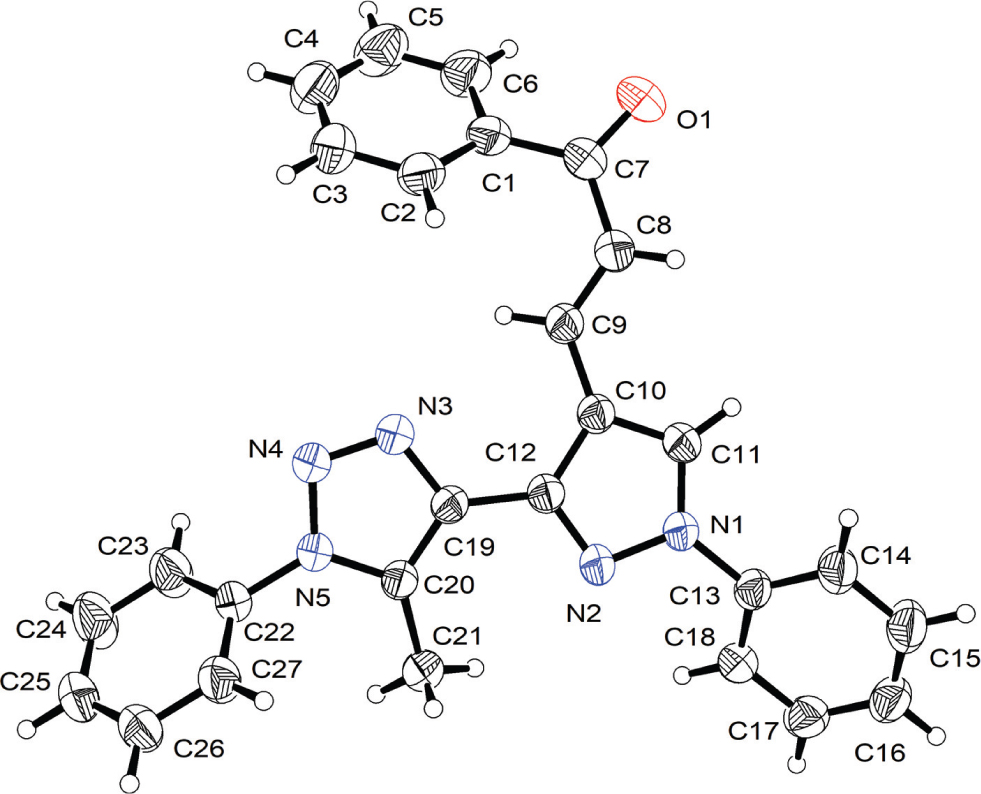
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.31 × 0.18 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 29.8°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 8540, 5164, 0.035 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2801 |
| N(param)refined: | 300 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.6089(3) | 0.1784(2) | −0.2761(2) | 0.0612(7) |
| C2 | 0.6412(4) | 0.3049(2) | −0.2969(2) | 0.0660(7) |
| H2 | 0.660160 | 0.353945 | −0.244058 | 0.079* |
| C3 | 0.6451(4) | 0.3581(3) | −0.3960(2) | 0.0796(9) |
| H3 | 0.667613 | 0.442744 | −0.409638 | 0.096* |
| C4 | 0.6162(4) | 0.2875(3) | −0.4738(2) | 0.0856(9) |
| H4 | 0.616829 | 0.324622 | −0.540299 | 0.103* |
| C5 | 0.5865(4) | 0.1624(3) | −0.4542(2) | 0.0895(10) |
| H5 | 0.569364 | 0.113822 | −0.507679 | 0.107* |
| C6 | 0.5816(4) | 0.1077(3) | −0.3566(2) | 0.0807(9) |
| H6 | 0.559874 | 0.022691 | −0.344140 | 0.097* |
| C7 | 0.6034(4) | 0.1137(2) | −0.1708(2) | 0.0652(7) |
| C8 | 0.5277(3) | 0.1846(2) | −0.08732(19) | 0.0585(7) |
| H8 | 0.545470 | 0.142525 | −0.022470 | 0.070* |
| C9 | 0.4355(3) | 0.3039(2) | −0.09493(17) | 0.0501(6) |
| H9 | 0.426345 | 0.349818 | −0.158716 | 0.060* |
| C10 | 0.3489(3) | 0.3679(2) | −0.01301(16) | 0.0467(6) |
| C11 | 0.3147(3) | 0.3126(2) | 0.08149(17) | 0.0511(6) |
| H11 | 0.346655 | 0.225166 | 0.105600 | 0.061* |
| C12 | 0.2738(3) | 0.5013(2) | −0.01143(16) | 0.0463(6) |
| C13 | 0.1591(3) | 0.3941(2) | 0.23171(17) | 0.0495(6) |
| C14 | 0.2429(4) | 0.3062(2) | 0.30552(19) | 0.0649(7) |
| H14 | 0.345146 | 0.256278 | 0.291775 | 0.078* |
| C15 | 0.1718(4) | 0.2936(3) | 0.4003(2) | 0.0737(8) |
| H15 | 0.226417 | 0.233793 | 0.450413 | 0.088* |
| C16 | 0.0224(4) | 0.3678(3) | 0.42142(19) | 0.0672(8) |
| H16 | −0.023686 | 0.358802 | 0.485630 | 0.081* |
| C17 | −0.0593(4) | 0.4558(2) | 0.3475(2) | 0.0657(7) |
| H17 | −0.161269 | 0.505861 | 0.361536 | 0.079* |
| C18 | 0.0098(3) | 0.4700(2) | 0.25212(18) | 0.0562(6) |
| H18 | −0.044530 | 0.530426 | 0.202291 | 0.067* |
| C19 | 0.2722(3) | 0.6090(2) | −0.09084(16) | 0.0456(6) |
| C20 | 0.1836(3) | 0.7342(2) | −0.09528(16) | 0.0464(6) |
| C21 | 0.0581(3) | 0.8011(2) | −0.02716(19) | 0.0603(7) |
| H21A | 0.112761 | 0.844419 | 0.020424 | 0.091* |
| H21B | 0.000916 | 0.737577 | 0.008784 | 0.091* |
| H21C | −0.020517 | 0.864517 | −0.066555 | 0.091* |
| C22 | 0.1777(3) | 0.9212(2) | −0.23063(17) | 0.0498(6) |
| C23 | 0.1120(4) | 0.9394(2) | −0.3261(2) | 0.0761(9) |
| H23 | 0.102721 | 0.867816 | −0.361756 | 0.091* |
| C24 | 0.0597(5) | 1.0649(3) | −0.3689(2) | 0.0902(11) |
| H24 | 0.014412 | 1.077883 | −0.433927 | 0.108* |
| C25 | 0.0733(4) | 1.1702(3) | −0.3176(2) | 0.0717(8) |
| H25 | 0.037417 | 1.254620 | −0.347327 | 0.086* |
| C26 | 0.1398(4) | 1.1517(2) | −0.2224(2) | 0.0722(8) |
| H26 | 0.149137 | 1.223692 | −0.187122 | 0.087* |
| C27 | 0.1931(4) | 1.0268(2) | −0.1782(2) | 0.0653(7) |
| H27 | 0.239114 | 1.014022 | −0.113464 | 0.078* |
| N1 | 0.2275(3) | 0.40624(17) | 0.13255(13) | 0.0491(5) |
| N2 | 0.1999(3) | 0.52498(16) | 0.07659(14) | 0.0511(5) |
| N3 | 0.3666(3) | 0.59442(17) | −0.17605(14) | 0.0529(5) |
| N4 | 0.3412(3) | 0.70498(18) | −0.23374(14) | 0.0546(5) |
| N5 | 0.2308(3) | 0.79058(17) | −0.18469(13) | 0.0487(5) |
| O1 | 0.6609(3) | −0.00381(18) | −0.15491(17) | 0.1037(8) |
Source of material
The title compound was synthesized from the reaction of equimolar quantities of 3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde and acetophenone in ethanol in presence of sodium hydroxide (10%) for 2 h at room temperature. The crude product was collected by filtration, washed with water and recrystallized from dimethylformamide to give colourless crystals (90%).
Experimental details
All hydrogen atoms were placed in calculated positions using a riding model. Methyl C—H bonds were fixed at 0.96 Å, with U(H) = 1.5 Ueq(C). C—H distances for sp2 hybridized groups were set to 0.93 Å and their Uiso(H) set to 1.2 times the Ueq(C). The high R1 value for all reflections is attributable to the weakness of data, particularly above 0.9 Å resolution.
Comment
Chalcones are an important class of compounds found in various natural products [5]. The synthetic methods for chalcone-containing compounds are simple, efficient, convenient, and high yielding [6]. Synthetic and natural chalcones show various interesting medicinal activities [7], [8]. They act as antibacterial, antiviral, antiulcer, antihelmintic, antiprotozoal, antioxidative, and insecticidal agents [9], [10], [11], [12], [13].
In the crystal structure, the asymmetric unit consists of one molecule (see the figure). The molecule comprises five ring systems, namely: A, phenyl (C1—C6); B, pyrazolyl (C10—C12,N1,N2); C, a second phenyl (C13—C18); D, triazolyl (C19—C21,N3—N5) and E, a third phenyl (C22—C27) group. The core of the molecule consists of rings B and D, which are almost coplanar with a twist angle of 10.35(12)°. The phenyl groups deviate from the core plane with interplanar angles A/B, B/C and D/E of 42.24(7)°, 33.54(11)° and 55.72(7)°, respectively.
The molecule displays an intramolecular C—H⋯N contact with a C21⋯N2 distance of 3.109(3) Å. An intermolecular C—H⋯O contact with a C11⋯O1 distance of 3.241(3) Å is also observed. Some related structures show similar features [14], [15].
Acknowledgements
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
References
1. Rigaku Oxford Diffraction: CrysAlisPRO. Rigaku Oxford Diffraction, Yarnton, England (2015).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
5. Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z.: Chalcone: a privileged structure in medicinal chemistry. Chem. Rev. 117 (2017) 7762–7810.10.1021/acs.chemrev.7b00020Search in Google Scholar PubMed PubMed Central
6. Gomes, M. N.; Muratov, E. N.; Pereira, M.: Peixoto, J. C.; Rosseto, L. P.; Cravo, P. V. L.; Andrade, C. H.; Neves, B. J.: Chalcone derivatives: promising starting points for drug design. Molecules 22 (2017) 1210.10.3390/molecules22081210Search in Google Scholar PubMed PubMed Central
7. Robinson, R. W.; Overmeyer, J. H.; Young, A. M.; Erhardt, P. W.; Maltese, W. A.: Synthesis and evaluation of indole-based chalcones as inducers of methuosis, a novel type of nonapoptotic cell death. J. Med. Chem. 55 (2012) 1940–1956.10.1021/jm201006xSearch in Google Scholar PubMed PubMed Central
8. Matos, M. J.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.: Potential pharmacological uses of chalcones: a patent review (from June 2011 − 2014). Expert Opin. Ther. Patents 25 (2015) 351–366.10.1517/13543776.2014.995627Search in Google Scholar PubMed
9. Rani, A.; Anand, A.; Kumar, K.; Kumar, V.: Recent developments in biological aspects of chalcones: the odyssey continues. Expert Opin. Drug Discov. 14 (2019) 249–288.10.1080/17460441.2019.1573812Search in Google Scholar PubMed
10. Andrade, J. T.; Santos, F. R. S.; Lima, W. G.; Sousa, C. D. F.; Oliveira, L. S. F. M.; Ribeiro, R. I. M. A.; Gomes, A. J. P. S.; Araújo M. G. F.; Villar, J. A. F. P.; Ferreira, J. M. S.: Design, synthesis, biological activity and structure-activity relationship studies of chalcone derivatives as potential anti-Candida agents. J. Antibiot. 71 (2018) 702–712.10.1038/s41429-018-0048-9Search in Google Scholar PubMed
11. Singh, P.; Anand, A.; Kumar, V.: Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem. 85 (2016) 758–777.10.1016/j.ejmech.2014.08.033Search in Google Scholar PubMed
12. Maydt, D.; De Spirt, S.; Muschelknautz, C.; Stahl, W.; Müller, T. J.: Chemical reactivity and biological activity of chalcones and other α,β-unsaturated carbonyl compounds. Xenobiotica 43 (2013) 711–718.10.3109/00498254.2012.754112Search in Google Scholar PubMed
13. Sahu, N. K.; Balbhadra, S. S.; Choudhary, J.; Kohli, D. V.: Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem. 19 (2012) 209–225.10.2174/092986712803414132Search in Google Scholar PubMed
14. El-Hiti, G. A.; Abdel-Wahab, B. F.; Hegazy, A. S.; Alamri, M.; Kariuki, B. M.: Crystal structure of 2-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1H-indene-1,3(2H)-dione, C28H19N5O2. Z. Kristallogr. NCS 232 (2017) 19–20.10.1515/ncrs-2016-0108Search in Google Scholar
15. El-Hiti, G. A.; Abdel-Wahab, B. F.; Alshammari, M. B.; Hegazy, A. S.; Kariuki, B. M.: Crystal structure of (E)-3-methyl-4-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1-phenyl-1H-pyrazol-5(4H)-one, C29H23N7O. Z. Kristallogr. NCS 232 (2017) 291–293.10.1515/ncrs-2016-0243Search in Google Scholar
©2019 Mohammed F. Alotibi et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2
Articles in the same Issue
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2

