Abstract
C13H12N2O3, monoclinic, P21/n (no. 14), a = 7.3322(5) Å, b = 8.0341(5) Å, c = 19.4479(14) Å, β = 95.775(2)°, V = 1139.82(13) Å3, Z = 4, Rgt(F) = 0.0533, wRref(F2) = 0.1335, T = 293(2) K.
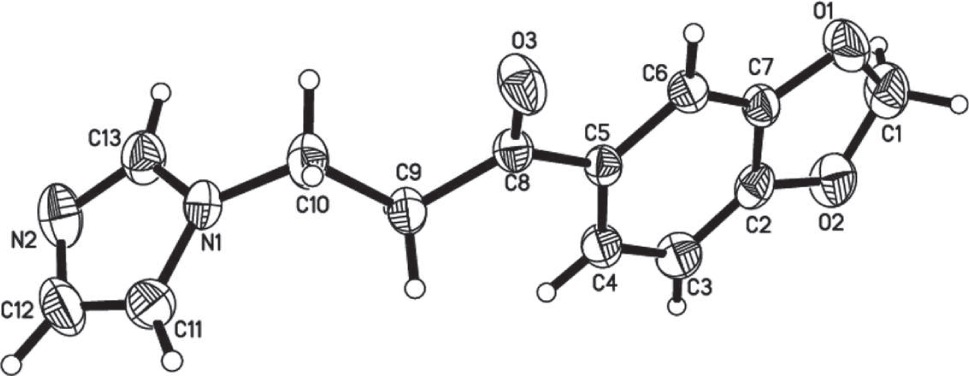
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.53 × 0.30 × 0.24 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.0 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 55°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 15327, 2619, 0.069 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1782 |
| N(param)refined: | 163 |
| Programs: | Bruker programs [26], SHELX [27, 28] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | −0.32584(19) | 0.3882(2) | 0.54491(7) | 0.0586(4) |
| O2 | −0.1784(2) | 0.18701(19) | 0.48625(7) | 0.0599(4) |
| O3 | 0.17088(19) | 0.70432(18) | 0.69870(7) | 0.0553(4) |
| N1 | 0.6623(2) | 0.55756(18) | 0.80766(7) | 0.0375(4) |
| N2 | 0.8445(3) | 0.4049(3) | 0.87925(10) | 0.0646(5) |
| C1 | −0.3535(3) | 0.2516(3) | 0.49832(11) | 0.0608(6) |
| H1A | −0.4189 | 0.2883 | 0.4551 | 0.073* |
| H1B | −0.4257 | 0.1660 | 0.5180 | 0.073* |
| C2 | −0.0534(3) | 0.2699(2) | 0.53079(9) | 0.0421(5) |
| C3 | 0.1311(3) | 0.2462(3) | 0.54163(10) | 0.0491(5) |
| H3A | 0.1900 | 0.1654 | 0.5177 | 0.059* |
| C4 | 0.2275(3) | 0.3490(2) | 0.59033(9) | 0.0418(5) |
| H4A | 0.3534 | 0.3347 | 0.5998 | 0.050* |
| C5 | 0.1409(2) | 0.4718(2) | 0.62493(8) | 0.0331(4) |
| C6 | −0.0492(2) | 0.4951(2) | 0.61174(9) | 0.0378(4) |
| H6A | −0.1097 | 0.5783 | 0.6337 | 0.045* |
| C7 | −0.1411(2) | 0.3902(2) | 0.56534(9) | 0.0395(4) |
| C8 | 0.2424(2) | 0.5806(2) | 0.67726(9) | 0.0347(4) |
| C9 | 0.4340(2) | 0.5335(2) | 0.70589(9) | 0.0387(4) |
| H9A | 0.5184 | 0.5596 | 0.6721 | 0.046* |
| H9B | 0.4394 | 0.4145 | 0.7142 | 0.046* |
| C10 | 0.4930(3) | 0.6241(3) | 0.77239(10) | 0.0478(5) |
| H10A | 0.5102 | 0.7410 | 0.7624 | 0.057* |
| H10B | 0.3964 | 0.6158 | 0.8029 | 0.057* |
| C11 | 0.8367(3) | 0.5899(3) | 0.79383(11) | 0.0513(5) |
| H11A | 0.8735 | 0.6622 | 0.7605 | 0.062* |
| C12 | 0.9454(3) | 0.4958(3) | 0.83824(12) | 0.0627(6) |
| H12A | 1.0727 | 0.4935 | 0.8404 | 0.075* |
| C13 | 0.6752(3) | 0.4464(3) | 0.85915(10) | 0.0497(5) |
| H13A | 0.5744 | 0.4033 | 0.8786 | 0.060* |
Source of material
A mixture of 1-(2H-1,3-benzodioxol-5-yl)ethanone (3.28 g, 20 mmol), dimethylamine hydrochloride (2.20 g, 27 mmol), paraformaldehyde (0.81 g, 9.0 mmol) in absolute ethanol (15 mL) and a catalytic amount of concentrated hydrochloric acid (0.5 mL) was heated to reflux for two hours. The reaction mixture was cooled and acetone (30 mL) was added. The precipitated Mannich base hydrochloride, namely 1-(2H-1,3-benzodioxol-5-yl)-3-(dimethylamino)propan-1-one hydrochloride was filtered off and dried. The Mannich base hydrochloride (2.58 g, 10 mmol) was dissolved in water (10 mL) and imidazole (1.36 g, 20 mmol) was added. The reaction mixture was heated to reflux for five hours, cooled and the precipitated solid was collected by filtration to give 1.15 g (47%) of the title ketone as a white solid (m.p. 423–425 K). Slow evaporation of an ethanolic solution of the title compound furnished its colourless single crystals.
Experimental details
Carbon-bound hydrogen atoms were placed in calculated positions and were included in the refinement using the riding model approximation, with Uiso(H) set to 1.2Ueq(C).
Discussion
Imidazole derivatives have been first synthesised in the 1840s. Their current importance is testified by their ubiquitousness in the fields of medicinal chemistry, material science and agrochemicals [1]. Imidazole-based compounds exhibit diverse biological activities anticancer [2], [3], [4], [5] antibacterial [6, 7] , antitubercular [8, 9] , antifungal [10, 11] , antiviral [12], [13], [14], anticonvulsant [15], [16], [17] and antiparkinson’s agents [18, 19] . The discovery of the broad spectrum antifungal drug clotrimazole sparked considerable interest in their antifungal properties. Thereafter, research focused on incorporating the imidazole pharmacophore in different chemical structures [20], [21], [22]. In addition, a literature search revealed that a plethora of bioactive molecules incorporate the 1,3-benzodioxole scaffold in their structure [23], [24], [25]. The title ketone is a hybrid structure bearing both the 1,3-benzodioxole and imidazole moieties in its structure and can be used as a precursor to synthesize a number of new bioactive surrogates.
In the crystal structure of the title compound, the asymmetric unit contains one independent molecule. The molecules are packed via several non-classical C—H ⋯O intermolecular hydrogen bonds to give a 3-D network.
Acknowledgement
This research project was supported by a grant from the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University.
References
1 Jin, Z.: Muscarine, imidazole, oxazole, and thiazole alkaloids. Nat. Prod. Rep. 28 (2011) 1143–1191.10.1039/c0np00074dSearch in Google Scholar PubMed
2 Li, Q. Y.; Deng, X. Q.; Zu, Y. G.; Lv, H.; Su, L.; Yao, L.; Zhang, Y.; Li, L.: Cytotoxicity and topo I targeting activity of substituted 10–nitrogenous heterocyclic aromatic group derivatives of SN-38. Eur. J. Med. Chem. 45 (2010) 3200–3206.10.1016/j.ejmech.2010.03.013Search in Google Scholar PubMed
3 Lu, Y.; Li, C. M.; Wang, Z.; Ross, C. R.; Chen, J.; Dalton, J. T.; Li, W.; Miller, D. D.: Discovery of 4-substituted methoxybenzoyl-aryl-thiazole as novel anticancer agents: synthesis, biological evaluation, and structure-activity relationships. J. Med. Chem. 52 (2009) 1701–1711.10.1021/jm801449aSearch in Google Scholar PubMed PubMed Central
4 Biersack, B.; Muthukumar, Y.; Schobert, R.; Sasse, F.: Cytotoxic and antivascular 1-methyl-4-(3-fluoro-4-methoxyphenyl)-5-(halophenyl)-imidazoles. Bioorg. Med. Chem. Lett. 21 (2011) 6270–6273.10.1016/j.bmcl.2011.09.005Search in Google Scholar PubMed
5 Chen, J.; Ahn, S.; Wang, J.; Lu, Y.; Dalton, J. T.; Miller, D. D.; Li, W.: Discovery of novel 2-aryl-4-benzoyl-imidazole (ABI-III) analogues targeting tubulin polymerization as antiproliferative agents. J. Med. Chem. 55 (2012) 7285–7289.10.1021/jm300564bSearch in Google Scholar PubMed PubMed Central
6 Sharma, S.; Gangal, S.; Rauf, A.: Convenient one-pot synthesis of novel 2-substituted benzimidazoles, tetrahydrobenzimidazoles and imidazoles and evaluation of their in vitro antibacterial and antifungal activities. Eur. J. Med. Chem. 44 (2009) 1751–1757.10.1016/j.ejmech.2008.03.026Search in Google Scholar PubMed
7 Aonofriesei, F.; Lupsor, S.: Inhibitory potential of a novel imidazole derivative as evaluated by time-kill and dehydrogenase activity. Curr. Microbiol. 66 (2013) 162–168.10.1007/s00284-012-0252-ySearch in Google Scholar PubMed
8 Stover, C. K.; Warrener, P.; VanDevanter, D. R.; Sherman, D. R.; Arain, T. M.; Langhorne, M. H.; Anderson, S. W.; Towell, J. A.; Yuan, Y.; McMurray, D. N.; Kreiswirth, B. N.: A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405 (2000) 962–966.10.1038/35016103Search in Google Scholar PubMed
9 Lima, C. H. S.; Henriques, M. G. M. O.; Candea, A. L. P.; Lourenco, M. C. S.; Bezerra, F. A. F. M.; Ferreira, M. L.; Kaiser, C. R.; de Souza, M. V. N.: Synthesis and antimycobacterial evaluation of N′-(E)-heteroaromaticpyrazine-2-carbohydrazide derivatives. Med. Chem. 7 (2011) 245–249.10.2174/157340611795564303Search in Google Scholar PubMed
10 Polak, A.: Oxiconazole, a new imidazole derivative. Evaluation of antifungal activity in vitro and in vivo. Arzneimittelforschung 32 (1981) 17–24.Search in Google Scholar
11 Rossello, A.; Bertini, S.; Lapucci, A.; Macchia, M.; Martinelli, A.; Rapposelli, S.; Herreros, E.; Macchia, B.: Synthesis, antifungal activity, and molecular modeling studies of new inverted oxime ethers of oxiconazole. J. Med. Chem. 45 (2002) 4903–4912.10.1021/jm020980tSearch in Google Scholar PubMed
12 Zhan, P.; Liu, X.; Zhu, J.; Fang, Z.; Li, Z.; Pannecouque, C.; De Clercq, E.: Synthesis and biological evaluation of imidazole thioacetanilides as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. 17 (2009) 5775–5781.10.1016/j.bmc.2009.07.028Search in Google Scholar PubMed
13 Basu, A.; Jasu, K.; Jayaprakash, V.; Mishra, N.; Ojha, P.; Bhattacharya, S.: Development of CoMFA and CoMSIA models of cytotoxicity data of anti-HIV-1-phenylamino-1H-imidazole derivatives. Eur. J. Med. Chem. 44 (2009) 2400–2407.10.1016/j.ejmech.2008.09.043Search in Google Scholar PubMed
14 Jia, W.; Zhao, Y.; Li, R.; Wu, Y.; Li, Z.; Gong, P.: Synthesis and in–vitro anti–hepatitis–B virus activity of 6H–[1]benzothiopyrano [4,3–b]quinolin–10–ols. Arch. Pharm. 342 (2009) 507–512.10.1002/ardp.200900070Search in Google Scholar PubMed
15 Karakurt, A.; Alagöz, M. A.; Sayoğlu, B.; Calış, U.; Dalkara, S.: Synthesis of some novel 1-(2-naphthyl)-2-(imidazol-1-yl)ethanone oxime ester derivatives and evaluation of their anticonvulsant activity. Eur. J. Med. Chem. 57 (2012) 275–282.10.1016/j.ejmech.2012.08.037Search in Google Scholar PubMed
16 Karakurt, A.; Ozalp, M.; Işık, Ş.; Stables, J. P.; Dalkara, S.: Synthesis, anticonvulsant and antimicrobial activities of some new 2-acetylnaphthalene derivatives. Bioorg. Med. Chem. 18 (2010) 2902–2911.10.1016/j.bmc.2010.03.010Search in Google Scholar PubMed
17 Caliş, U.; SeptioğluEbubekir, S.; Aytemir, M. D.: Synthesis and anticonvulsant evaluation of some novel (thio) semicarbazone derivatives of arylalkylimidazole. Arzneimittelforschung 61 (2011) 327–334.10.1055/s-0031-1296206Search in Google Scholar
18 Sugimoto, Y.; Kobayashi, K.; Asai, M.; Ohno, A.; Yamada, K.; Ozaki, S.; Ohta, H.; Okamoto, O.: Synthesis and biological evaluation of imidazole derivatives as novel NOP/ORL1 receptor antagonists: Exploration and optimization of alternative pyrazole structure. Bioorg. Med. Chem. Lett. 19 (2009) 4611–4616.10.1016/j.bmcl.2009.06.095Search in Google Scholar PubMed
19 Asproni, B.; Murineddu, G.; Pau, A.; Pinna, G. A.; Langgård, M.; Christoffersen, C. T.; Nielsen, J.; Kehler, J.: Synthesis and SAR study of new phenylimidazole-pyrazolo [1, 5-c] quinazolines as potent phosphodiesterase 10A inhibitors. Bioorg. Med. Chem. 19 (2011) 642–649.10.1016/j.bmc.2010.10.038Search in Google Scholar PubMed
20 Aboul-Enein, M. N.; El-Azzouny, A. A.; Attia, M. I.; Saleh, O. A.; Kansoh, A. L.: Synthesis and anti–candida potential of certain novel 1–[(3–substituted–3–phenyl) propyl]–1H–imidazoles. Arch. Pharm. 344 (2011) 794–801.10.1002/ardp.201000224Search in Google Scholar PubMed
21 De Vita, D.; Scipione, L.; Tortorella, S.; Mellini, P.; Di Rienzo, B.; Simonetti, G.; D’Auria, F. D.; Panella, S.; Cirilli, R.; Di Santo, R.; Palamara, A. T.: Synthesis and antifungal activity of a new series of 2-(1H-imidazol-1-yl)-1-phenylethanol derivatives. Eur. J. Med. Chem. 49 (2012) 334–342.10.1016/j.ejmech.2012.01.034Search in Google Scholar PubMed
22 El Hage, S.; Lajoie, B.; Feuillolay, C.; Roques, C.; Baziard, G.: Synthesis, antibacterial and antifungal activities of bifonazole derivatives. Arch. Pharm. 344 (2011) 402–410.10.1002/ardp.201000304Search in Google Scholar PubMed
23 Micale, N.; Zappalà, M.; Grasso, S.: Synthesis and cytotoxic activity of 1,3-benzodioxole derivatives. Farmaco 58 (2003) 351–355.10.1002/chin.200336096Search in Google Scholar
24 Leite, A. C. L.; da Silva, K. P.; de Souza, I. A.; de Araújo, J. M.; Brondani, D. J.: Synthesis, antitumour and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur. J. Med. Chem. 39 (2004) 1059–1065.10.1016/j.ejmech.2004.09.007Search in Google Scholar PubMed
25 Aboul-Enein, M. N.; El-Azzouny, A. A.; Attia, M. I.; Maklad, Y. A.; Amin, K. M.; Abdel-Rehim, M.; El-Behairy, M. F.: Design and synthesis of novel stiripentol analogues as potential anticonvulsants. Eur. J. Med. Chem. 47 (2012) 360–369.10.1016/j.ejmech.2011.11.004Search in Google Scholar PubMed
26 Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA, (2009).Search in Google Scholar
27 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
28 Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Cryst. C71 (2015), 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
©2017 Reem I. Al-Wabli et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16

