Abstract
The earth natural carrying capacity is being surpassed, and there is an urgent need to develop new alternatives, notably in regards to energy supplies, carbon dioxide emissions, and nitrogen supplies to the ecosystem. Hydrogen gas, produced from renewable energy by water electrolysis, may serve as a platform molecule for the 21st century low-carbon economy and electrification. The ability to utilise hydrogen metabolic processes is quite diverse, and this offers up a vast array of avenues for innovative biotechnological advancements and applications. A strategy focusing on the major role of hydrogen throughout the production of bio-based foundational element compounds through the hydrocarbon pathway would avoid the inherent low economic value of hydrocarbons in favour of products with greater value. Furthermore, hydrogen could serve as a crucial carbon-neutral source for the manufacture of third-generation proteins while allowing carbon capture and nutritional recovery immediately at the site of emission. Using these methods to deal with the seasonal changes in renewable energy sources makes the use of alternative energy as efficient as possible. The outcomes demonstrated the production technologies of bio-hydrogen is a good way to make renewable hydrogen that is both cost-effective and good for the environment compared to other ways of making hydrogen.
Introduction
With rising urbanisation, industrialization, and population expansion, it is anticipated that the world energy demand would rise by 56% between 2010 and 2040, from 553 quadrillion kJ to 855 quadrillion kJ (Hosseinzadeh et al. 2020). As the predominant sources of energy, fossil fuels such as coal and petroleum are considered nonrenewable. In addition, fossil fuel combustion in energy production and transportation releases numerous contaminants into the atmosphere, which would include greenhouse gases, monoxide, nitrogen oxides, particulate matter, and organic contaminants such as polycyclic aromatic hydrocarbons (Khlaifat et al. 2020). On all continents, the global warming caused by greenhouse gases emissions has had a variety of negative effects on human well-being, such as malnutrition and mental effects on health from flooding and droughts in China, South Africa, Bangladesh and Ethiopia, as well as respiratory and cardiovascular impacts of extreme weather events in Australia, western North America and western Europe (Huang et al. 2019a). A long-term changing climate has become a hazard to several pillars of well-being and human health (Watts et al. 2021). Not only are the released pollutants harmful and even carcinogenic, but they may also create hydrocarbon particulates with adverse health effects (Hassan et al. 2022a). These negative consequences are felt most strongly in metropolitan areas with a high population density, since it is projected that automobile emissions caused 387,000 premature deaths and $1 trillion in health damage worldwide in 2015 (Hassan 2022).
Currently, nearly 65% of the European Union total domestic consumption of renewable energy is bioenergy (Huang et al. 2021). It is anticipated that by 2040, renewable energy generation would supply roughly 51% of the world total energy demand and become the dominant energy source (Huang et al. 2021). In addition, freshwater deficiency is recognised as a significant issue around the globe today (Abbas et al. 2022). Moreover, waste materials and wastewater are now considered the most important environmental challenges; nonetheless, they may serve as good sources of biomass for energy recovery (Hassan and Jaszczur 2021). There are numerous technologies available to address each of these challenges separately, such as crop residues and municipal wastes for sewage disposal (Chen et al. 2020), advanced oxidation procedures for sewage water and water as a function, and electricity production from renewable energy resources such as solar and wave (Hassan et al. 2022b). Nevertheless, the creation of techniques that simultaneously address water and energy limits, as well as the health and environmental issues associated with municipal solid waste, is both exciting and crucial (Hosseinzadeh et al. 2021).
Hydrogen is a particularly intriguing energy carrier since its energy output is 2.75 times that of fossil fuels or 122 kJ/g. Hydrogen is clean energy since its combustion produces no CO2 or other hazardous pollutants, just water. In reality, the use of hydrogen as a fuel satisfies the zero-emissions goal that is now being pursued internationally. Increased interest has been shown in the generation of hydrogen from wastewater and waste, as well as from biomass and other renewable resources (Assawamongkholsiri et al. 2018). Some processes for producing hydrogen from bio-energy include photo, dark, and solid-state fermenting (Ceran et al. 2021), microbial desalination cells (Dessì et al. 2020), pyrolysis (Foong et al. 2021), incineration (Chen et al. 2020), and plasma (Byun et al. 2011). Many different types of waste and sewage have been investigated using these approaches. However, there is still a lack of information about which method, based on the full techno-economic evaluation and its influence on the internal and external factors, is the best option for hydrogen production and processing efficiency. Many articles have talked about different ways to make hydrogen, but techno-economic and environmental footprint analyses have not paid as much attention to new ways to make hydrogen, like shadowy and steady carbonation, microbial desalination cells, and erythrocytes. Also, do not know enough about the techno-economic assessment and environmental effects of more developed processes like pyrolysis and gasification to be able to choose the best way to make hydrogen from different types of bio-waste.
Figure 1 depicts the hydrogen energy system (Al-Alawi 2008). Currently, technology connected to the generation, storage, and transportation of hydrogen energy has grown fast throughout the globe, contributing to the shift towards clean and decarbonized future energy systems (Xu, Zhou, and Yu 2022).
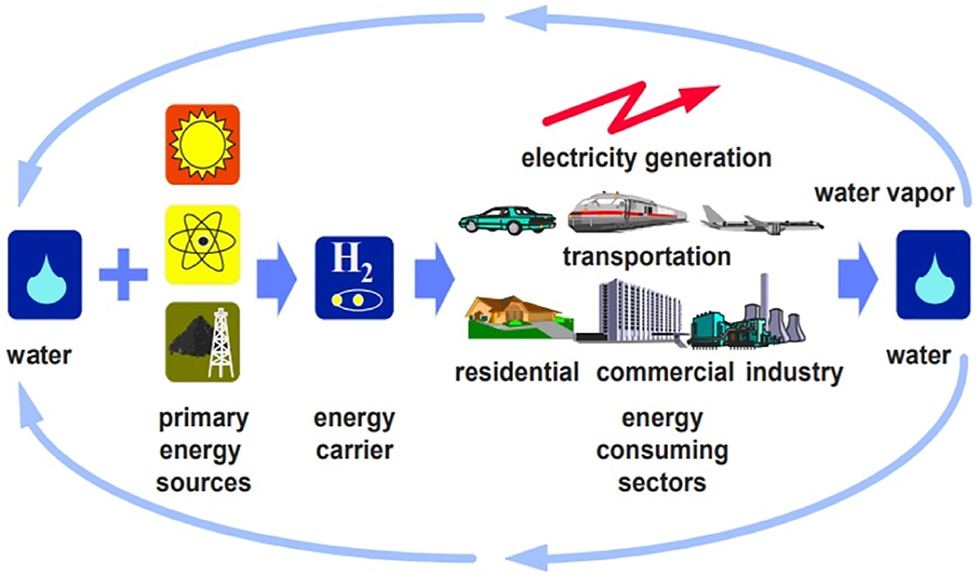
Hydrogen energy system (Hassan and Jaszczur 2021).
This article provided a complete review of biomass-to-hydrogen synthesis using a variety of unique and environmentally friendly production pathways, highlighting their strengths and shortcomings. When it comes to small-scale, dispersed facilities, the best way to make hydrogen depends a lot on the economic potential and distribution of raw materials.
The global carbon-neutral plan shows that using hydrogen is becoming more and more important. A detailed understanding of hydrogen generation, storage, and transportation is essential for creating efficient and environmentally friendly hydrogen use methods. The first step in this work was to determine the properties of various hydrogen generation systems, including both fossil and renewable non-fossil pathways. The merits, drawbacks, and future possibilities of each renewable hydrogen production technique were then clarified by further examining the categorization and research developments of bio-hydrogen production techniques. The current paper provides a short overview of the most cutting-edge methods for producing hydrogen, with a focus on biomass high-efficiency properties. It has been discovered that bio-hydrogen technological improvements offer a number of benefits and promising futures in advancing hydrogen energy economy realisation and environmental degradation reduction. This article will provide a thorough analysis of biomass-to-hydrogen synthesis using a variety of cutting-edge, environmentally friendly production methods, with a focus on their advantages and disadvantages. The most efficient way to produce hydrogen, particularly for those tiny, dispersed facilities, relies significantly on the distribution of raw materials and production capacity.
Adaptation to bioenergy
The impending environmental crisis and climate warming concerns, along with rising oil prices and diminishing fossil fuel resources, have attracted significant global interest in the creation of alternative, sustainable energy, carbon-neutral, and environmentally friendly fuels to meet the escalating energy demand. Bioenergy is an innovative and sustainable substitute for fossil fuels that can fight against the energy crisis and preserve the globe from environmental disaster. Bioenergy is believed to have the ability to offer carbon-free and renewable energy through sustainable means. It is a strategy to diversify energy sources to mitigate associated costs and to also help support the local regional economy, (International Energy Agency 2021). Due to its renewability, bioenergy generated from microbes is of tremendous relevance in the current global energy landscape. Bacteria possess adaptable and diversified metabolic machinery for converting and synthesising a number of organic compounds into numerous kinds of bioenergy. Establishing a feasible connection between an electron acceptor microbe and an electron sink is the fundamental principle behind the majority of bioenergy generating methods (Kumar, Kumar, and Pal 2021). Globally, considerable and intensive research is now being conducted on bioenergy production using renewable energy resources.
Origins of biological hydrogen production
Biohydrogen is a natural and transient byproduct of several metabolic activities mediated by microorganisms. Biohydrogen refers to the production of hydrogen gas by microbiological mechanisms or thermochemical processing of biomass. In addition to being referred to as biohydrogen, thermochemically generated hydrogen is also referred to as biohydrogen owing to the use of biomass as a substrate or feedstock. On the other hand, biohydrogen generation may occur through anaerobic/fermentation, chemiluminescence, enzymatic, and the following hypothesis: developed pathways, among others. In the last two decades, the scientific community throughout the world has shown a substantial interest in the biological pathways of hydrogen generation. During the preceding decade, hydrogen production research, both fundamental and applied, saw significant progress.
Biological hydrogen production mechanisms may be further subdivided into light-independent fermenting and light-dependent processes. The chemiluminescence process can be classified as either photosynthesis or fermentation, depending on the carbon source and development and manufacturing techniques used. Biophotolysis of water employing macroalgae and phytoplankton through both direct and indirect photosynthesis or photofermentation facilitated by photosynthetic organisms are examples of energy mechanisms. Dinoflagellates and diatoms produce hydrogen via both direct and indirect biophotolysis by consuming inorganic carbon dioxide in the presence of sunlight and water, whereas cyanobacteria generate hydrogen via photofermentation by consuming a wide range of substrates from inorganic to organic acids in the absence of light. However, the fermentative process is limited to anaerobic metabolism, in which anaerobic bacteria (mainly acidogenic bacteria) produce hydrogen via an acidogenesis process, coupled with volatile fatty acids and carbon dioxide-fermentation results in the formation of hydrogen in the absence of oxygen. In the lack of oxygen, both obligatory and aerotolerant bacteria are able to produce hydrogen. Microbial electroplating is an in-situ approach in which an external potential is given to microbial cells in order to increase biological hydrogen production. In vitro hydrogen synthesis is mediated by a synthetic enzyme system, which is one of the exciting approaches envisioned by scientists. The biochemistry and metabolism associated with biological pathways vary considerably according to the biocatalyst used, the operating parameters adapted, the environment utilised, and the potting medium utilised.
Hydrogen production from biomass
Bio-hydrogen generation is a method of generating hydrogen via chemical or biological processes, including solar energy absorption and transformation by living organisms (Kumar, Kumar, and Pal 2021). Bio-hydrogen mass production could be derived from a variety of sustainably sourced natural sources, including biomass feedstocks, agricultural leftovers, forestry wastes and leftovers, industrial and municipal trash (Kumar, Kumar, and Pal 2021). Typically, biomass fuel sources fall into five groups (Wang et al. 2018), which are outlined here. The first group is vegetation grass materials, such as rice husk, wheat crop residues, trees and shrubs, and energy vegetation (Zhang et al. 2020a); the second group is animal droppings, along with cow, pig, the third group is digestate generated by the food business, such as pomace, lees, oilseed rape cake, and groundnut cake; the fourth group is rubbish, including liquid waste and kitchen waste (Peng et al. 2017); and the fifth group contains other materials, such as biogas Some basic sources for biohydrogen generation are shown in Figure 2.
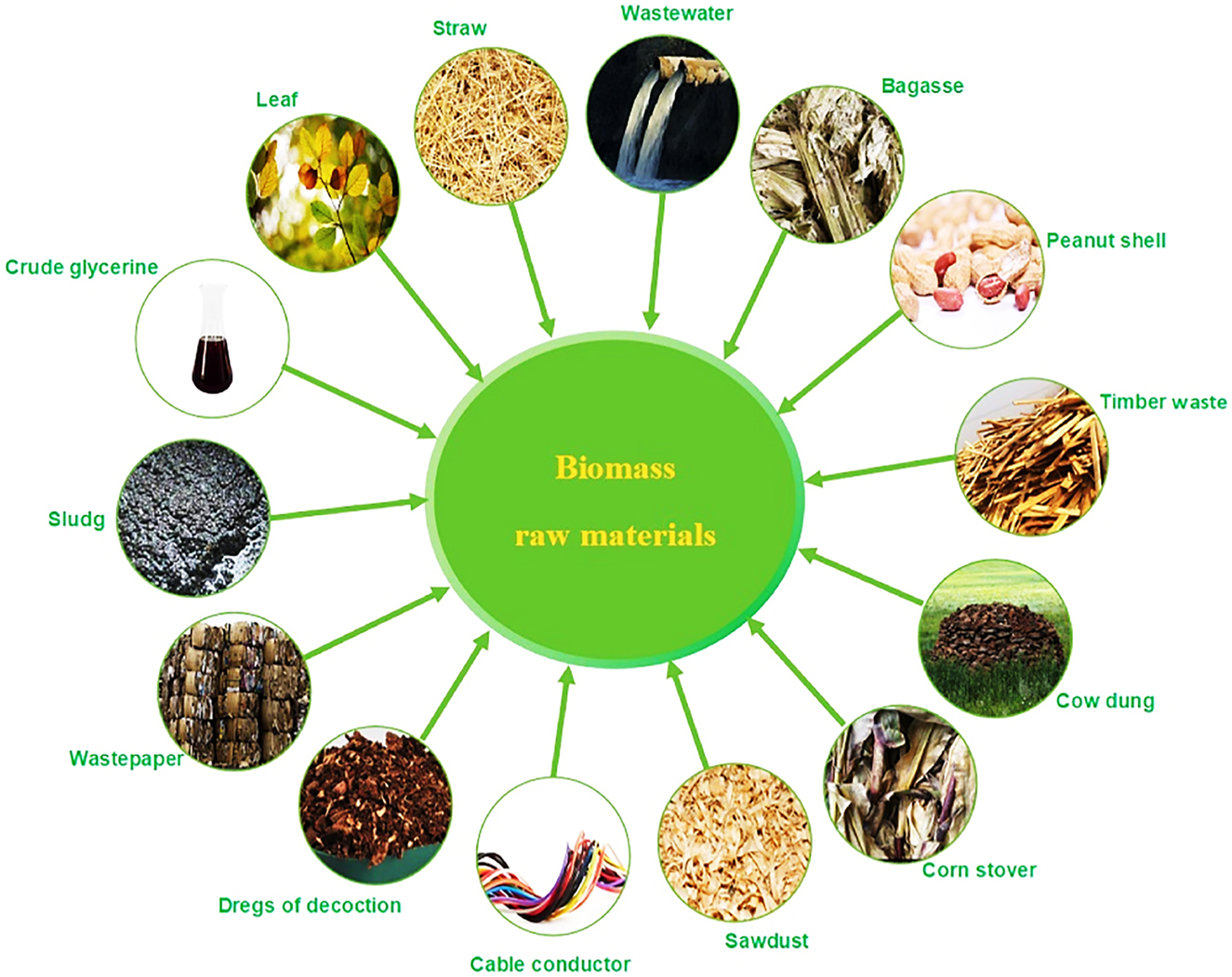
Raw biomass materials.
The carbon in biomass is not derived from fossil fuels but rather from carbon dioxide absorbed in the environment by plants. Carbon dioxide created during the biohydrogen generation process is a dioxide emissions method that involves the recycling of carbon components. As a result, bio-hydrogen generation is nearly carbon neutral when compared to traditional hydrogen manufacturing techniques (Hassan et al. 2022c). Therefore, hydrogen generation via biomass will finally meet the goal of environmentally friendly clean energy (Hassan et al. 2022d), and therefore, the accompanying technologies will have a high productive capacity and hence a bright future. In the next part, the features and research accomplishments of bio-hydrogen generation will be examined in detail, and its underlying concepts and methodologies will be contrasted.
Producing bio-hydrogen by renewable biomass
At present, 97% of the globe hydrogen is derived by fossil fuels, with steam methane reformation being the most prevalent technique of synthesis. Other renewable-energy-based alternatives, such as water electrolysis and hydrogen generation from bioenergy, namely hemicellulose feedstock, are now being evaluated in pilot-scale presentations or in the direction of commercialisation. The sources of lignocellulose (Jaszczur et al. 2020) include agricultural food wastes, marine byproducts, crop residues, and forest residues. An estimated 4.6 billion tonnes of hemicellulose agricultural residues are produced annually, of which 25% are used, and roughly 8 billion tonnes of grassland used to create energy (Hassan et al. 2022e). The affordability and quantity of biomass have drawn interest. The generation of hydrogen from biomass enhances production volume, adds to economic capacity, improves source flexibility, and decreases emissions of greenhouse gases. Carbon-neutral biomass is possible if maintained natural cycle. The carbon dioxide generated during the creation of hydrogen from biomass is used for plant growth during photosynthesis. Researchers are looking into a number of ways to make hydrogen from biomass, including biological conversions, thermochemical processes, and possibly biosynthesis with the help of an electrochemical reaction (Jaszczur and Hassan 2020).
Bio-hydrogen production technologies
The synthesis of bio-hydrogen involves biological and chemical processes. Essentially, it is based on photosynthesis-produced biomass and offers the benefits of enormous storage of raw materials, energy savings, and outstanding environmental performance. As a result, it has become a subject of significant study interest in the area of hydrogen production. The exact categorisation of bio-hydrogen generation is shown in Figure 3.
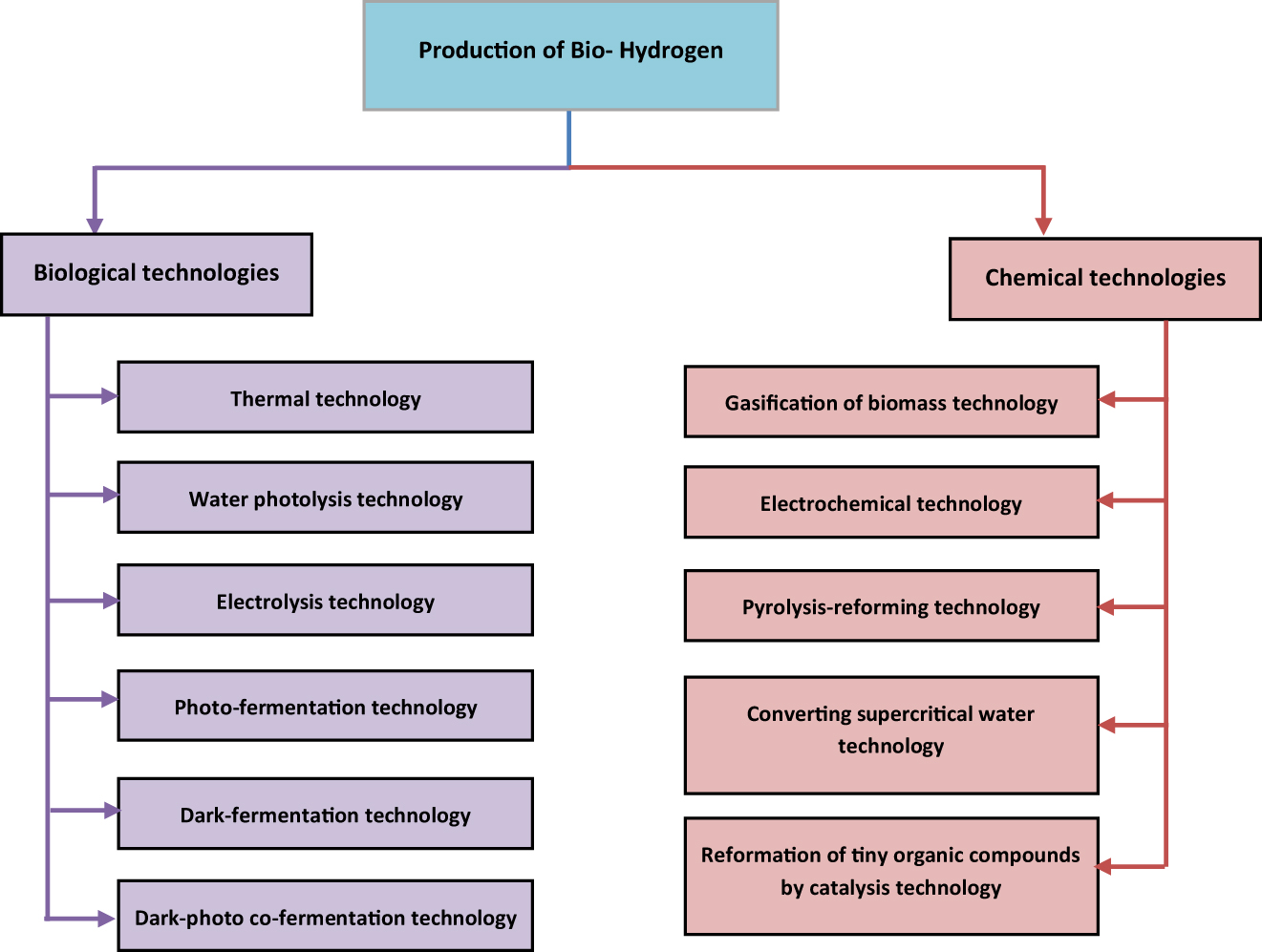
Depicts the categorization of biohydrogen generation.
Thermal technology
The most contemporary method of creating hydrogen from biomass is known as the thermal process. Complementary biofuel technologies, such as biomethane, were borrowed from steam methanol reformation to create the technology (Lepage et al. 2021). The three primary thermochemical processes are combustion, decomposition, and aqueous solution reformation (Stenberg et al. 2018).
Water photolysis technology
Producing hydrogen via water photocatalytic degradation refers to a technique of manufacturing hydrogen and oxygen by decomposing water through photosynthesis using microorganisms (Shi et al. 2019). Both blue and green microalgae have been investigated extensively in this field (Ghirardi et al. 2014). These two bacteria have modest dietary needs because they create hydrogen immediately by photocatalytic degradation using just air, water, simple inorganic ions, and light.
Water photolysis has attracted a lot of interest since it has long been thought of as an ecologically beneficial way to produce biological hydrogen. Scenedesmus obliquus, a kind of green algae, has been known to create hydrogen during metabolism since 1939, as indexed in (Gaffron and Rubin 1942). They found that microalgae fix carbon dioxide and absorb hydrogen under anaerobic circumstances. On the other hand, hydrogen may be created under light circumstances, but the process only takes a brief time (Greenbaum 1982). Some studies have revealed that a variety of green algae, including Chlamydomonas reinhardtii (Llama et al. 1979; Maione and Gibbs 1986) and Chlorellafiscal (Kessler 1973), may produce hydrogen. Simple prokaryotes called cyanobacteria may manufacture oxygen via photosynthesis and come in a variety of shapes and sizes (such as single cells, filamentous and colonies). In the genera Nostoc and Anabaena, cyanobacteria ability to produce hydrogen has been extensively explored (BEIKIN 1978). In order to raise the hydrogen enzyme resistance to oxygen and extend the time it takes to produce hydrogen while simultaneously increasing hydrogen production, much research is now being done in this area (Melo and Silva 2011). The primary advantage of producing hydrogen from water via water photolysis is that it can be done in an aqueous environment at room temperature and pressure (Akhlaghi and Najafpour-Darzi 2020).
Electrolysis technology
Electrolysis is a well investigated electrochemical process that breaks water molecules to produce hydrogen. The process occurs on a low-temperature fuel cell and relies on an electrical current flowing through water via a conductive electrolyte. This separates water into oxygen and hydrogen (Kothari, Buddhi, and Sawhney 2008). After separation, a speedy conversion produces pure hydrogen. In addition, there are no carbon, sulphury, or nitrogen byproducts, which reduces cleaning costs compared to gasification processes. The constraint on water splitting is based on thermodynamic features, since the usage of at least 45 kWh/kg of hydrogen necessitates acceptable hydrogen production (Show, Lee, and Zhang 2011).
Photo-fermentation technology
Under anaerobic and light circumstances, hydrogen is typically produced through photo-fermentation. A photosynthetic bacteria-initiated anaerobic fermentation process is catalyzed by employing light energy and the diminished ability to retrieve tiny molecular organic materials (Su et al. 2010). Currently, cyanobacteria often employ soybean waste, dairy waste, and starch waste as substrates for the generation of hydrogen (Show, Lee, and Zhang 2011). Rhodospillum rubrum, Rhodopseudomonas, RhodoDseudomonas capsulate, Thiocapsa Roseopersicina, etc. are the most frequently investigated hydrogen-producing photosynthesis microorganisms (Ren et al. 2011).
Numerous investigations have shown that nitrogen fixation hydrolysis reactions drive photosynthetic bacteria generation of hydrogen (Argun and Kargi 2011a). Only one photosynthetic system is present in photosynthetic bacteria, and organic molecules often serve as the electron donor. As a result, oxygen is often not used to generate hydrogen (Hassan et al. 2022f). The lack of oxygen release, as compared to producing hydrogen by water photolysis, reduces the problems associated with separating hydrogen from oxygen, considerably simplifying the manufacturing process (Li et al. 2007). All metabolic steps for photo-hydrogen fermentation generation may be stated as:
Cyanobacteria often employ amino substances as substrates to create hydrogen in anaerobic and ammonia circumstances (Kapdan et al. 2009). The following diagram illustrates the hydrogen generation process using lactic acid as a material:
For the creation of hydrogen, cyanobacteria may also use oligosaccharides like sucrose and polysaccharides like starch. The related chemical process is depicted below:
The change in the pH of the fluid, substrate content, type of fermentation microbe, light levels, etc. all have an impact on how quickly hydrogen is produced during photo-fermentation (Kapdan et al. 2009).
Local organic acids are produced by Clostridium and other aerobic microbes during metabolism, and organic acids are helpful for increasing the effectiveness of hydrogen synthesis. To increase the efficiency of hydrogen generation, several scientists have thus far grown composite formulations that combine clostridium and photosynthetic bacteria (Lu et al. 2012). Kawagoshi ultimately produced Rhodobacter Sphaeroides KUPB, a salt-tolerant photosynthetic bacterium, by continuous illumination, isolation, and purification (Kawagoshi et al. 2010) using combined acid as the substrate in his work.
In conclusion, the wide potential of hydrogen manufacturing technologies via photo-fermentation is boosting the efficiency of hydrogen generation by either applying gene technology or combining photosynthesis bacteria with other microbes. Additionally, a range of organic materials, including waste water and organic acid, may be used in the hydrogen manufacturing process (Argun and Kargi 2011b). Numerous sources are readily accessible, and no oxygen is used throughout the synthesis process (Asada et al. 2006a). But since light is needed for photo-fermentation to work, it is hard to do amplification studies (Rai and Singh 2016).
Dark-fermentation
The fermentation process is another name for biological hydrogen synthesis, which occurs during dark fermentation. The fundamental process is the fermentation of organic wastes by heterotrophic anaerobic bacteria in anaerobic environments (Brennan and Owende 2010; Lin et al. 2012). During the fermentation process, bacteria can break down many different types of base species to make hydrogen (Kumar et al. 2016; Kumar et al. 2017).
Aerotolerant and obligatory bacteria make up the majority of the anaerobic microorganisms that produce hydrogen from organic materials (Nandi and Sengupta 1998). In general, facultative anaerobic bacteria mostly consist of Enterobacter, Escherichia, and Klebsiella, while obligatory anaerobic microorganisms include Clostfidium, Desulfovibrio, and other bacteria (Delavar and Wang 2021). The four types of bacteria with the most research and the best ability to produce hydrogen at this time are Clostridium, Acinetobacter, Enterobacter escherichia, and Bacillus (Chen and Lin 2003; Xu, Mi, and Ren 2016). Under the influence of nitrogenase or hydroenzymes, shadowy bacteria may convert a wide range of substrates (etoac, lactic, cellulosic saccharides, disulfide, etc.) into hydrogen. The chemical process that uses glucose as a starting point to make hydrogen is shown below:
This technique of producing hydrogen has been the subject of several studies. According to Fang and Liu (2002), the amount of phosphorus in the reaction mechanism had a substantial impact on E. harbinenseB49 ability to grow and produce hydrogen. The results showed that at a phosphate concentration of 50 mmol/L, the potential for hydrogen generation may be maximized. For example, maltose was utilized as a matrix by Kumar and Das (2000) in reactor to create hydrogen, and after 8 h of hydrodynamic residence time, the hydrogen outcomes about 5.32 mol. Using glucose, Zhang et al. (2015) carried out fermentation at a ranged temperature and various pH levels. They discovered that when the pH hit 5.5, the rates of hydrogen generation and glucose uptake were at their maximum. When Oh et al. (2003) investigated the effects of pH adjustment and acid control on the effectiveness of dark fermentation in producing hydrogen, they discovered that acid injection might boost hydrogen production rate by 50%. It suggested that controlling acid levels might have a big impact on how much hydrogen is produced and how well dark fermentation works.
Currently, a reasonably advanced technique for producing biological hydrogen is dark fermentation. It benefits from a variety of substrates supplied; continuous synthesis of hydrogen requiring light; stable producing hydrogen, gentle reaction conditions, cheap cost, etc. However, this approach has certain drawbacks, including a poor rate of input material utilisation, blatant product inhibition, tail fluid that contaminates the climate (Yue et al. 2012). Furthermore, the shady hydrogen generation method has yet to be thoroughly evaluated economically (Pareek et al. 2020).
Dark-photo co-fermentation
A technique for manufacturing hydrogen by combining the benefits of hydrogen-producing bacteria from photo which is known as dark-photo co-fermentation (Ren, Guo, and Liu 2010). This method improves both the efficiency of producing hydrogen and the efficiency of converting substrates (Zhang et al. 2020b). The manufacturing process typically consists of two steps: Prior to dark fermentation, the biomass raw materials underwent pretreatment and some tail liquid was released. This process produced hydrogen. Furthermore, under the activation of a nitrogen enzyme, tiny organic in the dark-fermentation were employed as electron donors to create hydrogen by portrait (Dawood, Anda, and Shafiullah 2020; Momirlan and Veziroǧlu 1999). This technique is seen in Figure 4.
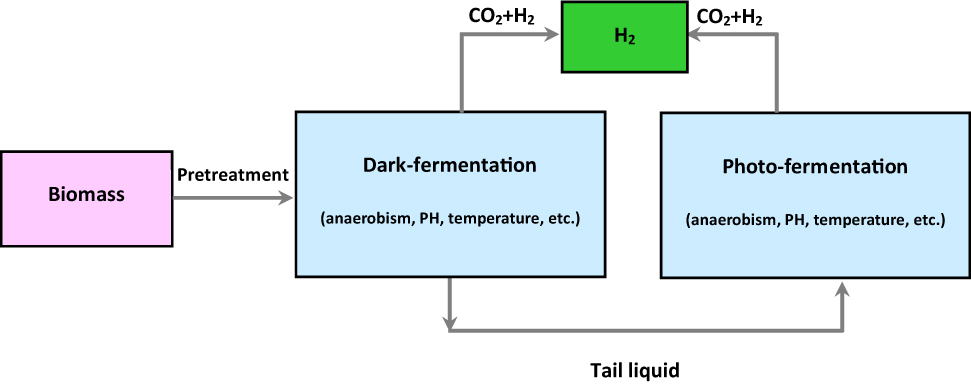
The dark-photo co-fermentation technology for producing hydrogen.
This shadowy founder method is very effective and inexpensive since it may considerably enhance the overall quantity of hydrogen (Hawkes et al. 2002; Nath and Das 2004). It has been extensively investigated how dark-photo founder might produce hydrogen. When Nath et al. (Nath, Kumar, and Das 2005) attempted to establish a picture using Arthrobacter sphaeroides O.U.001 on the compounds ontaining of Enterobacter, they discovered that the concentration was much greater than single process. In the dark-photo co-fermentation two-step of experiment, Tao et al. (2007) presented that utilizing sucrose as the substrate may greatly boost hydrogen production, with the highest producing hydrogen reaching up to 7.12 mol sucrose. After enzymatic hydrolysis of starch, Lo et al. (2008) combination of dark-photo founder and processes generated hydrogen (It is difficult to degrade). Indicating that the approach of two-step could enhance material efficiency using inexpensive raw ingredients for hydrogen production, the hydrogen production was capable of reaching 4.01 mol glucose. The two strains used in this procedure, however, need different growth and nutrient conditions, which suggests that each strain rate of hydrogen synthesis is variable, resulting in a bottleneck in producing hydrogen (Mishra et al. 2019). This method approach, which combines the benefits of dark-and photo-fermentation processes, can produce hydrogen from a variety of substrates, and as a result, it has some advancement potential and usage prospects in the advertising manufacturing of large amounts of hydrogen (Dinesh et al. 2020) and ecological sustainability (Asada et al. 2006b).
Chemical methods
The main non-living activity involved in biohydrogen generation is the chemical and thermochemical processing of biomass. Thermo-chemical processes use either combustion or decomposition to generate a hydrogen-rich stream of gas, sometimes described as a mixture of carbon monoxide and hydrogen (Lipman 2011). Thermo-chemical techniques for hydrogen generation use chemical reactions aided by heat to liberate hydrogen from hydrocarbons or water (Yildiz and Kazimi 2006). The thermochemical method is compatible with an extensive variety of materials. Gasification of biomass at temperatures of over 1000 K in the presence of oxygen and/or steam produces gas and char by partial oxidation and/or thermochemical conversion processes (Navarro et al. 2009). This method is superior to pyrolysis for hydrogen production. There has been combustion by partial oxidation for nearly 150 years (de Jong 2008). Low-temperature (1000 °C) gasification provides a substantial quantity of hydrocarbons, but high-temperature combustion yields essentially no hydrocarbons (Navarro et al. 2009). Pyrolysis enables the thermal degradation of biomass at 650–800 K in the absence of oxygen to produce liquid oils, solid carbon, and gas chemicals (Navarro et al. 2009). Hydrogen may be created immediately by rapid or flash decomposition if the maximum temperature and enough volatility phase retention time are present (Navarro et al. 2009). Gasification and pyrolysis followed by reformation of the carbohydrate component of the bio-oil are the two primary thermochemical processes employed for hydrogen generation (Balat 2010). In addition to pyrolysis and gasification, a water–gas shift is utilised to transform the reforming gas into hydrogen, and pressure-driven membrane absorption is used to purify the product (Saxena et al. 2008). In the absence of oxygen, a supercritical water state may convert biomass into fuel gases, which can be readily extracted from the water phase by cooling to room temperature (Navarro et al. 2009). The cost of hydrogen production through steam reforming combustion of wet biomass was several times that of hydrogen production via steam methane reformation (Saxena et al. 2008).
Gasification of biomass
The technique of pressing and producing biomass obscene resources in a gasification additive and then turning them into hydrogen-containing flammable gas via gasification is referred to as hydrogen generation by gasification (Karellas 2015; Zhang et al. 2019a). Tar and charcoal are byproducts of the gasification process that are unavoidable. Research on catalysts, such as natural ores and alkali metals, is currently under way to accelerate coke gasification and decrease the concentration of tar (Cummer and Brown 2005). The gasification of biomass used to produce hydrogen is seen in Figure 5.
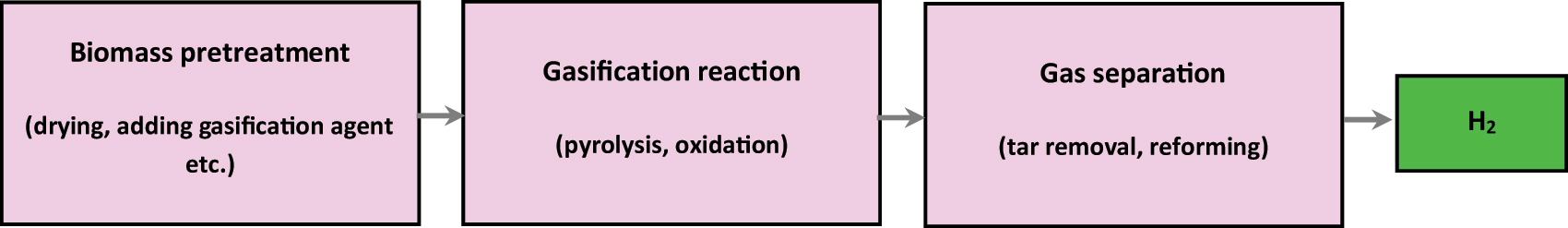
Illustrates the gasification of biomass to produce hydrogen.
Gasifying reagents (air, water vapor, oxygen, etc.) are necessary for biomass gasification in order to produce hydrogen (Puig-Arnavat, Bruno, and Coronas 2010). We may acquire a high hydrogen content by using water vapor as a gasification agent, however doing so results in significant energy consumption and gas generation with a high calorific value. The cost might be reduced by using air as the gasification agent, but at the expense of an unworkable low rate of hydrogen generation (Uddin et al. 2008). The process of creating hydrogen via biomass gasification involves several processes. The following is a list of the major replies (Uddin et al. 2008):
These processes suggest that throughout the hydrogen generation process, in addition to other small-molecule carbides. The typical temperature range for chemical reactions is 450–1000 °C (Huang et al. 2019b). When employing waste material as a raw resource in a fixed bed, Turn et al. (1998) examined the synthesis of hydrogen from biomass using a benchscale fluidized bed gasifier. Parametric experiments were conducted to assess the mpacts of heating rate, equivalency ratio, and steam to biomass ratio. The hydrogen highest yield, the capability of the gaseous state for hydrogen synthesis by moving carbon dioxide and steam reforming upper hydrocarbons, was calculated based on experimental observations of oxygen content and yield. Over the research conditions studied, the hydrogen yield potential demonstrated an equivalency ratio of 0 and a steam-to-biomass proportion of 1.7. This corresponds to 78% of the maximum possible output of 165 g hydrogen per kilogramme of dry, ash-free biomass for this fuel. A series of tests were conducted by Hamad et al. (2016) to study the impact of various operational variables on the effectiveness of the gasification. These were the oxygen-to-fuel equivalency ratio (0.12–0.4), the reaction temperature (707–850 °C), the response retention time (45–120 min), and the kind of catalyst. The results indicate that the produced hydrogen from the gasification of crop residues with calcium hydroxide has a higher ratio of hydrogen and CO (45 and 35%, respectively).
The observations shown in the table above show that the rate of hydrogen production rises as the temperature rises, causing the component concentration to vary significantly. The choice of the biomass natural resources, the gasification and catalyst as well as the temperature and gasification residence time, all have a significant impact on the rate of producing hydrogen utilizing biomass chemical combustion (Sikarwar et al. 2017; Zhang et al. 2019b). But getting the gaseous agent to a very high temperature and cleaning it would take a lot of energy, which means it would be expensive (Guo et al. 2022; Jalan and Srivastava 1999a).
Electrochemical technology
Electrolysis is a well investigated electrochemical process that breaks water molecules to produce hydrogen. The process occurs on a low-temperature fuel cell and relies on an electrical current flowing through water via electrolyte. This separates water into oxygen and hydrogen (Gallagher et al. 2017). After separation, a simple and speedy conversion produces pure hydrogen. In addition, there are no carbon, sulphury, or nitrogen byproducts, which reduces cleaning costs compared to gasification processes. The constraint on water splitting is based on thermodynamic features, since the usage of at least 46 kWh/kg of necessitates acceptable hydrogen production (Ganzoury and Allam 2015).
Pyrolysis-reforming
Biomass is cooked to a high temperature without the need for a gasification agent during the complicated process known as pyrolysis, which produces gas, solid and liquid via a sequence of chemical reactions and heat transfer (Jalan and Srivastava 1999b). The pyrolysis method of producing hydrogen is shown in Figure 6.
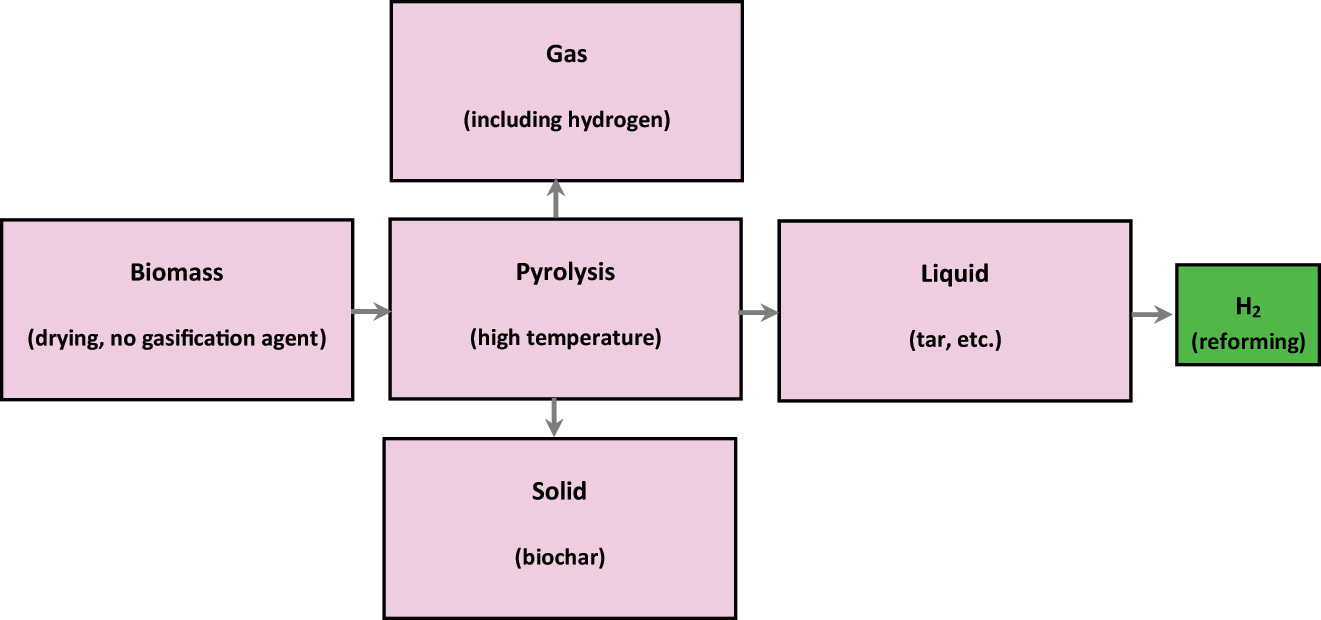
The pyrolysis of biomass to produce hydrogen.
Pyrolysis needs additional reforming to produce hydrogen because the high temperature that is maintained throughout the process encourages the development of tar, which is sticky and challenging to evaporate (Zhang et al. 2022). There are currently five ways of reforming fuels: steam reformation, water phase modernising, production of hydrogen reforming, chemical reforming, and photocatalyst reforming. One of them, chemical reformation, can separate hydrogen on-site and is a new way to make hydrogen that is good for the environment (Zeng and Gong 2015).
Qinglan et al. (2010) conducted the catalytic decomposition of biomass production using a dual-particle powder fluidised bed reactor in order to generate hydrogen-rich gas. The impact of key operating parameters on the yield and dispersion of gas products at low pressure and temperature were investigated. Ansari et al. (2014) used a twin bed reactor system, bagasse may be used efficiently as an alternative source of combustible gas and for the generation of hydrogen and syngas. Utilizing a catalyst in the second bed improves the efficacy of bagasse pyrolysis. Through microencapsulation synthesis of nano-catalysts, a gas containing more hydrogen and carbon monoxide and less hydrocarbons and carbon dioxide was created. Compared to the single bed post-transcriptional method, the microemulsion-made catalyst increased gas production from 0.4 m3/kg to 0.8 m3/kg and decreased yield (%) from 0.5 to 0.3 g. However, the heating value of the output gas stayed almost the same at 10–11 MJ/m3. Luo et al. (2017) presented a technique for concurrently producing glassy slag and reusing the heat for biomass pyrolysis processes to create hydrogen-rich gas. The impacts of a number of factors, including waste temperatures, mass ratio of waste to bioenergy, particle size, and angular velocity on pyrolysis extraction yield and gas properties were investigated. In addition, the photocatalytic efficiency of blast-furnace slag for enhancing tar cracking was investigated. Under the circumstances of 1000 °C waste heat and 0.6%, the biomass was completely pyrolyzed. At a ratio of 0.8%, biomass may be completely pyrolyzed at a slag temperature of 700 degrees Celsius. At a rotor speed of sixteen rpm/min, when slag particles in the reactor displayed a movement, the maximum gas output was achieved. During the pyrolysis process, blast-furnace waste displayed catalytic properties in tar cracking and reforming. In addition, lowering the particle size of the waste encouraged the production of more light gases and less char and condensation. During the later catalytic reforming process, however, the effect of slag particle size was no longer clear. Barbarias et al. (2019) developed, characterised, and studied the effectiveness of a series of two catalysts in relation to hydrogen production and tar reduction during combustion in a two-stage reaction mechanism. The results demonstrate that catalysts made by the sol-gel approach show more catalytic activity in relation to the formation of hydrogen and gas than catalysts prepared via insemination. Furthermore, filamentous graphene was more likely to form on the surface of reacted sol-gel catalysts than unstructured carbon on the interfaces of reacted saturated catalysts. Both types of carbon were made by the enhanced catalysts, but it depended on the type of metal and how it interacted with the phase. The catalyst is replenished between operations by coke ignition in the presence of the reformation reactor, utilising a series of air concentrations and a temperature ramping between 600 and 700 °C, as described by Barbarias et al. (2019). Due to the sintering of the catalyst surface, the catalysts do not completely regain their original activity during coke combustion, as shown by a number of analytical tests. In a subsequent oxidative pathway, this hardening process is gradually decreased, and the catalyst reaches a stable state. Waheed, Wu, and Williams (2016) investigated the effects of catalytic cracking temperature, steaming flow rate, and biomass particle size on syngas and hydrogen yields during the two-stage pyrolysis/catalytic reformation of coconut husk in a fixed-bed batch reactor. It may be deduced that the hydrogen yield per gramme of rice husks increased dramatically as the reformation temperature increased from 850 °C to 1050 °C, from 20.03 to 30.62 mmol hydrogen. The increased gas output was attributed to the endothermic production of a product reaction, the water gas reaction, and the steam reforming process. Oxidation and reduction, depolymerization, and thermal decomposition all played a big role in the increase of hydrogen from 53.95 to 65.18 vol% in the resultant gas.
Converting supercritical water
The term “production of hydrogen by steam reforming conversion” refers to a process that uses superheated water (p = 22.1 MPa, T = 374.2 °C) to create hydrogen-rich gases, including CH4, hydrogen, CO2, and CO and through raw material carbonization and steam reformation (Sheikhdavoodi et al. 2015). With this approach, the volume concentration of hydrogen gas products may surpass 60%, the rate of exchange of biomass can reach 100%, and the reaction does not produce tar or any other byproducts. The following is a list of the reaction equations for the hydrogen manufacturing process (Sheikhdavoodi et al. 2015):
Existing research shows that the duration of reaction, pressure, temperature, biomass content, oxidant concentrations, and type of catalysts all have an impact on the hydrogen generation efficiency via supercritical conversions of water (Kıpçak and Akgün 2015; Reddy et al. 2014). The method of catalytic biomass saturated water combustion was optimised by Kang et al. (2016). By evaluating catalysts utilising both lignin and cellulose as biomass models, the most effective catalysts were determined. Then, using waste biomass as feedstock, a Taguchi experimental design-based optimization study was conducted using wheat bran, canola powder, and American citizen grass. The effects of various factors are investigated. The proportional relevance of these factors for hydrogen generation is as follows: heat > catalytic load > catalyst type > feedstock category. For the synthesis of hydrogen, high temperatures (650 °C) and high catalyst loadings (100%) are advantageous. The average output of hydrogen from various waste biomass was as follows: majority of grass > canola powder > grain straw. Pine and wheat straw were pretreated using the catalytic impregnation technique of Hossain, Chowdhury, and Charpentier (2019), who subsequently converted supercharged water to hydrogen. The outcomes demonstrated that the pretreatment of raw materials had a positive impact on hydrogen generation. According to previous research, using the right catalysts to produce hydrogen from supercritical water may considerably increase the effectiveness of hydrogen production and gasification in an environment with low temperatures (Li and Guo 2019; Nanda et al. 2016). The catalysts utilized in this technique of hydrogen generation typically fall into one of several categories (Yanik et al. 2008). Huang et al. (2019b) discovered that the generation of hydrogen was greatly boosted. Because of their affordability and strong catalytic activity, which catalysts have been used in the majority of recent investigations. However, due to sintering and carbon deposition, the majority of catalysts are often rendered inactive during the creation of hydrogen (Guo et al. 2012; Kruse and Dinjus 2005). One of the most promising methods for producing hydrogen is the transformation of supercritical conditions. Wet materials may be fed straight into the hydrogen generation process. Additionally, it produces a lot of hydrogen and has a high reaction efficiency (Ge, Guo, and Jin 2020; Zhang et al. 2019c). The product is very simple to transport and store. But because of the high equipment requirements, it will cost a lot to invest in and maintain. Therefore, there are certain restrictions on its commercialization (Pei et al. 2008; Xu et al. 2019a).
Reformation of tiny organic compounds by catalysis
The biomass can be transformed into specific molecular precursors and then the molecules can be directly dispersed to each hydroformylation station because the production of bio-hydrogen for hydroformylation stations includes many technical issues (Okolie et al. 2020). This process may make transporting hydrogen less expensive. Gas-phase reformation and water-phase reforming are two ways that small-molecule reforming may produce hydrogen. The categorization of hydrogen generation by the reforming process of tiny organic compounds is shown in detail in Figure 7.
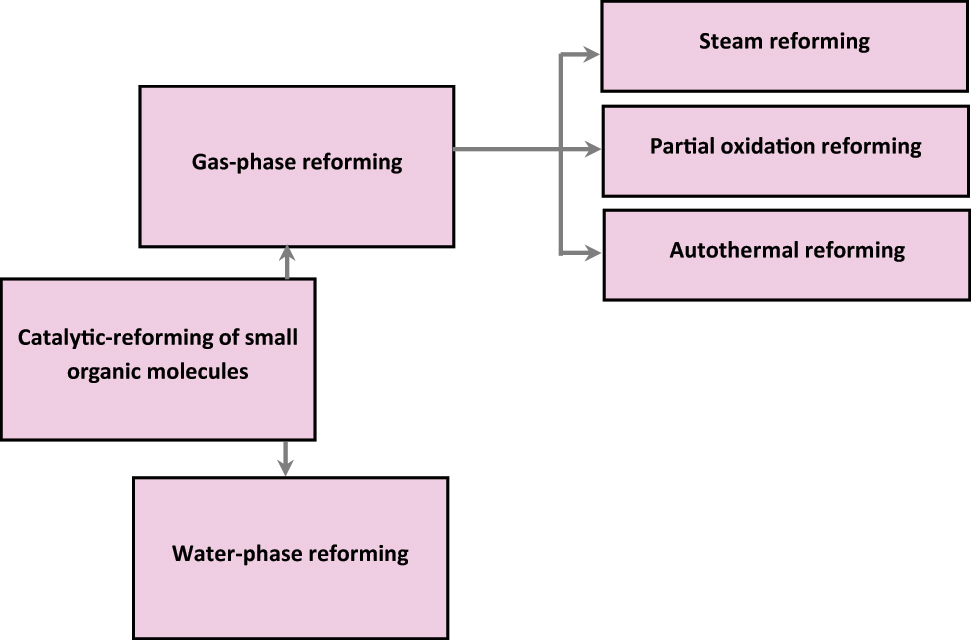
Catalytic-reforming of tiny organic compounds is classified.
Biofuel extraction from bioenergy appears as a potential option for resolving the current environmental disaster and ushering in a carbon-free future. Additionally, since this reaction is a highly endothermic process, heat from outside is needed (Xu et al. 2019b). The temperature of partial oxidation processes, which may reach more than 1000 °C, can typically be reached in fixed-bed tubular reactors operating under atmospheric pressure.
Numerous studies typically employed catalysts to lower the reaction temperatures (Brown 2001; Wang et al. 2009; Yurum 1996). According to Musso et al. (2022), hydrogen created by hydrogen production a biofuel obtained from biomass, including bio-oil, is an intriguing energy vector. The objective of this study is the synthesis of hydrogen by steam distillation of tiny organic modeling compounds found in the alkaline solution of bio-oil and, for the accuracy that is critical of a real system, the emulation of this culture supernatant by a representative combination of these compounds. Ternary combined oxides for Ni-La-Me catalysis were synthesised, characterised by X-ray diffraction, chemisorption isotherms, differential thermal, electrochemical impedance, and scanning electron microscopy, and then evaluated in steam reforming processes. Due to its inherent H2O adsorption process as well as a greater number of oxygen vacancies in its catalytic composition, the results show that it provides intriguing qualities for its possible use as an industrial catalyst for hydrogen generation through steam reforming. On the other hand, the hard metal interaction led to a decrease in catalytic activity as a result of less crystallite segregation from the nanocomposites structure. For autothermal reforming, pressure differences and temperatures ranging from 400 to 1200 °C are commonly used (Chen et al. 2010; Dauenhauer, Salge, and Schmidt 2006). Due to the Cu has high activity in water gas conversion reactions and potent anti-carbon deposition properties, it is used as a catalyst in the majority of studies (Vagia and Lemonidou 2008). Without extra heating, the autothermal reforming process may start up fast and continue indefinitely (Gallucci, Annaland, and Kuipers 2010). A number of requirements must be met for the water-phase haber process to produce hydrogen, including maintaining the raw material in an aqueous solution below 280 °C and maintaining a saturated vapor pressure just above that of water (Liu et al. 2008). Since evaporation is not required, hydrogen synthesis through the water phase is appropriate for biomass materials that are challenging to vaporize (Luo et al. 2010; Tanksale et al. 2007).
Commercialization challenges of bio-hydrogen
Hydrogen has been utilized in the refineries and industrial applications for many decades and has recently gained attention. From 2020 to 2030, the demand for hydrogen is projected to increase by around 5.48% yearly (Arun et al. 2022). To secure and keep the sustainability of hydrogen with minimal environmental effect, the generated hydrogen should include no or substantially low carbon emissions. One kilogramme of hydrogen contains the same amount of energy as one gallon of gasoline, which when burned generates 9.1 kg of carbon dioxide. Therefore, it was determined that the emissions from hydrocarbon fuels were more than those from hydrogen synthesis and the same was recommended (Jarunglumlert et al. 2018). The density of hydrogen generated from substrates is greater. The commercialisation and deployment of biofuel production on a wide scale are beset by formidable obstacles (Baeyens et al. 2020). Biohydrogen lack of distribution channels is the biggest obstacle to its commercialisation. Production, storage, transportation, distribution, and ultimate usage would be obstacles to biohydrogen commercialization.
Storage of biohydrogen
Typically, biohydrogen is maintained by decompression, cooling, or a hybrid approach. In addition to solids, liquids, and surface-based materials, material-based biohydrogen storage systems have been created. Additionally, biohydrogen may be stored locally or in bulk. Due to its low gravity, keeping biohydrogen presents difficulties since it demands a great deal of energy for compression. When storing biohydrogen in a stable state, pressure and temperature are required, and consideration must be given to design, social responsibility, legal difficulties, and economic effectiveness. Low material durability and its reactivity with chemicals pose safety risks. If biohydrogen is stored in large quantities, it could get dirty and need to be cleaned before it can be used.
Distribution and transportation
Typically, biohydrogen is carried through pipelines, high-pressure tube trucks, and liquified hydrogen tankers. When compared to other modes of transportation, pipeline transport was shown to be the least costly. Existing hydrogen transportation pipelines are inadequate to meet the demand for biohydrogen (Singh, Sarma, and Lal 2014). Due to biohydrogen inherent brittleness, existing gas pipes cannot be repurposed for its transmission. In addition, even a 5% concentration of biohydrogen in natural gas has an effect on the pipes durability (Hemmati et al. 2020). lack of hydrogen flow regulation at refuelling stations, which has an effect on absorption and system losses. Changes in temperature while transporting compressed hydrogen and the lack of recharging stations for biohydrogen are two big problems.
The use of hydrogen
Biohydrogen has various uses in the provision of energy, heating through combined heat and power units, powering telecom towers and automobile industries, etc. It is necessary to minimise the weight, quantity, and cost of pressurised hydrogen gas for cars and fuel cell installations (Walsh 2000). The efficiency, degradation problems, durability, and initial concentration of fuel cells must be enhanced, and the performances and health monitoring system must be optimised. It is necessary to increase the short run duration of fuel cells for portable electronics without altering their original dimensions.
The exergy efficiency, effective energy, production cost, of several hydrogen generation systems are compared in Table 1 (Tukenmez, Yilmaz, and Ozturk 2021). Values are standardized from 0 to 10, with 10 representing perfect performance and 0 representing subpar performance (zero cost and zero emissions).
Comparison methods for producing hydrogen (Tukenmez et al. 2021).
| Method | Exergy efficiency | Energy efficiency | Cost |
|---|---|---|---|
| Electrolysis | 2.5 | 5.3 | 7.34 |
| Plasma arc decomposition | 3.2 | 7 | 9.18 |
| Thermolysis | 4 | 5 | 6.12 |
| Thermochemical water splitting | 3 | 4.2 | 8.06 |
| Biomass conversion | 4.5 | 5.6 | 8.1 |
| Biomass gasification | 6 | 6.5 | 8.25 |
| Biomass reforming | 2.8 | 3.9 | 7.93 |
| PV electrolysis | 0.7 | 1.24 | 4.5 |
| Photocatalysis | 0.1 | 0.2 | 5.19 |
| Photoelectrochemical method | 0.15 | 0.7 | 0 |
| Dark fermentation | 1.1 | 1.3 | 7.52 |
| High-temperature electrolysis | 2.6 | 2.9 | 5.54 |
| Hybrid thermochemical cycles | 4.8 | 5.3 | 7.41 |
| Fossil fuel reforming | 4.6 | 8.3 | 9.28 |
| Biophotolysis | 1.3 | 1.4 | 7.27 |
| Photo-fermentation | 1.4 | 1.5 | 7.61 |
| Artificial photosynthesis | 0.8 | 0.9 | 7.54 |
| Photoelectrolysis | 0.34 | 0.78 | 7.09 |
The information may be summed up in accordance with the pertinent data in Table 1 and related studies: Plasma arc breakdown and hydrogen generation from fossil fuel reforming have the highest efficiency. The efficiency of producing hydrogen by means of long-term photocatalytic degradation, photocatalysis, and solar photovoltaic is generally low. However, bio-hydrogen generation has significantly increased energy efficiency.
Techno-economic evaluation of biohydrogen production
Economic analysis foretells the widespread economic demand for any technology (Hrbáčková et al. 2019). Using cost analysis, economic demand, and climatic data, is often done to assess the viability of the project (Hosseini 2022; Javaheri 2023; Závadský et al. 2019). The decision-making process for any project is straightforward when using the findings from these studies (Ondra, Tuček, and Rajnoha 2018). According to published research, the techno-economic assessment of each process was determined based on many assumptions, including degradation, income tax cost, ideal annualised, deflation, foundation discount rate, selling price, and consumables cost (Han et al. 2016; Zhang et al. 2013). Although there are various methods for predicting analytical studies, Aspen has lately attracted a lot of attention for its method of calculating the costs of capital, operations, accumulated cash flow, and profitability (Dokulil, Popesko, and Kadalová 2022). In addition to the aforementioned, the sensitivity evaluation was also carried out depending on the cost of the raw materials, the discount rates, and the biohydrogen minimum selling price (Edou and Onwudili 2022). The cost of producing biohydrogen may be used to establish the minimum selling price of any technology. For example, the processing cost is regarded as the least cost after subtracting a few allowances if the biohydrogen was produced by a thermochemical process. Based on the current pricing, the total cost must be comparable with the lowest-priced procedure on the market. Given that the cost of the raw materials constantly varies depending on the worldwide market, there needs to be some tolerance in the computation. Typically, they will maintain the tolerance at 20% while calculating. According to some notable research, anerobic fermentation and combustion are two processes that could be used to generate biohydrogen at a low cost (Hassan 2020). Additionally, the use of nanomaterials and the creation of CO2 have had an impact on the price of hydrogen (Abdulateef et al. 2021; Hassan 2021; Mahmod et al. 2021; Wagner et al. 2021). Additionally, there are certain influences on costs brought on by inflation. Feed cost increases, equipment and supplies, and labour costs are all impacted by inflation.
Conclusions
Although it is plausible that biomass gasification could result in the generation of hydrogen, the local biomass optimisation management approach based on the industry sector has received little attention. This paper presents an integrated optimization approach based upon this premise. The constitution of various biomasses paired with the gasifier at varying temperatures may have a significant effect on hydrogen generation. Consequently, it is required to anticipate their production under various gasification circumstances based on knowledge about the available biomass, which forms the basis for cost estimation. Considering the influence of local biomass on the environment and the ecology, this research also examined the return of various biomass products to the field and a generally steady supply of mixed supply circumstances using the procurement model. The inventory model was used to manage the inventory of a range of mixed biomass products in order to optimise the advantages of hydrogen generation. Due to the repetitive nature of biomass, fresh choices must be made each year for each region. As the generation of biomass hydrogen is associated with a range of byproducts, the related model for fine management should also take the costs and advantages of treating these gases into account. The key bullet points of this review are as follows:
The manufacture of hydrogen from fossil fuels is more energy and energy efficient, but at the expense of substantial environmental pollution. Despite poor energy and exergy efficiency, the electrolysis-based hydrogen manufacturing process has little environmental effect. The technique for producing hydrogen from biomass offers both benefits, namely adequate energy efficiency and reduced environmental effects.
The manufacture of bio-hydrogen may be fueled by a variety of raw sources and simultaneously create fewer pollutants. The chemical process provides a high hydrogen yield but requires a higher temperature and incurs a cost of hydrogen. The biological technologies are reliable and can make a lot of hydrogen at a high rate. It can also help meet goals for sustainable green energy.
The conventional manufacturing of hydrogen from fossil fuels creates more pollutions. The cost of producing hydrogen by electrolysis of water is significant. Bio-hydrogen generation is good for reducing carbon emissions and getting rid of carbon, but it also has a lot of room for growth.
The costs associated with liquefaction and compression for hydrogen storage are considerable. The physical hydrogen storage technique is hampered by a lack of appropriate materials and a poor level of efficiency. The bio-hydrogen storage techniques, have the benefits of high hydrogen storage effectiveness, low cost, and little environmental effect.
The fulfillment of the hydrogen economy requires the resolution of transportation concerns. The economic advantage will be enhanced if the hydrogen converting device can be installed at the hydrogen plants terminals in order to eliminate the hydrogen direct transit stage. Consequently, introducing biomass technologies into all facets of hydrogen consumption has increased economic consequences.
Future perspectives
There is broad agreement across numerous studies that using nanoparticles may greatly speed up the kinetics and yield of (bio)hydrogen generation, which provides a huge potential to lower production costs. Although there are still many obstacles to overcome, nanoscience continues to develop in a manner that will eventually make (bio)hydrogen a dependable replacement for fossil fuels. The complicated refinement of biowaste into a variety of high-value-added products and the recuperation of waste heat are prerequisites for it though.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Abbas, M. K., Q. Hassan, M. Jaszczur, Z. S. Al-Sagar, A. N. Hussain, A. Hasan, and A. Mohamad. 2022. “Energy Visibility of a Modeled Photovoltaic/diesel Generator Set Connected to the Grid.” Energy Harvesting and Systems 9 (1): 27–38, https://doi.org/10.1515/ehs-2021-0022.Suche in Google Scholar
Abdulateef, A. M., M. Jaszczur, Q. Hassan, R. Anish, H. Niyas, K. Sopian, and J. Abdulateef. 2021. “Enhancing the Melting of Phase Change Material Using a Fins–Nanoparticle Combination in a Triplex Tube Heat Exchanger.” Journal of Energy Storage 35: 102227, https://doi.org/10.1016/j.est.2020.102227.Suche in Google Scholar
Akhlaghi, N., and G. Najafpour-Darzi. 2020. “A Comprehensive Review on Biological Hydrogen Production.” International Journal of Hydrogen Energy 45 (43): 22492–512, https://doi.org/10.1016/j.ijhydene.2020.06.182.Suche in Google Scholar
Al-Alawi, M. T. 2008. “Hydrogen Production from Biomass.” In Sustainable Energy Production and Consumption, 273–80. Dordrecht: Springer.10.1007/978-1-4020-8494-2_18Suche in Google Scholar
Ansari, M. H., S. Jafarian, A. Tavasoli, A. Karimi, and M. Rashidi. 2014. “Hydrogen Rich Gas Production via Nano-Catalytic Pyrolysis of Bagasse in a Dual Bed Reactor.” Journal of Natural Gas Science and Engineering 19: 279–86.10.1016/j.jngse.2014.05.018Suche in Google Scholar
Argun, H., and F. Kargi. 2011. “Bio-hydrogen Production by Different Operational Modes of Dark and Photo-Fermentation: An Overview.” International Journal of Hydrogen Energy 36 (13): 7443–59, https://doi.org/10.1016/j.ijhydene.2011.03.116.Suche in Google Scholar
Argun, H., and F. Kargi. 2011. “Bio-Hydrogen Production by Different Operational Modes of Dark and Photo-Fermentation: An Overview.” International Journal of Hydrogen Energy 36 (13): 7443–59, https://doi.org/10.1016/j.ijhydene.2011.03.116.Suche in Google Scholar
Arun, J., T. Sasipraba, K. P. Gopinath, P. Priyadharsini, S. Nachiappan, N. Nirmala, and A. Pugazhendhi. 2022. “Influence of Biomass and Nanoadditives in Dark Fermentation for Enriched Bio-Hydrogen Production: A Detailed Mechanistic Review on Pathway and Commercialization Challenges.” Fuel 327: 125112, https://doi.org/10.1016/j.fuel.2022.125112.Suche in Google Scholar
Asada, T., P. Chen, C. Chiarella, and P. Flaschel. 2006. “Keynesian Dynamics and the Wage–Price Spiral: A Baseline Disequilibrium Model.” Journal of Macroeconomics 28 (1): 90–130, https://doi.org/10.1016/j.jmacro.2005.10.007.Suche in Google Scholar
Asada, Y., M. Tokumoto, Y. Aihara, M. Oku, K. Ishimi, T. Wakayama, and H. Kohno. 2006. “Hydrogen Production by Co-cultures of Lactobacillus and a Photosynthetic Bacterium, Rhodobacter Sphaeroides RV.” International Journal of Hydrogen Energy 31 (11): 1509–13, https://doi.org/10.1016/j.ijhydene.2006.06.017.Suche in Google Scholar
Assawamongkholsiri, T., A. Reungsang, P. Plangkang, and S. Sittijunda. 2018. “Repeated Batch Fermentation for Photo-Hydrogen and Lipid Production from Wastewater of a Sugar Manufacturing Plant.” International Journal of Hydrogen Energy 43 (7): 3605–17, https://doi.org/10.1016/j.ijhydene.2017.12.119.Suche in Google Scholar
Baeyens, J., H. Zhang, J. Nie, L. Appels, R. Dewil, R. Ansart, and Y. Deng. 2020. “Reviewing the Potential of Bio-Hydrogen Production by Fermentation.” Renewable and Sustainable Energy Reviews 131: 110023, https://doi.org/10.1016/j.rser.2020.110023.Suche in Google Scholar
Balat, M. 2010. “Thermochemical Routes for Biomass-Based Hydrogen Production.” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 32 (15): 1388–98, https://doi.org/10.1080/15567030802706796.Suche in Google Scholar
Barbarias, I., M. Artetxe, G. Lopez, A. Arregi, L. Santamaria, J. Bilbao, and M. Olazar. 2019. “Catalyst Performance in the HDPE Pyrolysis-Reforming under Reaction-Regeneration Cycles.” Catalysts 9 (5): 414, https://doi.org/10.3390/catal9050414.Suche in Google Scholar
BEIKIN, S. 1978. “Hydrogen Metabolism in the Facultative Anoxygenic Cyanobacteria Oscillatoria Limnetica and Aphanothece Halophytica.” Archives of Microbiology 116: 109–11.10.1007/BF00408741Suche in Google Scholar PubMed
Brennan, L., and P. Owende. 2010. “Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-products.” Renewable and Sustainable Energy Reviews 14 (2): 557–77, https://doi.org/10.1016/j.rser.2009.10.009.Suche in Google Scholar
Brown, L. F.. 2001. “A Comparative Study of Fuels for On-Board Hydrogen Production for Fuel-Cell-Powered Automobiles.” International Journal of Hydrogen Energy 26 (4): 381–97.10.1016/S0360-3199(00)00092-6Suche in Google Scholar
Byun, Y., M. Cho, J. W. Chung, W. Namkung, H. D. Lee, S. D. Jang, and S. M. Hwang. 2011. “Hydrogen Recovery from the Thermal Plasma Gasification of Solid Waste.” Journal of Hazardous Materials 190 (1–3): 317–23, https://doi.org/10.1016/j.jhazmat.2011.03.052.Suche in Google Scholar PubMed
Ceran, B., A. Mielcarek, Q. Hassan, J. Teneta, and M. Jaszczur. 2021. “Aging Effects on Modelling and Operation of a Photovoltaic System with Hydrogen Storage.” Applied Energy 297: 117161, https://doi.org/10.1016/j.apenergy.2021.117161.Suche in Google Scholar
Chen, C. C., and C. Y. Lin. 2003. “Using Sucrose as a Substrate in an Anaerobic Hydrogen-Producing Reactor.” Advances in Environmental Research 7 (3): 695–9, https://doi.org/10.1016/s1093-0191(02)00035-7.Suche in Google Scholar
Chen, W. H., M. R. Lin, J. J. Lu, Y. Chao, and T. S. Leu. 2010. “Thermodynamic Analysis of Hydrogen Production from Methane via Autothermal Reforming and Partial Oxidation Followed by Water Gas Shift Reaction.” International Journal of Hydrogen Energy 35 (21): 11787–97, https://doi.org/10.1016/j.ijhydene.2010.08.126.Suche in Google Scholar
Chen, G., I. A. Jamro, S. R. Samo, T. Wenga, H. A. Baloch, B. Yan, and W. Ma. 2020. “Hydrogen-rich Syngas Production from Municipal Solid Waste Gasification through the Application of Central Composite Design: An Optimization Study.” International Journal of Hydrogen Energy 45 (58): 33260–73, https://doi.org/10.1016/j.ijhydene.2020.09.118.Suche in Google Scholar
Cummer, K., and R. C. Brown. 2005. “Indirectly Heated Biomass Gasification Using a Latent-Heat Ballast—Part 3: Refinement of the Heat Transfer Model.” Biomass and Bioenergy 28 (3): 321–30, https://doi.org/10.1016/j.biombioe.2004.06.009.Suche in Google Scholar
Dauenhauer, P. J., J. R. Salge, and L. D. Schmidt. 2006. “Renewable Hydrogen by Autothermal Steam Reforming of Volatile Carbohydrates.” Journal of Catalysis 244 (2): 238–47, https://doi.org/10.1016/j.jcat.2006.09.011.Suche in Google Scholar
Dawood, F., M. Anda, and G. M. Shafiullah. 2020. “Hydrogen Production for Energy: An Overview.” International Journal of Hydrogen Energy 45 (7): 3847–69, https://doi.org/10.1016/j.ijhydene.2019.12.059.Suche in Google Scholar
de Jong, W. 2008. “Sustainable Hydrogen Production by Thermochemical Biomass Processing.” In Hydrogen Fuel, 197–238. CRC Press.10.1201/9781420045772.ch6Suche in Google Scholar
Delavar, M. A., and J. Wang. 2021. “Numerical Investigation of pH Control on Dark Fermentation and Hydrogen Production in a Microbioreactor.” Fuel 292: 120355.10.1016/j.fuel.2021.120355Suche in Google Scholar
Dessì, P., F. Asunis, H. Ravishankar, F. G. Cocco, G. De Gioannis, A. Muntoni, and P. N. Lens. 2020. “Fermentative Hydrogen Production from Cheese Whey with In-Line, Concentration Gradient-Driven Butyric Acid Extraction.” International Journal of Hydrogen Energy 45 (46): 24453–66.10.1016/j.ijhydene.2020.06.081Suche in Google Scholar
Dinesh, G. H., D. D. Nguyen, B. Ravindran, S. W. Chang, D. V. N. Vo, Q. V. Bach, and A. Arun. 2020. “Simultaneous Biohydrogen (H2) and Bioplastic (Poly-β-Hydroxybutyrate-PHB) Productions under Dark, Photo, and Subsequent Dark and Photo Fermentation Utilizing Various Wastes.” International Journal of Hydrogen Energy 45 (10): 5840–53, https://doi.org/10.1016/j.ijhydene.2019.09.036.Suche in Google Scholar
Dokulil, J., B. Popesko, and K. Kadalová. 2022. Factors with a Major Effect on the Budgetary Control Process–An Empirical Study from the Czech Republic. Amfiteatru Economic.10.24818/EA/2022/59/235Suche in Google Scholar
Edou, D. J. N., and J. A. Onwudili. 2022. “Comparative Techno-Economic Modelling of Large-Scale Thermochemical Biohydrogen Production Technologies to Fuel Public Buses: A Case Study of West Midlands Region of England.” Renewable Energy 189: 704–16.10.1016/j.renene.2022.02.074Suche in Google Scholar
Fang, H. H., and H. Liu. 2002. “Effect of pH on Hydrogen Production from Glucose by a Mixed Culture.” Bioresource Technology 82 (1): 87–93, https://doi.org/10.1016/s0960-8524(01)00110-9.Suche in Google Scholar PubMed
Foong, S. Y., Y. H. Chan, W. Y. Cheah, N. H. Kamaludin, T. N. B. T. Ibrahim, C. Sonne, and S. S. Lam. 2021. “Progress in Waste Valorization Using Advanced Pyrolysis Techniques for Hydrogen and Gaseous Fuel Production.” Bioresource Technology 320: 124299, https://doi.org/10.1016/j.biortech.2020.124299.Suche in Google Scholar PubMed
Gaffron, H., and J. Rubin. 1942. “Fermentative and Photochemical Production of Hydrogen in Algae.” The Journal of General Physiology 26 (2): 219–40, https://doi.org/10.1085/jgp.26.2.219.Suche in Google Scholar PubMed PubMed Central
Gallagher, M. J., C. Allen, J. T. Buchman, T. A. Qiu, P. L. Clement, M. O. Krause, and L. M. Gilbertson. 2017. “Research Highlights: Applications of Life-Cycle Assessment as a Tool for Characterizing Environmental Impacts of Engineered Nanomaterials.” Environmental Sciences: Nano 4 (2): 276–81, https://doi.org/10.1039/c7en90005h.Suche in Google Scholar
Gallucci, F., M. V. S. Annaland, and J. A. M. Kuipers. 2010. “Pure Hydrogen Production via Autothermal Reforming of Ethanol in a Fluidized Bed Membrane Reactor: A Simulation Study.” International Journal of Hydrogen Energy 35 (4): 1659–68, https://doi.org/10.1016/j.ijhydene.2009.12.014.Suche in Google Scholar
Ganzoury, M. A., and N. K. Allam. 2015. “Impact of Nanotechnology on Biogas Production: A Mini-Review.” Renewable and Sustainable Energy Reviews 50: 1392–404, https://doi.org/10.1016/j.rser.2015.05.073.Suche in Google Scholar
Ge, Z., L. Guo, and H. Jin. 2020. “Catalytic Supercritical Water Gasification Mechanism of Coal.” International Journal of Hydrogen Energy 45 (16): 9504–11, https://doi.org/10.1016/j.ijhydene.2020.01.245.Suche in Google Scholar
Ghirardi, M. L., P. W. King, D. W. Mulder, C. Eckert, A. Dubini, P. C. Maness, and J. Yu. 2014. “Hydrogen Production by Water Biophotolysis.” In Microbial BioEnergy: Hydrogen Production, 101–35. Dordrecht: Springer.10.1007/978-94-017-8554-9_5Suche in Google Scholar
Greenbaum, E. 1982. “Photosynthetic Hydrogen and Oxygen Production: Kinetic Studies.” Science 215 (4530): 291–3, https://doi.org/10.1126/science.215.4530.291.Suche in Google Scholar PubMed
Guo, S., L. Guo, C. Cao, J. Yin, Y. Lu, and X. Zhang. 2012. “Hydrogen Production from Glycerol by Supercritical Water Gasification in a Continuous Flow Tubular Reactor.” International Journal of Hydrogen Energy 37 (7): 5559–68, https://doi.org/10.1016/j.ijhydene.2011.12.135.Suche in Google Scholar
Guo, J. X., X. Tan, K. Zhu, and B. Gu. 2022. “Integrated Management of Mixed Biomass for Hydrogen Production from Gasification.” Chemical Engineering Research and Design 179: 41–55, https://doi.org/10.1016/j.cherd.2022.01.012.Suche in Google Scholar
Hamad, M. A., A. M. Radwan, D. A. Heggo, and T. Moustafa. 2016. “Hydrogen Rich Gas Production from Catalytic Gasification of Biomass.” Renewable Energy 85: 1290–300, https://doi.org/10.1016/j.renene.2015.07.082.Suche in Google Scholar
Han, W., Y. Y. Hu, S. Y. Li, F. F. Li, and J. H. Tang. 2016. “Biohydrogen Production from Waste Bread in a Continuous Stirred Tank Reactor: A Techno-Economic Analysis.” Bioresource Technology 221: 318–23, https://doi.org/10.1016/j.biortech.2016.09.055.Suche in Google Scholar PubMed
Hassan, Q. 2020. “Optimisation of Solar-Hydrogen Power System for Household Applications.” International Journal of Hydrogen Energy 45 (58): 33111–27, https://doi.org/10.1016/j.ijhydene.2020.09.103.Suche in Google Scholar
Hassan, Q. 2021. “Evaluation and Optimization of Off-Grid and On-Grid Photovoltaic Power System for Typical Household Electrification.” Renewable Energy 164: 375–90, https://doi.org/10.1016/j.renene.2020.09.008.Suche in Google Scholar
Hassan, Q. 2022. “Evaluate the Adequacy of Self-Consumption for Sizing Photovoltaic System.” Energy Reports 8: 239–54, https://doi.org/10.1016/j.egyr.2021.11.205.Suche in Google Scholar
Hassan, Q., and M. Jaszczur. 2021. “Self-Consumption and Self-Sufficiency Improvement for Photovoltaic System Integrated with Ultra-supercapacitor.” Energies 14 (23): 7888, https://doi.org/10.3390/en14237888.Suche in Google Scholar
Hassan, Q., M. Jaszczur, A. M. Abdulateef, J. Abdulateef, A. Hasan, and A. Mohamad. 2022a. “An Analysis of Photovoltaic/supercapacitor Energy System for Improving Self-Consumption and Self-Sufficiency.” Energy Reports 8: 680–95, https://doi.org/10.1016/j.egyr.2021.12.021.Suche in Google Scholar
Hassan, Q., M. Jaszczur, S. A. Hafedh, M. K. Abbas, A. M. Abdulateef, A. Hasan, and A. Mohamad. 2022b. “Optimizing a Microgrid Photovoltaic-Fuel Cell Energy System at the Highest Renewable Fraction.” International Journal of Hydrogen Energy 47 (28): 13710–31, https://doi.org/10.1016/j.ijhydene.2022.02.108.Suche in Google Scholar
Hassan, Q., B. Pawela, A. Hasan, and M. Jaszczur. 2022c. “Optimization of Large-Scale Battery Storage Capacity in Conjunction with Photovoltaic Systems for Maximum Self-Sustainability.” Energies 15 (10): 3845, https://doi.org/10.3390/en15103845.Suche in Google Scholar
Hassan, Q., M. Jaszczur, J. Teneta, M. K. Abbas, A. Hasan, and A. K. Al-Jiboory. 2022d. “Experimental Investigation for the Estimation of the Intensity of Solar Irradiance on Oblique Surfaces by Means of Various Models.” Energy Harvesting and Systems, https://doi.org/10.1515/ehs-2021-0087.Suche in Google Scholar
Hassan, Q., M. Jaszczur, A. K. Al-Jiboory, A. Hasan, and A. Mohamad. 2022e. “Optimizing of Hybrid Renewable Photovoltaic/wind Turbine/super Capacitor for Improving Self-Sustainability.” Energy Harvesting and Systems, https://doi.org/10.1515/ehs-2021-0095.Suche in Google Scholar
Hassan, Q., S. A. Hafedh, A. Hasan, and M. Jaszczur. 2022f. “Evaluation of Energy Generation in Iraqi Territory by Solar Photovoltaic Power Plants with a Capacity of 20 MW.” Energy Harvesting and Systems.10.1515/ehs-2021-0075Suche in Google Scholar
Hawkes, F. R., R. Dinsdale, D. L. Hawkes, and I. Hussy. 2002. “Sustainable Fermentative Hydrogen Production: Challenges for Process Optimisation.” International Journal of Hydrogen Energy 27 (11–12): 1339–47, https://doi.org/10.1016/s0360-3199(02)00090-3.Suche in Google Scholar
Hemmati, S., M. M. Elnegihi, C. H. Lee, D. Y. L. Chong, D. C. Foo, B. S. How, and C. Yoo. 2020. “Synthesis of Large-Scale Bio-Hydrogen Network Using Waste Gas from Landfill and Anaerobic Digestion: A P-Graph Approach.” Processes 8 (5): 505, https://doi.org/10.3390/pr8050505.Suche in Google Scholar
Hossain, M. Z., M. B. Chowdhury, and P. A. Charpentier. 2019. “Effect of Surface Acidity of Al2O3 Supported Metal Catalysts on Catalytic Activity and Carbon Deposition during SCWG of Glucose.” Biomass and Bioenergy 124: 142–50, https://doi.org/10.1016/j.biombioe.2019.04.005.Suche in Google Scholar
Hosseini, S. E. 2022. “Hydrogen Has Found its Way to Become the Fuel of the Future.” Future Energy 1 (3): 11–2, https://doi.org/10.55670/fpll.fuen.1.3.2.Suche in Google Scholar
Hosseinzadeh, A., J. L. Zhou, A. Altaee, M. Baziar, and D. Li. 2020. “Effective Modelling of Hydrogen and Energy Recovery in Microbial Electrolysis Cell by Artificial Neural Network and Adaptive Network-Based Fuzzy Inference System.” Bioresource Technology 316: 123967, https://doi.org/10.1016/j.biortech.2020.123967.Suche in Google Scholar PubMed
Hosseinzadeh, A., J. L. Zhou, A. H. Navidpour, and A. Altaee. 2021. “Progress in Osmotic Membrane Bioreactors Research: Contaminant Removal, Microbial Community and Bioenergy Production in Wastewater.” Bioresource Technology 330: 124998, https://doi.org/10.1016/j.biortech.2021.124998.Suche in Google Scholar PubMed
Hrbáčková, L., A. Stojanović, D. Tuček, and D. Hrušecká. 2019. “Environmental Aspects of Product Life Cycle Management and Purchasing Logistics: Current Situation in Large and Medium-Sized Czech Manufacturing Companies.” Acta Polytechnica Hungarica 16 (7): 79–94.10.12700/APH.16.7.2019.7.5Suche in Google Scholar
Huang, Y., N. C. Surawski, B. Organ, J. L. Zhou, O. H. Tang, and E. F. Chan. 2019. “Fuel Consumption and Emissions Performance under Real Driving: Comparison between Hybrid and Conventional Vehicles.” Science of the Total Environment 659: 275–82, https://doi.org/10.1016/j.scitotenv.2018.12.349.Suche in Google Scholar PubMed
Huang, J., C. Zhu, X. Lian, H. Feng, J. Sun, L. Wang, and H. Jin. 2019. “Catalytic Supercritical Water Gasification of Glucose with In-Situ Generated Nickel Nanoparticles for Hydrogen Production.” International Journal of Hydrogen Energy 44 (38): 21020–9, https://doi.org/10.1016/j.ijhydene.2019.04.184.Suche in Google Scholar
Huang, Y., N. C. Surawski, Y. Zhuang, J. L. Zhou, and G. Hong. 2021. “Dual Injection: An Effective and Efficient Technology to Use Renewable Fuels in Spark Ignition Engines.” Renewable and Sustainable Energy Reviews 143: 110921, https://doi.org/10.1016/j.rser.2021.110921.Suche in Google Scholar
Jalan, R. K., and V. K. Srivastava. 1999. “Studies on Pyrolysis of a Single Biomass Cylindrical Pellet—Kinetic and Heat Transfer Effects.” Energy Conversion and Management 40 (5): 467–94, https://doi.org/10.1016/s0196-8904(98)00099-5.Suche in Google Scholar
Jalan, R. K., and V. K. Srivastava. 1999. “Studies on Pyrolysis of a Single Biomass Cylindrical Pellet—Kinetic and Heat Transfer Effects.” Energy Conversion and Management 40 (5): 467–94, https://doi.org/10.1016/s0196-8904(98)00099-5.Suche in Google Scholar
Jarunglumlert, T., C. Prommuak, N. Putmai, and P. Pavasant. 2018. “Scaling-up Bio-Hydrogen Production from Food Waste: Feasibilities and Challenges.” International Journal of Hydrogen Energy 43 (2): 634–48, https://doi.org/10.1016/j.ijhydene.2017.10.013.Suche in Google Scholar
Jaszczur, M., and Q. Hassan. 2020. “An Optimisation and Sizing of Photovoltaic System with Supercapacitor for Improving Self-Consumption.” Applied Energy 279: 115776, https://doi.org/10.1016/j.apenergy.2020.115776.Suche in Google Scholar
Jaszczur, M., Q. Hassan, P. Palej, and J. Abdulateef. 2020. “Multi-Objective Optimisation of a Micro-grid Hybrid Power System for Household Application.” Energy 202: 117738, https://doi.org/10.1016/j.energy.2020.117738.Suche in Google Scholar
Javaheri, V.. 2023. “Steel Pipeline for the Hydrogen Storage and Delivery: Metallurgical Viewpoint for Finnish Ecosystem.” Future Technology 2 (1): 58–61, https://doi.org/10.55670/fpll.futech.2.1.4.Suche in Google Scholar
Kang, K., R. Azargohar, A. K. Dalai, and H. Wang. 2016. “Hydrogen Production from Lignin, Cellulose and Waste Biomass via Supercritical Water Gasification: Catalyst Activity and Process Optimization Study.” Energy Conversion and Management 117: 528–37, https://doi.org/10.1016/j.enconman.2016.03.008.Suche in Google Scholar
Kapdan, I. K., F. Kargi, R. Oztekin, and H. Argun. 2009. “Bio-Hydrogen Production from Acid Hydrolyzed Wheat Starch by Photo-Fermentation Using Different Rhodobacter Sp.” International Journal of Hydrogen Energy 34 (5): 2201–7, https://doi.org/10.1016/j.ijhydene.2009.01.017.Suche in Google Scholar
Karellas, S. 2015. “Hydrogen Production from Biomass Gasification.” In Production of Hydrogen from Renewable Resources, 97–117. Dordrecht: Springer.10.1007/978-94-017-7330-0_4Suche in Google Scholar
Kawagoshi, Y., Y. Oki, I. Nakano, A. Fujimoto, and H. Takahashi. 2010. “Biohydrogen Production by Isolated Halotolerant Photosynthetic Bacteria Using Long-Wavelength Light-Emitting Diode (LW-LED).” International Journal of Hydrogen Energy 35 (24): 13365–9, https://doi.org/10.1016/j.ijhydene.2009.11.121.Suche in Google Scholar
Kessler, E. 1973. “Effect of Anaerobiosis on Photosynthetic Reactions and Nitrogen Metabolism of Algae with and without Hydrogenase.” Archiv für Mikrobiologie 93 (2): 91–100, https://doi.org/10.1007/bf00424940.Suche in Google Scholar
Khlaifat, N., A. Altaee, J. Zhou, Y. Huang, and A. Braytee. 2020. “Optimization of a Small Wind Turbine for a Rural Area: A Case Study of Deniliquin, New South Wales, Australia.” Energies 13 (9): 2292, https://doi.org/10.3390/en13092292.Suche in Google Scholar
Kıpçak, E., and M. Akgün. 2015. “Hydrogen Production by Supercritical Water Gasification of Biomass.” In Production of Hydrogen from Renewable Resources, 179–220. Dordrecht: Springer.10.1007/978-94-017-7330-0_7Suche in Google Scholar
Kothari, R., D. Buddhi, and R. L. Sawhney. 2008. “Comparison of Environmental and Economic Aspects of Various Hydrogen Production Methods.” Renewable and Sustainable Energy Reviews 12 (2): 553–63, https://doi.org/10.1016/j.rser.2006.07.012.Suche in Google Scholar
Kruse, A., and E. Dinjus. 2005. “Influence of Salts during Hydrothermal Biomass Gasification: The Role of the Catalysed Water-Gas Shift Reaction.” Zeitschrift für Physikalische Chemie 219 (3): 341–66, https://doi.org/10.1524/zpch.219.3.341.59177.Suche in Google Scholar
Kumar, N., and D. Das. 2000. “Enhancement of Hydrogen Production by Enterobacter cloacae IIT-BT 08.” Process Biochemistry 35 (6): 589–93, https://doi.org/10.1016/s0032-9592(99)00109-0.Suche in Google Scholar
Kumar, G., G. Zhen, T. Kobayashi, P. Sivagurunathan, S. H. Kim, and K. Q. Xu. 2016. “Impact of pH Control and Heat Pre-treatment of Seed Inoculum in Dark H2 Fermentation: A Feasibility Report Using Mixed Microalgae Biomass as Feedstock.” International Journal of Hydrogen Energy 41 (7): 4382–92, https://doi.org/10.1016/j.ijhydene.2015.08.069.Suche in Google Scholar
Kumar, G., P. Sivagurunathan, A. Pugazhendhi, N. B. D. Thi, G. Zhen, K. Chandrasekhar, and A. Kadier. 2017. “A Comprehensive Overview on Light Independent Fermentative Hydrogen Production from Wastewater Feedstock and Possible Integrative Options.” Energy Conversion and Management 141: 390–402, https://doi.org/10.1016/j.enconman.2016.09.087.Suche in Google Scholar
Kumar, R., A. Kumar, and A. Pal. 2021. “An Overview of Conventional and Non-conventional Hydrogen Production Methods.” Materials Today Proceedings 46: 5353–9, https://doi.org/10.1016/j.matpr.2020.08.793.Suche in Google Scholar
Lepage, T., M. Kammoun, Q. Schmetz, and A. Richel. 2021. “Biomass-to-hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment.” Biomass and Bioenergy 144: 105920, https://doi.org/10.1016/j.biombioe.2020.105920.Suche in Google Scholar
Li, S., and L. Guo. 2019. “Stability and Activity of a Co-precipitated Mg Promoted Ni/Al2O3 Catalyst for Supercritical Water Gasification of Biomass.” International Journal of Hydrogen Energy 44 (30): 15842–52, https://doi.org/10.1016/j.ijhydene.2018.08.205.Suche in Google Scholar
Li, Y. F., N. Q. Ren, Y. Chen, and G. X. Zheng. 2007. “Ecological Mechanism of Fermentative Hydrogen Production by Bacteria.” International Journal of Hydrogen Energy 32 (6): 755–60, https://doi.org/10.1016/j.ijhydene.2006.08.004.Suche in Google Scholar
Lin, C. Y., C. H. Lay, B. Sen, C. Y. Chu, G. Kumar, C. C. Chen, and J. S. Chang. 2012. “Fermentative Hydrogen Production from Wastewaters: A Review and Prognosis.” International Journal of Hydrogen Energy 37 (20): 15632–42, https://doi.org/10.1016/j.ijhydene.2012.02.072.Suche in Google Scholar
Lipman, T. 2011. “An Overview of Hydrogen Production and Storage Systems with Renewable Hydrogen Case Studies.” Clean Energy States Alliance 32.Suche in Google Scholar
Liu, S., K. Zhang, L. Fang, and Y. Li. 2008. “Thermodynamic Analysis of Hydrogen Production from Oxidative Steam Reforming of Ethanol.” Energy and Fuels 22 (2): 1365–70, https://doi.org/10.1021/ef700614d.Suche in Google Scholar
Llama, M. J., J. L. Serra, K. K. Rao, and D. O. Hall. 1979. “Isolation and Characterization of the Hydrogenase Activity from the Non-heterocystous Cyanobacterium Spirulina Maxima.” FEBS Letters 98 (2): 342–6, https://doi.org/10.1016/0014-5793(79)80213-6.Suche in Google Scholar PubMed
Lo, Y. C., S. D. Chen, C. Y. Chen, T. I. Huang, C. Y. Lin, and J. S. Chang. 2008. “Combining Enzymatic Hydrolysis and Dark–Photo Fermentation Processes for Hydrogen Production from Starch Feedstock: A Feasibility Study.” International Journal of Hydrogen Energy 33 (19): 5224–33, https://doi.org/10.1016/j.ijhydene.2008.05.014.Suche in Google Scholar
Lu, L., D. Xing, B. Liu, and N. Ren. 2012. “Enhanced Hydrogen Production from Waste Activated Sludge by Cascade Utilization of Organic Matter in Microbial Electrolysis Cells.” Water Research 46 (4): 1015–26, https://doi.org/10.1016/j.watres.2011.11.073.Suche in Google Scholar PubMed
Luo, N., K. Ouyang, F. Cao, and T. Xiao. 2010. “Hydrogen Generation from Liquid Reforming of Glycerin over Ni–Co Bimetallic Catalyst.” Biomass and Bioenergy 34 (4): 489–95, https://doi.org/10.1016/j.biombioe.2009.12.013.Suche in Google Scholar
Luo, S., J. Fu, Y. Zhou, and C. Yi. 2017. “The Production of Hydrogen-Rich Gas by Catalytic Pyrolysis of Biomass Using Waste Heat from Blast-Furnace Slag.” Renewable Energy 101: 1030–6, https://doi.org/10.1016/j.renene.2016.09.072.Suche in Google Scholar
Mahmod, S. S., J. M. Jahim, P. M. Abdul, A. A. I. Luthfi, and M. S. Takriff. 2021. “Techno-economic Analysis of Two-Stage Anaerobic System for Biohydrogen and Biomethane Production from Palm Oil Mill Effluent.” Journal of Environmental Chemical Engineering 9 (4): 105679, https://doi.org/10.1016/j.jece.2021.105679.Suche in Google Scholar
Maione, T. E., and M. Gibbs. 1986. “Hydrogenase-mediated Activities in Isolated Chloroplasts of Chlamydomonas Reinhardii.” Plant Physiology 80 (2): 360–3, https://doi.org/10.1104/pp.80.2.360.Suche in Google Scholar PubMed PubMed Central
Melo, M. D. O., and L. A. Silva. 2011. “Photocatalytic Production of Hydrogen: An Innovative Use for Biomass Derivatives.” Journal of the Brazilian Chemical Society 22: 1399–406, https://doi.org/10.1590/s0103-50532011000800002.Suche in Google Scholar
Mishra, P., S. Krishnan, S. Rana, L. Singh, M. Sakinah, and Z. Ab Wahid. 2019. “Outlook of Fermentative Hydrogen Production Techniques: An Overview of Dark, Photo and Integrated Dark-Photo Fermentative Approach to Biomass.” Energy Strategy Reviews 24: 27–37, https://doi.org/10.1016/j.esr.2019.01.001.Suche in Google Scholar
Momirlan, M., and T. Veziroǧlu. 1999. “Recent Directions of World Hydrogen Production.” Renewable and Sustainable Energy Reviews 3 (2–3): 219–31, https://doi.org/10.1016/s1364-0321(98)00017-3.Suche in Google Scholar
Musso, M., S. Veiga, F. Perdomo, T. Rodríguez, N. Mazzei, B. Decarlini, and J. Bussi. 2022. “Hydrogen Production via Steam Reforming of Small Organic Compounds Present in the Aqueous Fraction of Bio-Oil over Ni-La-Me Catalysts (Me= Ce, Ti, Zr),” Biomass Conversion and Biorefinery 1–17.10.1007/s13399-021-02296-xSuche in Google Scholar
Nanda, S., S. N. Reddy, A. K. Dalai, and J. A. Kozinski. 2016. “Subcritical and Supercritical Water Gasification of Lignocellulosic Biomass Impregnated with Nickel Nanocatalyst for Hydrogen Production.” International Journal of Hydrogen Energy 41 (9): 4907–21, https://doi.org/10.1016/j.ijhydene.2015.10.060.Suche in Google Scholar
Nandi, R., and S. Sengupta. 1998. “Microbial Production of Hydrogen: An Overview.” Critical Reviews in Microbiology 24 (1): 61–84, https://doi.org/10.1080/10408419891294181.Suche in Google Scholar PubMed
Nath, K., and D. Das. 2004. “Improvement of Fermentative Hydrogen Production: Various Approaches.” Applied Microbiology and Biotechnology 65 (5): 520–9, https://doi.org/10.1007/s00253-004-1644-0.Suche in Google Scholar PubMed
Nath, K., A. Kumar, and D. Das. 2005. “Hydrogen Production by Rhodobacter Sphaeroides Strain OU 001 Using Spent Media of Enterobacter cloacae Strain DM11.” Applied Microbiology and Biotechnology 68 (4): 533–41, https://doi.org/10.1007/s00253-005-1887-4.Suche in Google Scholar PubMed
Navarro, R. M., M. C. Sanchez-Sanchez, M. C. Alvarez-Galvan, F. Del Valle, and J. L. G. Fierro. 2009. “Hydrogen Production from Renewable Sources: Biomass and Photocatalytic Opportunities.” Energy & Environmental Science 2 (1): 35–54, https://doi.org/10.1039/b808138g.Suche in Google Scholar
Oh, Y. K., E. H. Seol, J. R. Kim, and S. Park. 2003. “Fermentative Biohydrogen Production by a New Chemoheterotrophic Bacterium Citrobacter Sp. Y19.” International Journal of Hydrogen Energy 28 (12): 1353–9, https://doi.org/10.1016/s0360-3199(03)00024-7.Suche in Google Scholar
Okolie, J. A., S. Nanda, A. K. Dalai, F. Berruti, and J. A. Kozinski. 2020. “A Review on Subcritical and Supercritical Water Gasification of Biogenic, Polymeric and Petroleum Wastes to Hydrogen-Rich Synthesis Gas.” Renewable and Sustainable Energy Reviews 119: 109546, https://doi.org/10.1016/j.rser.2019.109546.Suche in Google Scholar
Ondra, P., D. Tuček, and R. Rajnoha. 2018. “The Empirical Quality Management Practices Study of Industrial Companies in the Czech Republic.” Polish Journal of Management Studies 17, https://doi.org/10.17512/pjms.2018.17.2.16.Suche in Google Scholar
Pareek, A., R. Dom, J. Gupta, J. Chandran, V. Adepu, and P. H. Borse. 2020. “Insights into Renewable Hydrogen Energy: Recent Advances and Prospects.” Materials Science for Energy Technologies 3: 319–27, https://doi.org/10.1016/j.mset.2019.12.002.Suche in Google Scholar
Pei, A., R. Zhang, H. Jin, and L. Guo. 2008. “Screening and Study of Catalysts for Hydrogen Production by Peanut Shell Gasification in Supercritical Water.” Journal of Xi’an Jiaotong University.Suche in Google Scholar
Peng, K., G. Morrow, Z. Xiaolei, W. Tipeng, Z. Tan, and J. Agarwal. 2017. “Systematic Comparison of Hydrogen Production from Fossil Fuels and Biomass Resources.” International Journal of Agricultural and Biological Engineering 10 (6): 192–200, https://doi.org/10.25165/j.ijabe.20171006.2990.Suche in Google Scholar
Puig-Arnavat, M., J. C. Bruno, and A. Coronas. 2010. “Review and Analysis of Biomass Gasification Models.” Renewable and Sustainable Energy Reviews 14 (9): 2841–51, https://doi.org/10.1016/j.rser.2010.07.030.Suche in Google Scholar
Qinglan, H., W. Chang, L. Dingqiang, W. Yao, L. Dan, and L. Guiju. 2010. “Production of Hydrogen-Rich Gas from Plant Biomass by Catalytic Pyrolysis at Low Temperature.” International Journal of Hydrogen Energy 35 (17): 8884–90, https://doi.org/10.1016/j.ijhydene.2010.06.039.Suche in Google Scholar
Rai, P. K., and S. P. Singh. 2016. “Integrated Dark-And Photo-Fermentation: Recent Advances and Provisions for Improvement.” International Journal of Hydrogen Energy 41 (44): 19957–71, https://doi.org/10.1016/j.ijhydene.2016.08.084.Suche in Google Scholar
Reddy, S. N., S. Nanda, A. K. Dalai, and J. A. Kozinski. 2014. “Supercritical Water Gasification of Biomass for Hydrogen Production.” International Journal of Hydrogen Energy 39 (13): 6912–26, https://doi.org/10.1016/j.ijhydene.2014.02.125.Suche in Google Scholar
Ren, N. Q., W. Q. Guo, and B. F. Liu. 2010. “Development and Application Prospect of Bio-Hydrogen Production Technology.” Harbin Gongye Daxue Xuebao 42 (6): 855–63.Suche in Google Scholar
Ren, N., W. Guo, B. Liu, G. Cao, and J. Ding. 2011. “Biological Hydrogen Production by Dark Fermentation: Challenges and Prospects towards Scaled-Up Production.” Current Opinion in Biotechnology 22 (3): 365–70, https://doi.org/10.1016/j.copbio.2011.04.022.Suche in Google Scholar PubMed
Saxena, R. C., D. Seal, S. Kumar, and H. B. Goyal. 2008. “Thermo-Chemical Routes for Hydrogen Rich Gas from Biomass: A Review.” Renewable and Sustainable Energy Reviews 12 (7): 1909–27, https://doi.org/10.1016/j.rser.2007.03.005.Suche in Google Scholar
Sheikhdavoodi, M. J., M. Almassi, M. Ebrahimi-Nik, A. Kruse, and H. Bahrami. 2015. “Gasification of Sugarcane Bagasse in Supercritical Water; Evaluation of Alkali Catalysts for Maximum Hydrogen Production.” Journal of the Energy Institute 88 (4): 450–8, https://doi.org/10.1016/j.joei.2014.10.005.Suche in Google Scholar
Shi, Y., A. F. Yang, C. S. Cao, and B. Zhao. 2019. “Applications of MOFs: Recent Advances in Photocatalytic Hydrogen Production from Water.” Coordination Chemistry Reviews 390: 50–75, https://doi.org/10.1016/j.ccr.2019.03.012.Suche in Google Scholar
Show, K. Y., D. J. Lee, and Z. P. Zhang. 2011. “Production of Biohydrogen: Current Perspectives and Future Prospects.” Biofuels: 467–79, https://doi.org/10.1016/b978-0-12-385099-7.00021-8.Suche in Google Scholar
Sikarwar, V. S., M. Zhao, P. S. Fennell, N. Shah, and E. J. Anthony. 2017. “Progress in Biofuel Production from Gasification.” Progress in Energy and Combustion Science 61: 189–248, https://doi.org/10.1016/j.pecs.2017.04.001.Suche in Google Scholar
Singh, S., P. M. Sarma, and B. Lal. 2014. “Biohydrogen Production by Thermoanaerobacterium Thermosaccharolyticum TERI S7 from Oil Reservoir Flow Pipeline.” International Journal of Hydrogen Energy 39 (9): 4206–14, https://doi.org/10.1016/j.ijhydene.2013.12.179.Suche in Google Scholar
Stenberg, V., M. Rydén, T. Mattisson, and A. Lyngfelt. 2018. “Exploring Novel Hydrogen Production Processes by Integration of Steam Methane Reforming with Chemical-Looping Combustion (CLC-SMR) and Oxygen Carrier Aided Combustion (OCAC-SMR).” International Journal of Greenhouse Gas Control 74: 28–39, https://doi.org/10.1016/j.ijggc.2018.01.008.Suche in Google Scholar
Su, H., J. Cheng, J. Zhou, W. Song, and K. Cen. 2010. “Hydrogen Production from Water Hyacinth through Dark-And Photo-Fermentation.” International Journal of Hydrogen Energy 35 (17): 8929–37, https://doi.org/10.1016/j.ijhydene.2010.06.035.Suche in Google Scholar
Tanksale, A., Y. Wong, J. N. Beltramini, and G. Q. Lu. 2007. “Hydrogen Generation from Liquid Phase Catalytic Reforming of Sugar Solutions Using Metal-Supported Catalysts.” International Journal of Hydrogen Energy 32 (6): 717–24, https://doi.org/10.1016/j.ijhydene.2006.08.009.Suche in Google Scholar
Tao, Y., Y. Chen, Y. Wu, Y. He, and Z. Zhou. 2007. “High Hydrogen Yield from a Two-step Process of Dark-And Photo-Fermentation of Sucrose.” International Journal of Hydrogen Energy 32 (2): 200–6, https://doi.org/10.1016/j.ijhydene.2006.06.034.Suche in Google Scholar
Tukenmez, N., F. Yilmaz, and M. Ozturk. 2021. “Thermodynamic Performance Assessment of a Geothermal Energy Assisted Combined System for Liquid Hydrogen Generation.” International Journal of Hydrogen Energy 46 (57): 28995–9011, https://doi.org/10.1016/j.ijhydene.2021.02.215.Suche in Google Scholar
Turn, S., C. Kinoshita, Z. Zhang, D. Ishimura, and J. Zhou. 1998. “An Experimental Investigation of Hydrogen Production from Biomass Gasification.” International Journal of Hydrogen Energy 23 (8): 641–8, https://doi.org/10.1016/s0360-3199(97)00118-3.Suche in Google Scholar
Uddin, M. A., H. Tsuda, S. Wu, and E. Sasaoka. 2008. “Catalytic Decomposition of Biomass Tars with Iron Oxide Catalysts.” Fuel 87 (4–5): 451–9.10.1016/j.fuel.2007.06.021Suche in Google Scholar
Vagia, E. C., and A. A. Lemonidou. 2008. “Thermodynamic Analysis of Hydrogen Production via Autothermal Steam Reforming of Selected Components of Aqueous Bio-Oil Fraction.” International Journal of Hydrogen Energy 33 (10): 2489–500, https://doi.org/10.1016/j.ijhydene.2008.02.057.Suche in Google Scholar
Wagner, J., P. Petera, B. Popesko, P. Novák, and K. Šafr. 2021. “Usefulness of the Budget: The Mediating Effect of Participative Budgeting and Budget-Based Evaluation and Rewarding.” Baltic Journal of Management 16 (4): 602–20, https://doi.org/10.1108/bjm-02-2020-0049.Suche in Google Scholar
Waheed, Q. M., C. Wu, and P. T. Williams. 2016. “Pyrolysis/reforming of Rice Husks with a Ni–Dolomite Catalyst: Influence of Process Conditions on Syngas and Hydrogen Yield.” Journal of the Energy Institute 89 (4): 657–67, https://doi.org/10.1016/j.joei.2015.05.006.Suche in Google Scholar
Walsh, L. J. 2000. “Safety Issues Relating to the Use of Hydrogen Peroxide in Dentistry.” Australian Dental Journal 45 (4): 257–69, https://doi.org/10.1111/j.1834-7819.2000.tb00261.x.Suche in Google Scholar PubMed
Wang, H., X. Wang, M. Li, S. Li, S. Wang, and X. Ma. 2009. “Thermodynamic Analysis of Hydrogen Production from Glycerol Autothermal Reforming.” International Journal of Hydrogen Energy 34 (14): 5683–90, https://doi.org/10.1016/j.ijhydene.2009.05.118.Suche in Google Scholar
Wang, H., J. Xu, L. Sheng, X. Liu, Y. Lu, and W. Li. 2018. “A Review on Bio-Hydrogen Production Technology.” International Journal of Energy Research 42 (11): 3442–53, https://doi.org/10.1002/er.4044.Suche in Google Scholar
Watts, N., M. Amann, N. Arnell, S. Ayeb-Karlsson, J. Beagley, K. Belesova, and A. Costello. 2021. “The 2020 Report of the Lancet Countdown on Health and Climate Change: Responding to Converging Crises.” The Lancet 397 (10269): 129–70, https://doi.org/10.1016/s0140-6736(20)32290-x.Suche in Google Scholar
Xu, J. F., Y. T. Mi, and N. Q. Ren. 2016. “Buffering Action of Acetate on Hydrogen Production by Ethanoligenens Harbinense B49.” Electronic Journal of Biotechnology 23: 7–11, https://doi.org/10.1016/j.ejbt.2016.07.002.Suche in Google Scholar
Xu, D., Y. Xiong, S. Zhang, and Y. Su. 2019. “The Influence of Preparation Method of Char Supported Metallic Ni Catalysts on the Catalytic Performance for Reforming of Biomass Tar.” International Journal of Energy Research 43 (13): 6922–33, https://doi.org/10.1002/er.4709.Suche in Google Scholar
Xu, D., Y. Xiong, S. Zhang, and Y. Su. 2019. “The Influence of Preparation Method of Char Supported Metallic Ni Catalysts on the Catalytic Performance for Reforming of Biomass Tar.” International Journal of Energy Research 43 (13): 6922–33, https://doi.org/10.1002/er.4709.Suche in Google Scholar
Xu, X., Q. Zhou, and D. Yu. 2022. “The Future of Hydrogen Energy: Bio-Hydrogen Production Technology.” International Journal of Hydrogen Energy, https://doi.org/10.1016/j.ijhydene.2022.07.261.Suche in Google Scholar
Yanik, J., S. Ebale, A. Kruse, M. Saglam, and M. Yüksel. 2008. “Biomass Gasification in Supercritical Water: II. Effect of Catalyst.” International Journal of Hydrogen Energy 33 (17): 4520–6, https://doi.org/10.1016/j.ijhydene.2008.06.024.Suche in Google Scholar
Yildiz, B., and M. S. Kazimi. 2006. “Efficiency of Hydrogen Production Systems Using Alternative Nuclear Energy Technologies.” International Journal of Hydrogen Energy 31 (1): 77–92, https://doi.org/10.1016/j.ijhydene.2005.02.009.Suche in Google Scholar
Yue, X. F., H. Y. Sun, X. X. Zhao, and L. Q. Zhao. 2012. “Research Progress of Food Waste Fermentation for Bio-Hydrogen Production.” Advanced Materials Research 550: 569–73, https://doi.org/10.4028/www.scientific.net/amr.550-553.569.Suche in Google Scholar
Yurum, Y. 1996. “Hydrogen Energy System: Production and Utilization of Hydrogen and Future Aspects.” Fuel and Energy Abstracts 3 (37): 182, https://doi.org/10.1016/0140-6701(96)88527-4.Suche in Google Scholar
Závadský, J., V. Korenková, Z. Závadská, J. Kadárová, and D. Tuček. 2019. “Competences in the Quality Management System Evaluation Based on the Most Worldwide Used Key Performance Indicators.” Calitatea 20 (169): 29–41.Suche in Google Scholar
Zeng, L., and J. L. Gong. 2015. “Advances in Chemical Looping Reforming for Direct Hydrogen Production.” CIE Journal 66 (8): 2854–62.Suche in Google Scholar
Zhang, Y., T. R. Brown, G. Hu, and R. C. Brown. 2013. “Comparative Techno-Economic Analysis of Biohydrogen Production via Bio-Oil Gasification and Bio-Oil Reforming.” Biomass and Bioenergy 51: 99–108, https://doi.org/10.1016/j.biombioe.2013.01.013.Suche in Google Scholar
Zhang, J. N., Y. H. Li, H. Q. Zheng, Y. T. Fan, and H. W. Hou. 2015. “Direct Degradation of Cellulosic Biomass to Bio-Hydrogen from a Newly Isolated Strain Clostridium Sartagoforme FZ11.” Bioresource Technology 192: 60–7, https://doi.org/10.1016/j.biortech.2015.05.034.Suche in Google Scholar PubMed
Zhang, Y., P. Xu, S. Liang, B. Liu, Y. Shuai, and B. Li. 2019. “Exergy Analysis of Hydrogen Production from Steam Gasification of Biomass: A Review.” International Journal of Hydrogen Energy 44 (28): 14290–302, https://doi.org/10.1016/j.ijhydene.2019.02.064.Suche in Google Scholar
Zhang, Y., P. Xu, S. Liang, B. Liu, Y. Shuai, and B. Li. 2019. “Exergy Analysis of Hydrogen Production from Steam Gasification of Biomass: A Review.” International Journal of Hydrogen Energy 44 (28): 14290–302, https://doi.org/10.1016/j.ijhydene.2019.02.064.Suche in Google Scholar
Zhang, Y., L. Li, P. Xu, B. Liu, Y. Shuai, and B. Li. 2019. “Hydrogen Production through Biomass Gasification in Supercritical Water: A Review from Exergy Aspect.” International Journal of Hydrogen Energy 44 (30): 15727–36, https://doi.org/10.1016/j.ijhydene.2019.01.151.Suche in Google Scholar
Zhang, T., D. Jiang, H. Zhang, Y. Jing, N. Tahir, Y. Zhang, and Q. Zhang. 2020. “Comparative Study on Bio-Hydrogen Production from Corn Stover: Photo-Fermentation, Dark-Fermentation and Dark-Photo Co-fermentation.” International Journal of Hydrogen Energy 45 (6): 3807–14, https://doi.org/10.1016/j.ijhydene.2019.04.170.Suche in Google Scholar
Zhang, T., D. Jiang, H. Zhang, Y. Jing, N. Tahir, Y. Zhang, and Q. Zhang. 2020. “Comparative Study on Bio-Hydrogen Production from Corn Stover: Photo-Fermentation, Dark-Fermentation and Dark-Photo Co-fermentation.” International Journal of Hydrogen Energy 45 (6): 3807–14, https://doi.org/10.1016/j.ijhydene.2019.04.170.Suche in Google Scholar
Zhang, B., Q. Lu, W. Huang, Y. Chen, W. Yan, B. Yu, and J. Zhang. 2022. “Hydrogen Production via In-Line Pyrolysis-Reforming of Organic Solid Waste Enhanced by Steel Slags.” International Journal of Hydrogen Energy 47 (10): 6605–19, https://doi.org/10.1016/j.ijhydene.2021.12.065.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Solar photovoltaic-integrated energy storage system with a power electronic interface for operating a brushless DC drive-coupled agricultural load
- Analysis of 1-year energy data of a 5 kW and a 122 kW rooftop photovoltaic installation in Dhaka
- Reviews
- Real yields and PVSYST simulations: comparative analysis based on four photovoltaic installations at Ibn Tofail University
- A comprehensive approach of evolving electric vehicles (EVs) to attribute “green self-generation” – a review
- Exploring the piezoelectric porous polymers for energy harvesting: a review
- A strategic review: the role of commercially available tools for planning, modelling, optimization, and performance measurement of photovoltaic systems
- Comparative assessment of high gain boost converters for renewable energy sources and electrical vehicle applications
- A review of green hydrogen production based on solar energy; techniques and methods
- A review of green hydrogen production by renewable resources
- A review of hydrogen production from bio-energy, technologies and assessments
- A systematic review of recent developments in IoT-based demand side management for PV power generation
- Research Articles
- Hybrid optimization strategy for water cooling system: enhancement of photovoltaic panels performance
- Solar energy harvesting-based built-in backpack charger
- A power source for E-devices based on green energy
- Theoretical and experimental investigation of electricity generation through footstep tiles
- Experimental investigations on heat transfer enhancement in a double pipe heat exchanger using hybrid nanofluids
- Comparative energy and exergy analysis of a CPV/T system based on linear Fresnel reflectors
- Investigating the effect of green composite back sheet materials on solar panel output voltage harvesting for better sustainable energy performance
- Electrical and thermal modeling of battery cell grouping for analyzing battery pack efficiency and temperature
- Intelligent techno-economical optimization with demand side management in microgrid using improved sandpiper optimization algorithm
- Investigation of KAPTON–PDMS triboelectric nanogenerator considering the edge-effect capacitor
- Design of a novel hybrid soft computing model for passive components selection in multiple load Zeta converter topologies of solar PV energy system
- A novel mechatronic absorber of vibration energy in the chimney
- An IoT-based intelligent smart energy monitoring system for solar PV power generation
- Large-scale green hydrogen production using alkaline water electrolysis based on seasonal solar radiation
- Evaluation of performances in DI Diesel engine with different split injection timings
- Optimized power flow management based on Harris Hawks optimization for an islanded DC microgrid
- Experimental investigation of heat transfer characteristics for a shell and tube heat exchanger
- Fuzzy induced controller for optimal power quality improvement with PVA connected UPQC
- Impact of using a predictive neural network of multi-term zenith angle function on energy management of solar-harvesting sensor nodes
- An analytical study of wireless power transmission system with metamaterials
- Hydrogen energy horizon: balancing opportunities and challenges
- Development of renewable energy-based power system for the irrigation support: case studies
- Maximum power point tracking techniques using improved incremental conductance and particle swarm optimizer for solar power generation systems
- Experimental and numerical study on energy harvesting performance thermoelectric generator applied to a screw compressor
- Study on the effectiveness of a solar cell with a holographic concentrator
- Non-transient optimum design of nonlinear electromagnetic vibration-based energy harvester using homotopy perturbation method
- Industrial gas turbine performance prediction and improvement – a case study
- An electric-field high energy harvester from medium or high voltage power line with parallel line
- FPGA based telecommand system for balloon-borne scientific payloads
- Improved design of advanced controller for a step up converter used in photovoltaic system
- Techno-economic assessment of battery storage with photovoltaics for maximum self-consumption
- Analysis of 1-year energy data of a 5 kW and a 122 kW rooftop photovoltaic installation in Dhaka
- Shading impact on the electricity generated by a photovoltaic installation using “Solar Shadow-Mask”
- Investigations on the performance of bottle blade overshot water wheel in very low head resources for pico hydropower
- Solar photovoltaic-integrated energy storage system with a power electronic interface for operating a brushless DC drive-coupled agricultural load
- Numerical investigation of smart material-based structures for vibration energy-harvesting applications
- A system-level study of indoor light energy harvesting integrating commercially available power management circuitry
- Enhancing the wireless power transfer system performance and output voltage of electric scooters
- Harvesting energy from a soldier's gait using the piezoelectric effect
- Study of technical means for heat generation, its application, flow control, and conversion of other types of energy into thermal energy
- Theoretical analysis of piezoceramic ultrasonic energy harvester applicable in biomedical implanted devices
- Corrigendum
- Corrigendum to: A numerical investigation of optimum angles for solar energy receivers in the eastern part of Algeria
- Special Issue: Recent Trends in Renewable Energy Conversion and Storage Materials for Hybrid Transportation Systems
- Typical fault prediction method for wind turbines based on an improved stacked autoencoder network
- Power data integrity verification method based on chameleon authentication tree algorithm and missing tendency value
- Fault diagnosis of automobile drive based on a novel deep neural network
- Research on the development and intelligent application of power environmental protection platform based on big data
- Diffusion induced thermal effect and stress in layered Li(Ni0.6Mn0.2Co0.2)O2 cathode materials for button lithium-ion battery electrode plates
- Improving power plant technology to increase energy efficiency of autonomous consumers using geothermal sources
- Energy-saving analysis of desalination equipment based on a machine-learning sequence modeling
Artikel in diesem Heft
- Solar photovoltaic-integrated energy storage system with a power electronic interface for operating a brushless DC drive-coupled agricultural load
- Analysis of 1-year energy data of a 5 kW and a 122 kW rooftop photovoltaic installation in Dhaka
- Reviews
- Real yields and PVSYST simulations: comparative analysis based on four photovoltaic installations at Ibn Tofail University
- A comprehensive approach of evolving electric vehicles (EVs) to attribute “green self-generation” – a review
- Exploring the piezoelectric porous polymers for energy harvesting: a review
- A strategic review: the role of commercially available tools for planning, modelling, optimization, and performance measurement of photovoltaic systems
- Comparative assessment of high gain boost converters for renewable energy sources and electrical vehicle applications
- A review of green hydrogen production based on solar energy; techniques and methods
- A review of green hydrogen production by renewable resources
- A review of hydrogen production from bio-energy, technologies and assessments
- A systematic review of recent developments in IoT-based demand side management for PV power generation
- Research Articles
- Hybrid optimization strategy for water cooling system: enhancement of photovoltaic panels performance
- Solar energy harvesting-based built-in backpack charger
- A power source for E-devices based on green energy
- Theoretical and experimental investigation of electricity generation through footstep tiles
- Experimental investigations on heat transfer enhancement in a double pipe heat exchanger using hybrid nanofluids
- Comparative energy and exergy analysis of a CPV/T system based on linear Fresnel reflectors
- Investigating the effect of green composite back sheet materials on solar panel output voltage harvesting for better sustainable energy performance
- Electrical and thermal modeling of battery cell grouping for analyzing battery pack efficiency and temperature
- Intelligent techno-economical optimization with demand side management in microgrid using improved sandpiper optimization algorithm
- Investigation of KAPTON–PDMS triboelectric nanogenerator considering the edge-effect capacitor
- Design of a novel hybrid soft computing model for passive components selection in multiple load Zeta converter topologies of solar PV energy system
- A novel mechatronic absorber of vibration energy in the chimney
- An IoT-based intelligent smart energy monitoring system for solar PV power generation
- Large-scale green hydrogen production using alkaline water electrolysis based on seasonal solar radiation
- Evaluation of performances in DI Diesel engine with different split injection timings
- Optimized power flow management based on Harris Hawks optimization for an islanded DC microgrid
- Experimental investigation of heat transfer characteristics for a shell and tube heat exchanger
- Fuzzy induced controller for optimal power quality improvement with PVA connected UPQC
- Impact of using a predictive neural network of multi-term zenith angle function on energy management of solar-harvesting sensor nodes
- An analytical study of wireless power transmission system with metamaterials
- Hydrogen energy horizon: balancing opportunities and challenges
- Development of renewable energy-based power system for the irrigation support: case studies
- Maximum power point tracking techniques using improved incremental conductance and particle swarm optimizer for solar power generation systems
- Experimental and numerical study on energy harvesting performance thermoelectric generator applied to a screw compressor
- Study on the effectiveness of a solar cell with a holographic concentrator
- Non-transient optimum design of nonlinear electromagnetic vibration-based energy harvester using homotopy perturbation method
- Industrial gas turbine performance prediction and improvement – a case study
- An electric-field high energy harvester from medium or high voltage power line with parallel line
- FPGA based telecommand system for balloon-borne scientific payloads
- Improved design of advanced controller for a step up converter used in photovoltaic system
- Techno-economic assessment of battery storage with photovoltaics for maximum self-consumption
- Analysis of 1-year energy data of a 5 kW and a 122 kW rooftop photovoltaic installation in Dhaka
- Shading impact on the electricity generated by a photovoltaic installation using “Solar Shadow-Mask”
- Investigations on the performance of bottle blade overshot water wheel in very low head resources for pico hydropower
- Solar photovoltaic-integrated energy storage system with a power electronic interface for operating a brushless DC drive-coupled agricultural load
- Numerical investigation of smart material-based structures for vibration energy-harvesting applications
- A system-level study of indoor light energy harvesting integrating commercially available power management circuitry
- Enhancing the wireless power transfer system performance and output voltage of electric scooters
- Harvesting energy from a soldier's gait using the piezoelectric effect
- Study of technical means for heat generation, its application, flow control, and conversion of other types of energy into thermal energy
- Theoretical analysis of piezoceramic ultrasonic energy harvester applicable in biomedical implanted devices
- Corrigendum
- Corrigendum to: A numerical investigation of optimum angles for solar energy receivers in the eastern part of Algeria
- Special Issue: Recent Trends in Renewable Energy Conversion and Storage Materials for Hybrid Transportation Systems
- Typical fault prediction method for wind turbines based on an improved stacked autoencoder network
- Power data integrity verification method based on chameleon authentication tree algorithm and missing tendency value
- Fault diagnosis of automobile drive based on a novel deep neural network
- Research on the development and intelligent application of power environmental protection platform based on big data
- Diffusion induced thermal effect and stress in layered Li(Ni0.6Mn0.2Co0.2)O2 cathode materials for button lithium-ion battery electrode plates
- Improving power plant technology to increase energy efficiency of autonomous consumers using geothermal sources
- Energy-saving analysis of desalination equipment based on a machine-learning sequence modeling


