Abstract
C12H16Br2, monoclinic, P21/c (no. 14), a = 7.9423(5) Å, b = 4.4163(3) Å, c = 16.8127(10) Å, β = 94.534(4)°, V = 587.87(6) Å3, Z = 2, Rgt (F) = 0.0376, wRref (F 2) = 0.1016, T = 173 K.
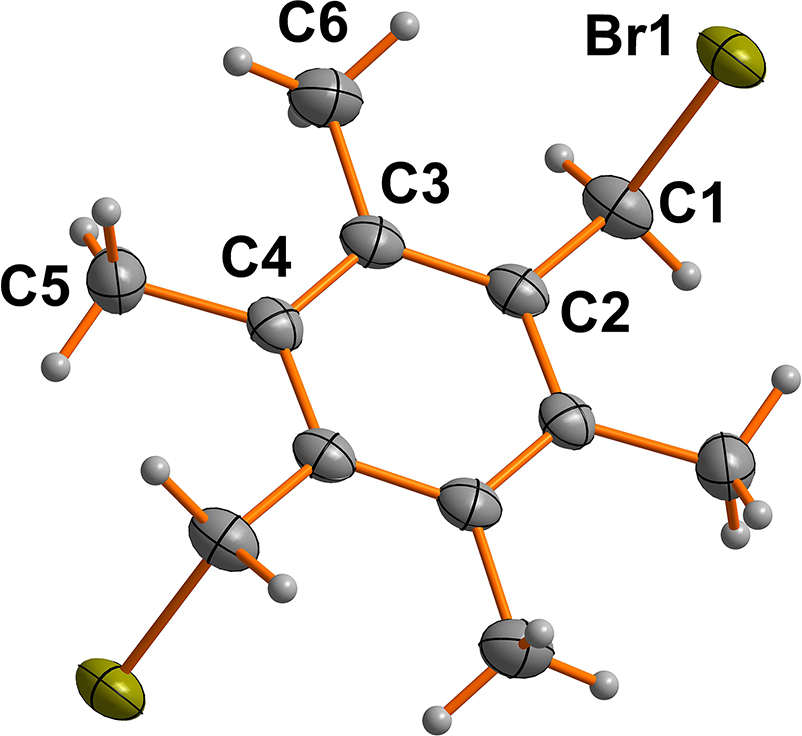
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block |
| Size: | 0.12 × 0.11 × 0.10 mm |
| Wavelength: | Ga Kα radiation (1.34139 Å) |
| μ: | 5.57 mm−1 |
| Diffractometer, scan mode: | Bruker D8 VENTURE Metaljet PHOTON II, φ and ω |
| θ max, completeness: | 60.3°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 4095, 1333, 0.039 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 1066 |
| N(param)refined: | 66 |
| Programs: | Bruker [1], Olex2 [2, 3], SHELX [4, 5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Br1 | 0.86356 (5) | 0.34688 (10) | 0.68181 (2) | 0.03993 (19) |
| C1 | 0.6912 (5) | 0.1198 (8) | 0.6139 (2) | 0.0344 (8) |
| H1A | 0.747474 | −0.040050 | 0.584292 | 0.041* |
| H1B | 0.611904 | 0.020101 | 0.648147 | 0.041* |
| C2 | 0.5945 (4) | 0.3245 (8) | 0.5557 (2) | 0.0277 (7) |
| C3 | 0.6510 (4) | 0.3664 (8) | 0.4794 (2) | 0.0291 (7) |
| C4 | 0.5549 (4) | 0.5378 (8) | 0.42288 (19) | 0.0281 (7) |
| C5 | 0.6072 (5) | 0.5692 (11) | 0.3389 (2) | 0.0410 (9) |
| H5A | 0.507074 | 0.605676 | 0.302307 | 0.061* |
| H5B | 0.662945 | 0.382554 | 0.323535 | 0.061* |
| H5C | 0.685578 | 0.739705 | 0.336443 | 0.061* |
| C6 | 0.8168 (5) | 0.2266 (10) | 0.4600 (2) | 0.0399 (9) |
| H6A | 0.899374 | 0.245834 | 0.506158 | 0.060* |
| H6B | 0.858855 | 0.331573 | 0.414151 | 0.060* |
| H6C | 0.799434 | 0.011979 | 0.447000 | 0.060* |
Source of material
1.4-Bis(bromomethyl)-2,3,5,6-tetramethylbenzene was synthesized and carried out according to the literature [6]. In brief, 13.42 g (0.1 mol) of durene were first dissolved in 50 mL of hot glacial acetic acid before paraformaldehyde 6.15 g (0.20 mol) and 40 mL of a 31% HBr/acetic acid solution were added. The mixture was kept for 8 h at 120 °C and then poured into 100 mL of water. The product was filtered off on a G3 glass frit and dried in vacuum.
Experimental details
All the H atoms were placed geometrically and refined without any constraints or restraints.
Comment
Bromomethyl-substituted benzene derivatives are important synthetic materials for construction of heterocyclic frameworks which have been extensively and widely used [6]. 1.4-Bis(bromomethyl)-2,3,5,6-tetramethylbenzene was one of the used compounds that is important. For example, 1.4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene can be used for the construction of a new organic piperidine derivative named as 4-methyl-1-({2,3,5,6-tetramethyl-4-[(4-methylpiperidinyl) methyl]phenyl}methyl)piperidine, which possesses better antidiabetic, antioxidant and DNA binding abilities [7]. Similarly, Caroline Daisy et al. reported the synthesis of the biologically important 2-(2,4,6-trimethylbenzylthio)benzo[d]thiazole by using 1.4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene [8]. Liu et al. reported two macrocyclic bis-benzimidazolium salts based sensors for anions sensing prepared by using 1.4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene [9]. However, there is no report on crystal structure of the title compound.
The title compound crystalizes in the monoclinic space group P21/c. The asymmetric unit is defined by one half of the title molecule. The molecules reside around an inversion center of the aforementioned space group (see the Figure). Bond lengths and angles in crystal structure are within normal ranges [10].
Funding source: Natural Science Foundation of Shaanxi Science and Technology Department https://orcid.org/0000-0003-1491-050X
Award Identifier / Grant number: 2022JQ-127
Funding source: Special Scientific Research Project of Shaanxi Education Department https://orcid.org/0000-0003-1491-050X
Award Identifier / Grant number: 21JK0955
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This project was funded Natural Science Foundation of Shaanxi Science and Technology Department (No. 2022JQ-127) and the Special Scientific Research Project of Shaanxi Education Department (No: 21JK0955). This work was supported by the the Youth Innovation Team of Shaanxi Universities.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K., Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment-Olex2 dissected. Acta Crystallogr. 2015, A71, 59–75; https://doi.org/10.1107/s2053273314022207.Search in Google Scholar
3. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
6. van der Made, A. W., van der Made, R. H. A convenient procedure for bromomethylation of aromatic compounds. Selective mono-, bis-, or trisbromomethylation. J. Org. Chem. 1993, 58, 1262–1263; https://doi.org/10.1021/jo00057a046.Search in Google Scholar
7. Nandini Asha, R., Sankarganesh, M., Bhuvanesh, N., Ravindran Durai Nayagam, B. Synthesis, structural, spectral, antidiabetic, DNA interactions and molecular docking investigations of a piperidine derivative. J. Mol. Struct. 2022, 1250, 131692; https://doi.org/10.1016/j.molstruc.2021.131692.Search in Google Scholar
8. Daisy, C., Nandini Asha, R., Suresh Kumar, G. S., Vadivel, E., Bhuvanesh, N., Ravindran Durai Nayagam, B. Experimental and theoretical studies of 2-mercaptobenzothiazole with 2-bromomethylmesitylene and 1,4-bis(bromomethyl) durene. J. Mol. Struct. 2022, 2020, 128894.10.1016/j.molstruc.2020.128894Search in Google Scholar
9. Liu, Y., Zhao, Z., Huo, R., Liu, Q. Two macrocycle-based sensors for anions sensing. Sci. Rep. 2019, 9, 502; https://doi.org/10.1038/s41598-018-36916-w.Search in Google Scholar PubMed PubMed Central
10. Basaran, R., Dou, S.-Q., Weiss, A. On the persistence of molecular symmetry in the solid state and the role of molecular group dipole moments. Centrosymmetry in bis-(chloromethyl)benzenes. Ber. Bunsenges. Phys. Chem. 1992, 96, 1688–1698; https://doi.org/10.1002/bbpc.19920961131.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

