Abstract
C15H15N3O2, orthorhombic, Pca21 (no. 29), a = 7.9831(2) Å, b = 10.6486(3) Å, c = 15.7222(4) Å, V = 1336.53(6) Å3, Z = 4, Rgt(F) = 0.0340, wRref(F2) = 0.0799, T = 100 K.
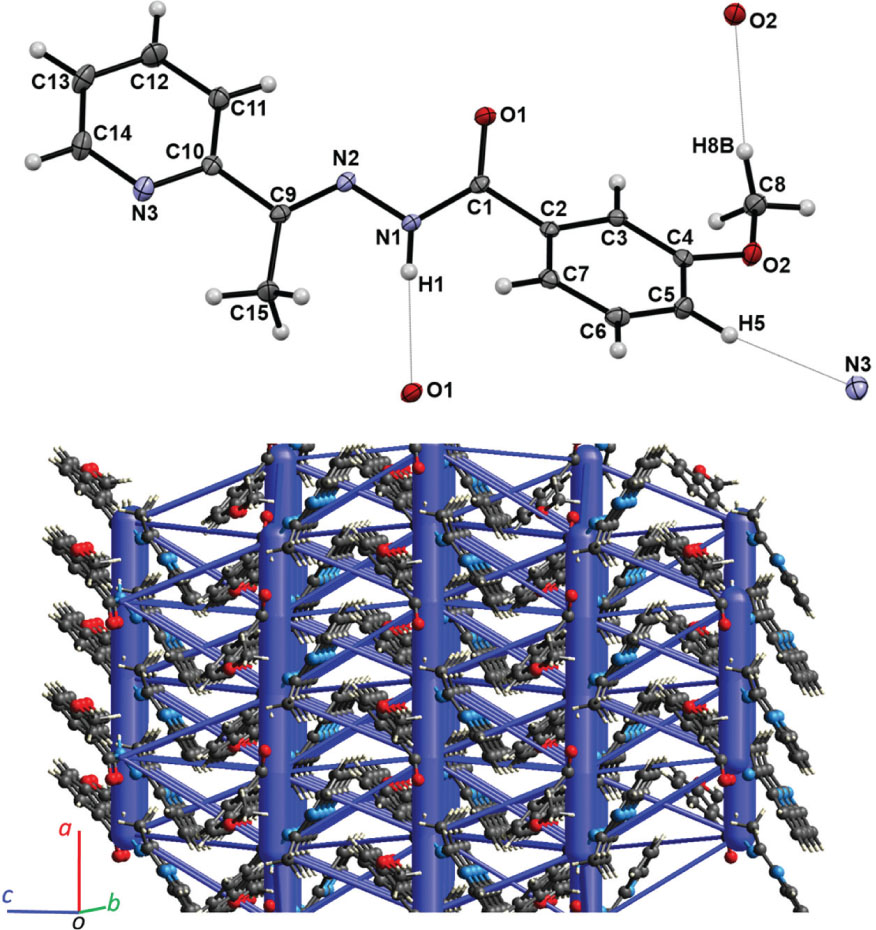
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.28 × 0.22 × 0.09 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 29.7°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 34842, 3790, 0.049 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3385 |
| N(param)refined: | 186 |
| Programs: | Bruker [1], SHELX [2], [3], Olex2 [4], Crystal Explorer [5], Mercury [6] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.65856(15) | 0.64702(11) | 0.53292(9) | 0.0163(3) |
| O2 | 1.13033(16) | 0.94314(11) | 0.64302(8) | 0.0172(3) |
| N1 | 0.81973(18) | 0.47369(13) | 0.50812(9) | 0.0123(3) |
| N2 | 0.69467(17) | 0.42449(13) | 0.45744(9) | 0.0122(3) |
| N3 | 0.5631(2) | 0.13527(14) | 0.37099(10) | 0.0164(3) |
| C1 | 0.7893(2) | 0.58803(16) | 0.54361(10) | 0.0115(3) |
| C2 | 0.9256(2) | 0.63898(15) | 0.59947(11) | 0.0114(3) |
| C3 | 0.9580(2) | 0.76818(15) | 0.59386(11) | 0.0120(3) |
| C4 | 1.0831(2) | 0.81951(16) | 0.64451(11) | 0.0132(3) |
| C5 | 1.1709(2) | 0.74460(17) | 0.70209(11) | 0.0156(3) |
| C6 | 1.1347(2) | 0.61830(17) | 0.70823(12) | 0.0157(3) |
| C7 | 1.0126(2) | 0.56340(15) | 0.65637(10) | 0.0134(3) |
| C8 | 1.0461(2) | 1.02502(16) | 0.58479(12) | 0.0190(4) |
| C9 | 0.7113(2) | 0.31023(15) | 0.43228(11) | 0.0123(3) |
| C10 | 0.5685(2) | 0.26067(16) | 0.38103(10) | 0.0125(3) |
| C11 | 0.4476(2) | 0.34046(17) | 0.34663(11) | 0.0169(4) |
| C12 | 0.3153(3) | 0.28838(19) | 0.30172(13) | 0.0231(4) |
| C13 | 0.3086(3) | 0.15935(19) | 0.29134(14) | 0.0233(4) |
| C14 | 0.4345(3) | 0.08763(17) | 0.32668(13) | 0.0198(4) |
| C15 | 0.8537(2) | 0.22489(17) | 0.45435(14) | 0.0225(4) |
| H1 | 0.916(3) | 0.438(2) | 0.5112(14) | 0.015* |
| H3 | 0.895440 | 0.819679 | 0.556100 | 0.014* |
| H5 | 1.255451 | 0.780516 | 0.736965 | 0.019* |
| H6 | 1.193383 | 0.567925 | 0.748208 | 0.019* |
| H7 | 0.989501 | 0.476039 | 0.660006 | 0.016* |
| H8A | 1.093279 | 1.109719 | 0.589322 | 0.029* |
| H8B | 0.926414 | 1.027556 | 0.598559 | 0.029* |
| H8C | 1.060933 | 0.993830 | 0.526631 | 0.029* |
| H11 | 0.455732 | 0.428818 | 0.353844 | 0.020* |
| H12 | 0.230380 | 0.340505 | 0.278372 | 0.028* |
| H13 | 0.219625 | 0.121204 | 0.260656 | 0.028* |
| H14 | 0.429805 | −0.000820 | 0.319174 | 0.024* |
| H15A | 0.877896 | 0.231453 | 0.515294 | 0.034* |
| H15B | 0.953112 | 0.249219 | 0.421713 | 0.034* |
| H15C | 0.823311 | 0.138099 | 0.440495 | 0.034* |
Source of material
To a suspension of 831 mg (5 mmoles) m-anisic hydrazide in 15 mL 90% aqueous EtOH were added 606 mg (561 μL, 5 mmoles) 2-acetylpyridine, and the reaction mixture was stirred for 6 h at 70 °C. The resulting clear solution was brought to 4 °C and left for 2 days to deposit crystals as colorless prisms.
Experimental details
The hydrazide H1 atom was located in difference-Fourier maps while all other hydrogen atoms were initially placed in calculated positions. Thermal parameters were constrained to ride on the carrier atoms (Uiso(methine H) = 1.2Ueq and Uiso(methyl H) = 1.5Ueq). Due to a high uncertainty on the Flack parameter (0.279), no attempt to model inversion twinning was made.
Comment
One particular interest to hydrazide-hydrazones, a versatile group of organic molecules, stems from their high affinity to iron, which makes these structures potential antimicrobial [7], [8], anticancer [9], or neuroprotective [10] agents. As a part of our search for inhibitors of cytotoxic virulence factors from drug-resistant bacteria [11], we have prepared the title compound, a structural analogue of a series of hydrazide-hydrazones that are pharmacologically active in vivo.
The title compound crystallizes in the orthorhombic Pca21 space group, with four equivalent molecules per unit cell. The asymmetric unit of the title structure contains one molecule of 2-acetylpyridine m-anisoyl hydrazone (2APymAH), shown in the upper part of the figure. The valence bond lengths and angles are in the expected ranges. The major portion of the molecule is approximately flat: all but three atoms, O1, H8C, and H15B, are located within 1 Å of the mean molecular plane. The pyridyl and the anisyl ring planes are at 10.0° and 13.3° to the molecular plane, respectively. The conventional hydrogen bonding in crystal structure of 2APymAH is limited to only one intermolecular heteroatom contact (N1⋯O1 = 3.020(2) Å, H1⋯O1 = 2.17(2) Å, N1—H1⋯O1 = 174(2)°) shown in the upper part of the figure. In the crystal, infinite chains of the H-bonds propagate in the [100] direction. In addition, two short intermolecular contacts of the C—H⋯O/N type, which satisfy to the directionality condition (angle C—H⋯O/N > 120°) and which are shown in the figure as dotted lines, may contribute to the stability of the molecular packing in the 2APymAH crystal, as well. The crystal structure lacks any strong π-π stacking interactions, with the shortest contact between the pirydyl (ring 1) and the anisyl (ring 2) rings Cg1⋯Cg2′ = 5.1198(11) Å (′ = −1/2 + x, 1 − y, z). In addition, a short C5—H5⋯Cg1′′ (′′ = 2 − x, 1 − y, 1/2 + z) contact is present in the crystal structure, as well.
To account for all interactions involved in the build-up of the crystal structure, we have performed DFT calculations, at the B3LYP/6-31G(d,p) theory level [5], [12], of the electrostatic, dispersion, polarization, and repulsion energies in the 2APymAH crystal structure. According to the calculations, the interactions from hydrogen-bonded pairs of molecules contributed about 50% to the lattice energy, with the dispersion energy providing most for the attractive forces between neighbouring molecules of 2APymAH (i.e. Eelstat = −54 kJ/mol, Edisp = −75.2 kJ/mol for x + 1/2, −y, z). The spatial distribution of the energetically most significant interactions is illustrated in the lower part of the figure, showing the interactions energy framework as blue cylinders penetrating the crystal lattice of 2APymAH. The cylinders connect centroids of the interacting molecules, and their diameters are proportional to the total energies of the interactions, with the 8 kJ/mol cut-off, for clarity. The most extensive intermolecular interactions occur in the H-bonding direction parallel to [100]. To estimate the lattice energy, all total energies of unique pairwise interactions between molecules were summed up, thus yielding Elattice = −164 kJ/mol for the 2APymAH crystal.
Acknowledgements
We gratefully acknowledge financial support by the University of Missouri Agriculture Experiment Station Chemical Laboratories and by the National Institute of Food and Agriculture (grant MO-HABC0002).
References
1. Bruker Axs Inc., Apex3 v. 2016.9-0 and SAINT v. 8.37A. Madison, WI, USA (2016).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
5. MacKenzie, C. F.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A.: CrystalExplorer model energies and energy frameworks: extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 4 (2017) 575–587.10.1107/S205225251700848XSearch in Google Scholar PubMed PubMed Central
6. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A.: Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 41 (2008) 466–470.10.1107/S0021889807067908Search in Google Scholar
7. Narang, R.; Narasimhan, B.; Sharma, S.: A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 19 (2012) 569–612.10.2174/092986712798918789Search in Google Scholar PubMed
8. Rzhepishevska, O.; Hakobyan, S.; Ekstrand-Hammarstroem, B.; Nygren, Y.; Karlsson, T.; Bucht, A.; Elofsson, M.; Boily, J.-F.; Ramstedt, M.: The gallium(III)-salicylidene acylhydrazide complex shows synergistic anti-biofilm effect and inhibits toxin production by Pseudomonas aeruginosa. J. Inorg. Biochem. 138 (2014) 1–8.10.1016/j.jinorgbio.2014.04.009Search in Google Scholar PubMed
9. Lovejoy, D. B.; Richardson, D. R.: Iron chelators as anti-neoplastic agents: Current developments and promise of the PIH class of chelators. Curr. Med. Chem. 10 (2003) 1035–1049.10.2174/0929867033457557Search in Google Scholar PubMed
10. Richardson, D. R.: Novel chelators for central nervous system disorders that involve alterations in the metabolism of iron and other metal ions. Ann. N. Y. Acad. Sci. 1012 (2004) 326–341.10.1196/annals.1306.026Search in Google Scholar PubMed
11. Mossine, V. V.; Waters, J. K.; Chance, D. L.; Mawhinney, T. P.: Transient proteotoxicity of bacterial virulence factor pyocyanin in renal tubular epithelial cells induces ER-related vacuolation and can be efficiently modulated by iron chelators. Toxicol. Sci. 154 (2016) 403–415.10.1093/toxsci/kfw174Search in Google Scholar PubMed PubMed Central
12. Thomas, S. P.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A.: Accurate lattice energies for molecular crystals from experimental crystal structures. J. Chem. Theory Comput. 14 (2018) 1614–1623.10.1021/acs.jctc.7b01200Search in Google Scholar PubMed
©2020 Valeri V. Mossine et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4
Articles in the same Issue
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4

