Abstract
Microbial diseases remain a leading cause of death worldwide, and the emergence of new infections underscores the need for innovative treatments. Plant-based sources for antimicrobial drugs are gaining popularity due to their fewer side effects compared to synthetic drugs. Corn silk (CS), an ancient medicinal plant, has been used for thousands of years to treat ailments such as edema and cystitis. It is a rich source of vitamins and minerals and acts as an anti-hypertensive, anti-diabetic, anti-cancerous, antioxidant, and antimicrobial agent. Its bioactive components, which include phenolic acids, flavonoids, tannins, alkaloids, terpenes, and saponins, are responsible for these therapeutic benefits. Numerous studies have examined the antibacterial effectiveness of diverse CS extracts, unveiling several methods by which CS demonstrates its antimicrobial properties. These mechanisms include the inhibition of energy production within microbial cells, interference with DNA and protein synthesis, prevention of biofilm formation, disruption of cell wall synthesis, and direct disruption of the cell wall structure. CS effectively blocks microbial growth and multiplication by focusing on these vital processes. Therefore, the purpose of this review is to provide a comprehensive summary of the different bioactive compounds found in CS, as well as their mechanisms of action against microbes. Understanding these mechanisms highlights CS’s potential as a potent source for developing new antimicrobial medications and supplements, offering a natural and effective alternative in the fight against infectious diseases.
Abbreviations
- CS
-
Corn silk
- FMCSS
-
Fresh matured corn silk sample
- DMCSS
-
Dried matured corn silk sample
- CE
-
Consecutive extraction
- IE
-
Individual extraction
- QS
-
Quorum sensing
- ATP
-
Adenosine triphosphate
- HIV
-
Human immunodeficiency viruses
- HSV
-
Herpes simplex virus
- SARS
-
Severe acute respiratory syndrome
- EPI
-
Epigenetics
- PEDV
-
Porcine epidemic diarrhea virus
- UTI
-
Urinary tract infection
- MFC
-
Minimum fungicidal concentration
- MIC
-
Minimum inhibition concentration
- UAE
-
Ultrasound assisted extraction
- MAE
-
Microwave-assisted extraction
- TZP
-
Piperacillin-tazobactam
1 Introduction
Microorganisms pose major problems to public health and the infectious diseases caused by them are a significant cause of morbidity and mortality around the world. Although antibiotics play an important role in the treatment of infectious diseases, the incidence of antibiotic resistance has surged over the past decades [1]. The alarming severity of drug resistance has led many scientists to search for new and alternative antimicrobial sources. A wide range of medicinal plants are a rich source of antimicrobials and they possess different medicinal properties against microorganisms [2]. Consequently, strong efforts are being made to utilize those medicinal plants as medicines in treating microbial infections.
Corn silk (CS) (Stigma maydis L.) is the stigma and style of the maize flower which is obtained as a by-product of corn cultivation. It has numerous biochemical nutrient compounds like proteins, carbohydrates, vitamins, and mineral salts and is also rich in B complex vitamins, vitamin A, vitamin K, and minerals like sodium and potassium [3]. CS also contains several bioactive compounds such as steroids, alkaloids, anthocyanins, saponins, carotenoids, and phenolic compounds which possess cooperative effects on physical health [4]. Many countries like China, the United States, and France have been using CS for the treatment of prostate disorders, kidney stones, obesity, urinary infections, and bedwetting. Extracts of CS help in reducing the deteriorating effects of diabetes, hypertension, and high blood pressure [5]. Scientifically, it has been examined that CS inhibits the α-amylase activity and retard the digestion of starch as well as restrains the increase of post-meal blood sugar [6]. It helps to reduce high blood pressure by regulating the electrolyte balance through the release of potassium and sodium in the urine [7]. CS, rich in flavonoids and phenolic compounds, acts as an excellent source of antioxidants and enhances the scavenging activity of harmful free radicals [8]. The anthocyanins present in some colored CSs exhibit higher antioxidant, anticarcinogenic, and anti-inflammatory activity [9]. CS can also regulate cell death by modulating different signaling cascades thereby supporting the nervous system [10].
Besides the numerous health properties of CS, it is known to hold a strong antimicrobial activity against different microorganisms. Several studies have been conducted to expose its potential to inhibit microbial strains. The ethanolic extract of CS has been found to inhibit the growth of Pseudomonas aeruginosa and Staphylococcus aureus which are one of the most common infections causing microbes such as urinary tract, respiratory and gastrointestinal infections [11]. The antibacterial effects of maize silk extracts were shown to be attributed to the presence of components such as steroids, flavonoids, tannins, and saponins, which were examined by phytochemical analysis [12]. Furthermore, optimised ethanol extracts of CS showed hypoglycemic action and efficiency against Bacillus subtilis, boosting its potential for antibacterial and health-promoting properties [13]. CS when used in making wine and vinegar produced a number of volatile compounds such as 1-butanol, 3-methyl-acetone, 1-butanol, 3-methyl-hexanoic acid and almost all of them showed antimicrobial properties when tested [14]. In an in vitro study, it was found that using 10% of CS extract as mouth gargles significantly reduced the decay-causing bacteria (S. mutans) as compared to the commercially available mouth gargles [15].
In general, the presence of certain bioactive compounds and the chemical composition of CS are mainly accountable for their healthcare applications and antimicrobial effect [16]. So, this review will highlight the different bioactive compounds found in CS and their antimicrobial mechanism against microorganisms. It will also reveal the antimicrobial efficacy of various CS extracts and will provide insight into how CS can become an operative antimicrobial agent in the preparation of new drugs.
2 Statement of novelty
The major focus of this review is toward utilization of agricultural waste that contains good amounts of biologically active components. Various studies have observed the antibacterial qualities of maize silk, even though numerous studies have examined its nutritional composition, therapeutic properties, and product development. This study outlines the mechanisms underlying the bioactive components in maize silk and covers the body of research on the material’s antimicrobial properties. Given the rising issue of microbial resistance, this study outlines research needs for future investigations and underlines the potential of CS for creating antimicrobial medications against bacteria resistance.
2.1 Methodology
The intense literature search and screening for the review article was done on Google Scholar, Pub Med, Springer, ResearchGate, and ScienceDirect as the major database for a comprehensive search of peer-reviewed journals. The cross references of articles were explored to search for related articles for the study. Keywords used for the search were CS, bioactive compounds, phytochemicals, microbial inhibition, and anti-microbial properties of CS. The search discovered 66 articles published between 2010 and 2023.
3 Bioactive compounds present in CS
Phytochemicals, or bioactive substances found in plants, are secondary metabolites that may have toxicological or pharmacological effects on humans [17]. These substances are produced along with primary metabolic substances and are essential for the growth, reproduction, and interactions of plants with the environment. Phytochemicals are also responsible for several microbiological and nutraceutical properties of food-based products and are commonly used for the preparation of dyes, flavors, fragrances, and insecticides [18]. Secondary metabolites are entirely accumulated in different parts of the plants and the amount present in them plays a pivotal role in treating many health-related diseases. Most profusely available classes of plant phytochemicals are alkaloids, tannins, saponins, flavonoids, terpenoids, steroids, and glycosides and are known to serve as an antioxidant, antiviral, anti-diabetic, anticancer, and anti-inflammatory agents [19]. CS is a major plant ingredient containing numerous biologically active compounds and has been used in various therapeutic methods for over thousands of years. It is rich in flavonoids, alkaloids, steroids, tannins, terpenoids, saponins, organic acids, and volatile oils [20,21]. The complete extraction of all the bioactive phytochemicals by using a single extraction method is very difficult and uncertain [22]. Therefore, various solvent extracts such as aqueous, alcohol, acetone, and hexane are used for extracting all the phytochemicals from CS as shown in Figure 1.
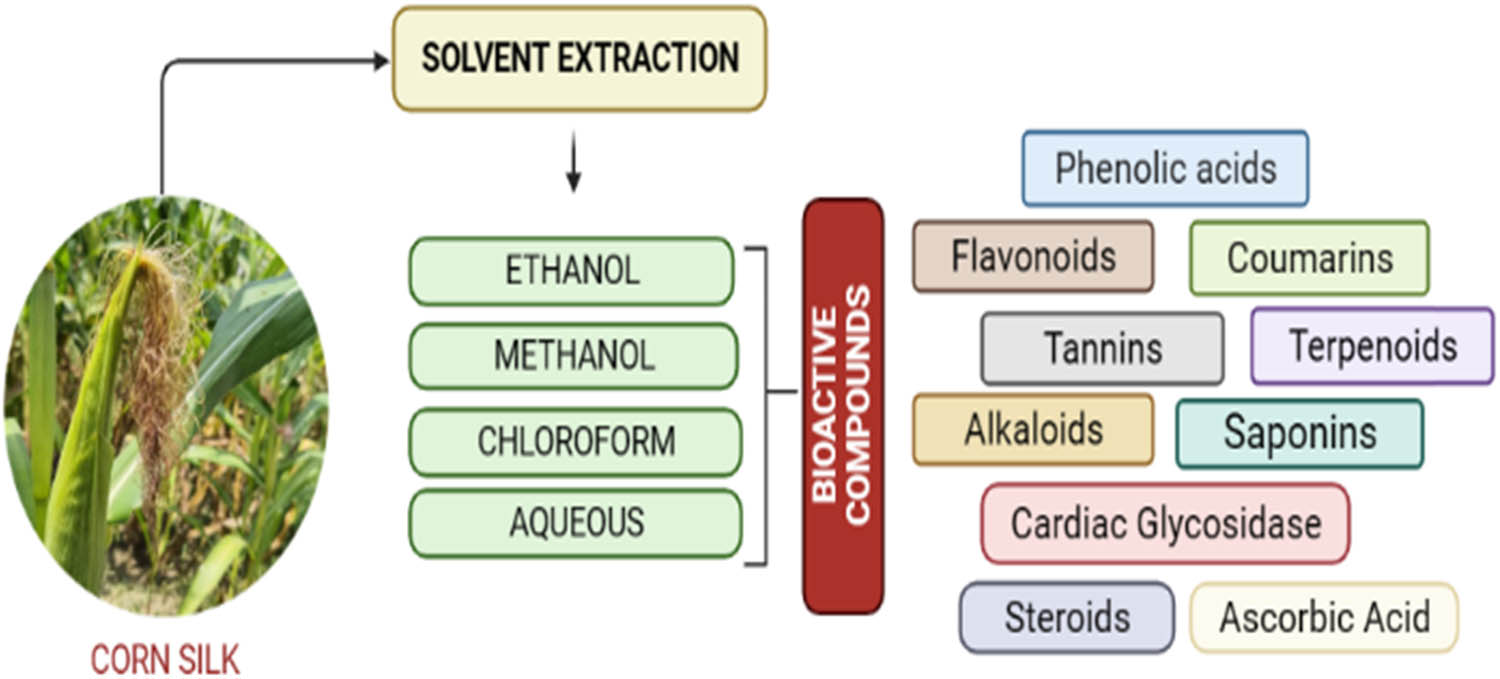
Phytochemical constituents found in various CS extracts. Source: Figure prepared by the authors.
A qualitative analysis of phytochemicals using ethanol, methanol, and chloroform-based CS extract was conducted by Morshed and Islam [23]. The main phytochemical constituents subjected to analysis were glycosides, terpenoids, flavonoids, tannins, steroids, phenols, saponins, amino acids, sugars, and carbohydrates. It was observed that flavonoids and glycosides were found in all three extracts whereas sugars and steroids were only found in ethanol and methanol-based extracts. However, terpenoids, phenols, saponins, amino acids, carbohydrates, and tannins were not found in any of the extracts. In another study conducted by Emmanuel et al. [24], the methanol extract of a fresh matured corn silk sample (FMCSS) and dried matured corn silk sample (DMCSS) were taken to check the occurrence of phenols, alkaloids, cardiac glycosides, flavonoids, terpenes, steroids, glycosides, tannins, anthraquinones, saponins, balsams, triterpenoids, phlobatannins, resins, and volatile oils. It was observed that phenol and volatile oil were not present in the fresh sample but were present in the dried sample and resin was only present in the fresh sample, whereas alkaloids, cardiac glycosides, saponins, flavonoids, steroids, tannins, glycosides, and balsams were present in both samples, and terpenoids, triterpenoids, phlobatannins, and cardenolides were not found in any of the samples. Solihah et al. [25] used the aqueous as well as methanolic extract of Malaysian CS for preliminary screening of phytochemical constituents. The results disclosed that phenols, flavonoids, alkaloids, saponins, phlobatannins, tannins, and cardiac glycosides were present in both extracts, whereas anthraquinones and terpenoids were noticed in methanolic extract alone. Besides these, sterols and protein-xanthoprotein were not found in any of the extracts as shown in Table 1.
Comparison of the phytochemicals found in different varieties of CS using different methods of extraction
| Variety of CSs | Type of CS extract | Phytochemical profile | References |
|---|---|---|---|
| CV. Mohar | Ethanol | Flavonoids, glycosides, steroids, and sugar | [23] |
| Methanol | Flavonoids, glycosides, steroids, and sugar | ||
| Chloroform | Flavonoids and glycosides | ||
| — | Methanol | Tannins, saponins, steroids, alkaloids, flavonoids, glycosides, cardiac glycosides, balsams, phenol, volatile oil, and resin | [24] |
| Malaysian CS | Methanol | Phenols, flavonoids, alkaloids, saponins, phlobatannins, tannins, cardiac glycosides, anthraquinones, and terpenoids | [25] |
| Aqueous | Phenols, flavonoids, alkaloids, saponins, phlobatannins, tannins, and cardiac glycosides | ||
| Purple waxy corn, white waxy corn, and super sweet corn | Methanol | Total phenolic content, total flavonoid content, and total anthocyanin content | [26] |
| Baby corn (Pacific 271 and Zeba SG 17 hybrid) | Ethanol | Flavonoids, tannins, terpenoids, steroids, and total phenolic content | [4] |
| Aqueous | Flavonoids, tannins, and total phenolic content | ||
| P.1543 | Consecutive extraction (CE), Individual extraction (IE), and consecutive extraction of crude methanolic extraction (CECME) | Phenolic acids, flavonoids, ascorbic acid, tannins, and cardiac glycosides | [22] |
| Purple, green, pink, and yellow (ZP Exp, ZP 555, ZP 341, ZP 366) | Methanol | Total phenolics, flavonoids, anthocyanins, and proanthocyanidins | [27] |
| — | Hydro-ethanolic extract | Tannins, catechic tannins, flavonoids leucoanthocyans, sterols and terpens, heterosid sterodic, coumarins, alkaloids, cardiac glycosides, oses, and holosides and anthocyanins | [28] |
| Aqueous extract | Tannins, catechic tannins, flavonoids leucoanthocyans, coumarins, alkaloids, cardiac glycosides, mucilage, and anthocyanins | ||
| Brazilian CS | Ethanolic extract | Flavonoids, tannins, phenols and terpenoids | [29] |
Sarepoua et al. [26] estimated the total phenolic, flavonoid, and anthocyanin content in the silk of purple waxy corn, white waxy corn, and super sweet corn at various stages of maturity. Methanolic extracts of CS samples were prepared using a modified method and it was found that purple waxy corn had higher than 100 µg GAE/g of total phenolic content whereas in white waxy corn and super sweet corn, the total phenolic content had less than 100 µg GAE/g. The highest total flavonoid concentration was found in purple CS, while super sweet CS with an intermediate level, and white waxy CS had the lowest. Similarly, super sweet CS and white waxy CS had lower anthocyanin contents, whereas purple waxy CS had the greatest (23.9–46.0 µg C3G/g). Moreover, the phytochemical analysis of two baby CS varieties Pacific 271 and Zeba SG 17 hybrid was done in a study conducted by Limmatvapirat et al. [4]. The ethanolic and aqueous extracts of both varieties were made and the result showed the presence of flavonoids and tannins in both the extracts. However, terpenoids and steroids were only found in ethanol extracts and alkaloids were not detected in any of them. Furthermore, the study presented that the amount of total phenolic contents and the total flavonoid contents in 40% v/v ethanol extracts were significantly higher than those of aqueous extracts. Nawaz et al. [22] conducted a study to obtain a high yield of phytochemicals using the best extraction method. CS variety (P.1543) was used for extraction by various methods such as consecutive extraction, individual extraction (IE), and consecutive extraction of crude methanolic extraction. The presence of flavonoids, tannins, phenolic acids, cardiac glycosides, and ascorbic acid was confirmed through phytochemical screening but saponins were absent in all three extracts. The amount of total phenolic content (0.11 ± 0.02–2.34 ± 0.3 g/100 g dw), tannins (0.031 ± 0.013–2.276 ± 0.12 g/100 g dw), flavonoid (0.03 ± 0.005–1.65 ± 0.12 g/100 g dw), and ascorbic acid (0.008 ± 0.001–0.164 ± 0.017 g/100 g dw) and their extraction yield was found to be comparatively higher in IE method.
Apart from this, the amount of total phenolics, flavonoids, anthocyanins, and proanthocyanidins of purple, green, pink, and yellow CSs was estimated at different maturity stages in a study led by Žilić et al. [27]. The observation depicted that flavonoids were present in all the varieties of CS and anthocyanins were only present in purple and pink CS which also contributes to their color, whereas proanthocyanidins were only found in purple-colored corn. Also, the phenolic and flavonoid content was found to be 2–4 folds higher in fresh silks as compared to the mature ones. Another study presented by Ammor et al. [28] showed the presence of phytoconstituents such as alkaloids, flavonoids, anthocyanins, heterosid sterodic, coumarins, leucoanthocyans, cardiac glycosides, and tannins in both aqueous and hydro ethanolic extracts of stigmata of Zea mays. While, terpenoids, sterols, oses, and holosides were only found in hydro-ethanolic extract, and mucilage was found only in aqueous extract. Nevertheless, saponosids, gallic tannins, and triterpens heterosids were not found in any of the extracts. A qualitative phytochemical screening was done on the ethanolic CS extract by Azevedo et al. [29] to identify its phytoconstituents including, alkaloids, flavonoids, phenols, tannins saponins, carbohydrates, terpenoids, and anthraquinones. The result showed positive for flavonoids, tannins, phenols, and terpenoids but negative for other constituents.
4 Antimicrobial properties of phytochemicals present in CS
CS being a major source of bioactive metabolites possesses various antimicrobial and antioxidant functions and several CS extracts have shown antibacterial activity against common pathogens and food spoilage bacteria [30]. The phytochemical composition of CS is directly linked to its antimicrobial effect and several researchers are trying to investigate the efficiency of those bioactive compounds as antimicrobial agents and how they combat the growth of different microbial strains [31]. In most cases, the plant extracts contain complex bioactive ingredients that act synergistically in one way or the other to kill the microorganism. The cytoplasmic membrane is the main target site of bioactive compounds and they disrupt its structural integrity, functionality, and permeability in different ways. Certain phytochemicals can also inhibit the efflux pump and normal cell communication or quorum sensing (QS) [32]. QS is related to cell-cell interactions and through which the bacterial cells determine their cell density [33]. Besides this, some phytochemicals can inhibit cell wall construction, inhibit the formation of biofilm, and inhibit microbial DNA replication or energy synthesis and some can even induce reactive oxygen species production in the microorganism [34]. Figure 2 shows the antimicrobial mechanism of certain bioactive compounds that are found in CS.
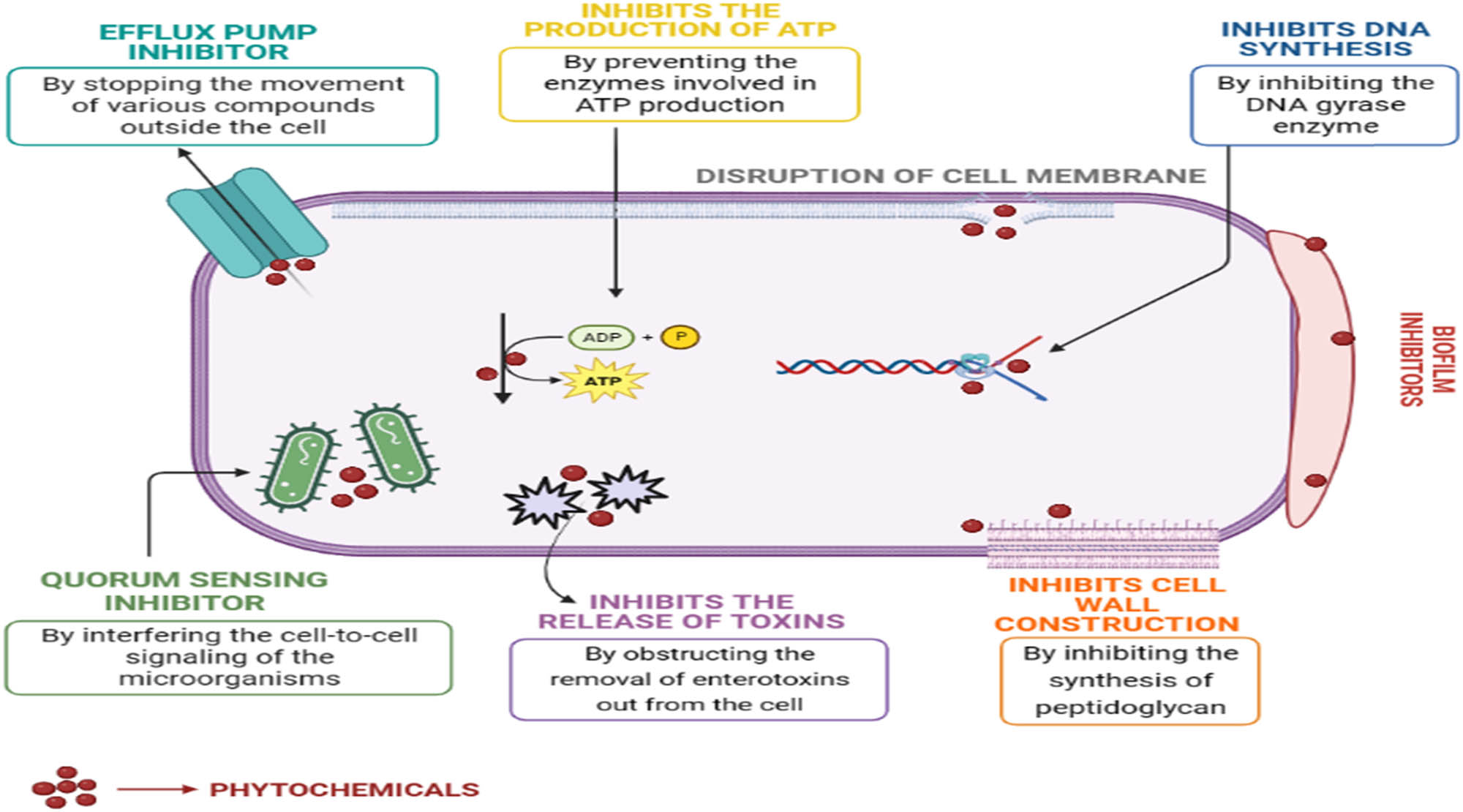
Antimicrobial mechanism of action of phytochemicals that are conspicuously found in CS. Source: Figure prepared by the authors.
4.1 Phenolics and polyphenols
Phenolic chemicals found in hundreds of plant extracts have shown strong antibacterial action against a wide range of microorganisms For example, chemicals extracted from nutmeg fruit seed kernels have shown potent antibacterial properties against 29 types of gram-negative bacteria [35]. The presence of galloyl groups and the arrangement of hydroxyl or methoxyl groups contribute to this activity. When phenolic chemicals interact with the cell membranes of bacteria, the cell wall is disrupted and the contents of the cell are released. They have the ability to inhibit the production of proteins, change metabolic pathways, and destroy bacterial cells. Furthermore, polyphenols can influence the formation of biofilms by QS or structural modifications, and they can prevent DNA synthesis by specifically targeting DNA gyrase [36]. While some phenolic compounds have antifungal effects against a variety of human infections, others, like β-caryophyllene, eugenol, 2-heptanone, and eugenyl acetate, also display antimicrobial action against fungi such as Candida albicans, Aspergillus niger, Aspergillus flavus, and Alternaria solani [37]. Furthermore, polyphenols inhibit the expression of virus proteins on cell surfaces and stop viruses from entering cells, thereby exhibiting antiviral properties [38]. The next sections examine the antibacterial mechanisms of the main phenolic compounds present in CS extracts.
4.2 Phenolic acids
The antimicrobial activity of phenolic acid is associated with the number and position of the hydroxyl group on the aromatic ring. Phenolic acids are capable of permeabilizing and destabilizing the cytoplasmic membrane of microorganisms and they can inhibit the synthesis of nucleic acids in bacteria. They can prevent free radical generation by inhibiting the enzymes like lipoxygenases, cytochrome P450, xanthine oxidase, and cyclooxygenase [39]. Phenolic acid (p-coumaric acid) has shown antibacterial action counter to both the gram-negative and gram-positive bacteria by increasing their bacterial membrane permeability and by binding to the phosphate anion of their DNA. Another phenolic acid (chlorogenic acid) was reported to have a bactericidal effect against Stenotrophomonas maltophilia, Klebsiella pneumoniae, Helicobacter pylori, Escherichia coli, Staphylococcus epidermidis, and S. aureus because of its inhibitory effect against multidrug efflux system and on biofilm formation [40].
4.3 Flavonoids
Flavonoids are important secondary metabolites exhibiting antimicrobial activity due to their ability to inhibit microbial growth through various mechanisms, including the inhibition of cell envelope synthesis, nucleic acid synthesis, ATP synthesis, bacterial efflux pumps, and bacterial toxins [1]. Flavonoids can also form complexes with bacterial cell walls and can disrupt microbial membranes. Some flavonoids can obstruct the enzymes involved in the formation of fatty acids, while others can lead to bacterial cell wall destruction by inhibiting the synthesis of peptidoglycan, which is an important component of the cell wall. They enhance the membrane permeability in some bacteria which is consistent with their effect on efflux-pump inhibitors and anti-biofilm formation [28]. Based on different backbone structures, flavonoids can be classified into flavonols, flavanols, flavones, flavanones, isoflavonoids, and anthocyanidins. All of these have shown good antibacterial properties against both gram-negative and gram-positive bacteria likely due to their ability to form complexes with cell membranes and proteins, damaging the lipid layer formation, inhibiting biofilms and energy metabolism, or by binding the enterotoxins [41].
4.4 Coumarins
The structural characteristics of coumarins, such as the presence of heterocyclic ring and free hydroxyl group, are responsible for their antimicrobial property [42]. Several coumarin derivatives have shown good antibacterial effects against E. coli, S. aureus, B. subtilis, and antifungal effects against C. albicans and A. niger [43]. Likewise, coumarins extract from medicinal plants have found to be effective against Enterobacter aerogenes, B. subtilis, Salmonella enterica Typhi, S. aureus, K. pneumoniae, H. pylori, and Enterobacter cloacae. Some coumarins can powerfully inhibit DNA gyrase and efflux pump systems and they also can inhibit the development of biofilm formation, QS network, and virulence factor production [26].
4.5 Tannins
Tannins contribute to the strong antibacterial activity due to the presence of phenolic hydroxyls and their other structural properties. They can inhibit cell wall synthesis by inactivating the enzymes or by directly binding to the peptidoglycan layer. Tannins can chelate ferric iron and thus make it unavailable for bacterial growth. Tannins can also inhibit the biosynthesis of fatty acids and can act as QS inhibitors [44]. The antimicrobial mode of action of tannins is related to their ability to inactivate microbial adhesins, enzymes, and cell envelope transport proteins and other proteins. Apart from this, tannins are capable of performing physiological functions such as host-mediated tumor activity, phagocytic cell activation, and anti-infective actions. For instance, the tannin of Sorghum has shown antimicrobial activity against certain bacteria like S. aureus, Salmonella typhimurium and fungi like A. niger, A. flavus, and Saccharomyces cerevisae [45].
4.6 Terpenes or terpenoids
Terpenoids or terpenes, also called isoprenoids, are the major bioactive compounds that have acted as great antimicrobial agents against several microorganisms due to their lipophilic characteristics [46]. They are known to have effective activity against bacteria, viruses, fungi, and protozoa. Although the antimicrobial action of terpenoids is not fully understood, it is assumed that they can disrupt the cell membrane by disrupting the lipophilic compounds [33]. Terpenoids can diffuse into the phospholipid bilayer of bacteria and can alter the difference in ATP concentration both inside and outside the cell. It leads to the disorder of the cell membrane and thus shows the bactericidal effect [47]. Terpenes can cause cell death in resistant microbes by disrupting their membrane permeability and obstructing their cell growth. Additionally, terpenes like carvacrol, geraniol, and thymol exhibit strong antibiofilm activity against various bacterial and fungal biofilms [48]. For instance, petalostemumol (terpenoid) had shown excellent activity against B. subtilis and S. aureus as compared to gram-negative bacteria as well as C. albicans. Two diterpenes isolates were found to inhibit the growth of S. aureus, Vibrio cholerae, P. aeruginosa, and Candida spp. [49]. Sesquiterpene (terpene) can inhibit the mycelial growth of plant pathogenic fungi such as Sclerotinina sclerotiorum and can also produce a total inhibition of spore germination on Fusarium graminearum, Pyricularia oryzae, and Gloeosporium fructigenum [50]. Some studies have identified that the presence of terpenes could even inhibit HIV-1 by creating a cytotoxic environment and by resisting its proliferation. Besides, terpenes have shown the inhibition of several viruses, such as retroviruses, murine leukemic virus, and simian immunodeficiency virus [51].
4.7 Saponins
Saponins are structurally diverse compounds that have demonstrated distinct antimicrobial properties against a variety of gram-positive and negative bacteria, fungi, and yeast [52]. A crude saponin extract was tested for antibacterial infection against five bacterial species (P. aeruginosa, K. pneumoniae, B. subtilis, S. aureus, E. coli) and it showed an active antibacterial effect against all of them. Some studies have shown the antibiotic effect of saponins on S. aureus because they can reduce the production of α-toxin and α-hemolysin and can inhibit the formation of biofilms. Saponins exert antiviral action against several strains of viruses such as poliovirus, herpes simplex virus, A and B influenza virus, SARS, and HIV either by interfering with the attachment of the virus to the cells or by affecting the genomic replication of it [53]. Saponins are involved in membrane disruption of cells that increases its permeability and they can also bind with the sterol lipids in the membrane which forces it to curve leading to disruption of pore formation [54]. The antifungal property of saponins is related to their ability to form complexes with sterols, leading to pore formation and causing the loss of membrane integrity [55].
4.8 Alkaloids
Alkaloids are heterocyclic nitrogen compounds that show antimicrobial functions through various mechanisms. Alkaloids cause cell death by damaging their cell structure and by acting as an inhibitor for protein and DNA synthesis. They possess potent Epigenetics activity and they can also inhibit DNA replication by inhibiting type II topoisomerase enzymes [56]. Alkaloids exhibit antimicrobial activity by intercalating into the cell wall and DNA of bacteria. For instance, berberine (alkaloid) can intercalate with DNA and increase the membrane permeability of bacteria by disrupting its membrane structure [57]. They are also capable of inhibiting toxins, QS, and virulence gene expression and are involved in destructing enzyme production and hindering biofilm formation [58].
Alkaloids such as sanguinarine, piperine, and quinine possess a broad spectrum of antibacterial action against E. coli, B. subtilis, and S. aureus [59]. The alkaloid fractions isolated from Strychnos potatorum L.f. (Loganiaceae) at the tested concentrations showed considerable antimicrobial activity against bacteria and fungi. Further, they significantly inhibited the growth of Proteus vulgaris, S. aureus, V. cholerae, S. typhimurium, Mycobacterium tuberculosis, C. albicans, and A. niger [60]. Some studies have reported that about 43 alkaloids can exhibit antiviral activity against the influenza virus. It can be due to the induction of interferons of the immune system or by enhanced activity of macrophages to destroy the virus. Alkaloids obtained from various plant extracts are known to display antiviral activity against dengue virus and porcine epidemic diarrhea virus [61].
5 Antimicrobial activity of CS extracts
The high phytochemical profile of CS and its efficient bactericidal action reveals that it has great potential to kill several microorganisms. Many studies have been investigated to know the antimicrobial action of CS against most common pathogens and food spoilage microbes. Thus, Tables 2 and 3 show how the different solvent extracts of CS exhibit a wide range of antimicrobial activity against the various strains of bacteria and fungi respectively.
Antibacterial properties of CS using different types of extract
| Type of extract | Bacterial species | Inhibition zone (mm) | Concentration of extract/minimum inhibition concentration (MIC) | Positive control | References |
|---|---|---|---|---|---|
| Ethanol extract | E. coli | 3–10 | 700 (µg) | — | [62] |
| S. aureus | |||||
| Porteus mirabilis | 4–15 | 600 (µg) | |||
| Ethanol extract | S. aureus | 19 | 500 mg/mL | Andrographis paniculata | [63] |
| Bacillus coli | 15 | >500 mg/mL | |||
| P. aeruginosa | 20 | >500 mg/mL | |||
| Pet-ether extract | Bacillus cereus | 12 | 25 mg/mL | Gentamycin (50 µg/mL) | [64] |
| B. subtilis | 11 | ||||
| S. aureus | 10 | ||||
| P. aeruginosa | 8 | ||||
| E. aerogenes | 8 | ||||
| Salmonella typhi | 9 | ||||
| Salmonella paratyphi | 8 | ||||
| E. coli | 0 | ||||
| Shigella sonnei | 10 | ||||
| Shigella flexneri | 5 | ||||
| P. mirabilis | 11 | ||||
| Chloroform extract | B. cereus | 11 | 25 mg/mL | ||
| B. subtilis | 11 | ||||
| S. aureus | 4 | ||||
| P. aeruginosa | 0 | ||||
| E. aerogenes | 7 | ||||
| S. typhi | 0 | ||||
| S. paratyphi | 0 | ||||
| E. coli | 0 | ||||
| S. sonnei | 8 | ||||
| Shigella flexneri | 0 | ||||
| P. vulgaris | 0 | ||||
| P. mirabilis | 0 | ||||
| Methanol extract | B. cereus | 10 | 25 mg/mL | ||
| B. subtilis | 11 | ||||
| S. aureus | 8 | ||||
| P. aeruginosa | 10 | ||||
| E. aerogenes | 11 | ||||
| S. typhi | 11 | ||||
| S. paratyphi | 7 | ||||
| E. coli | 0 | ||||
| S. sonnei | 10 | ||||
| Shigella flexneri | 7 | ||||
| P. vulgaris | 8 | ||||
| P. mirabilis | 6 | ||||
| Ethanol extract | B. cereus | 13 | 10 mg/mL | Streptomycin (10 mg/mL) | [23] |
| B. subtilis | 12 | ||||
| S. aureus | 11 | ||||
| P. aeruginosa | 9 | ||||
| S. sonnei | 11 | ||||
| Shigella flexneri | 8 | ||||
| P. vulgaris | 12 | ||||
| P. mirabilis | 12 | ||||
| E. aerogenes | 10 | ||||
| S. typhi | 10 | ||||
| S. paratyphi | 9 | ||||
| E. coli | 0 | ||||
| Chloroform extract | B. cereus | 12 | 10 mg/mL | ||
| B. subtilis | 12 | ||||
| S. aureus | 5 | ||||
| P. aeruginosa | 0 | ||||
| S. sonnei | 9 | ||||
| Shigella flexneri | 0 | ||||
| P. vulgaris | 0 | ||||
| P. mirabilis | 0 | ||||
| E. aerogenes | 8 | ||||
| S. typhi | 0 | ||||
| S. paratyphi | 0 | ||||
| E. coli | 0 | ||||
| Methanol extract | B. cereus | 12 | 10 mg/mL | ||
| B. subtilis | 12 | ||||
| S. aureus | 10 | ||||
| P. aeruginosa | 12 | ||||
| S. sonnei | 11 | ||||
| Shigella flexneri | 8 | ||||
| P. vulgaris | 9 | ||||
| P. mirabilis | 7 | ||||
| E. aerogenes | 12 | ||||
| S. typhi | 12 | ||||
| S. paratyphi | 6 | ||||
| E. coli | 0 | ||||
| Ultrasound assisted extraction (UAE) | B. cereus | — | 62.5 (µg/mL) | Chloramphenicol and Ampicillin | [16] |
| B. subtilis | 62.5 (µg/mL) | ||||
| S. aureus | 125 (µg/mL) | ||||
| Enterococcus fecalis | 62.5 (µg/mL) | ||||
| P. aeruginosa | 125 (µg/mL) | ||||
| Shigella flexneri | 125 (µg/mL) | ||||
| P. mirabilis | 250 (µg/mL) | ||||
| E. coli | 62.5 (µg/mL) | ||||
| S. sonnei | 62.5 (µg/mL) | ||||
| S. typhimurium | 62.5 (µg/mL) | ||||
| Salmonella enteritidis | 125 (µg/mL) | ||||
| K. pneumoniae | 125 (µg/mL) | ||||
| Morganella morganii | 62.5 (µg/mL) | ||||
| E. aerogenes | 125 (µg/mL) | ||||
| Hexane extract | E. coli | — | ≥1,024 (µg/mL) | — | [65] |
| S. aureus | ≥1,024 (µg/mL) | ||||
| P. aeruginosa | ≥1,024 (µg/mL) | ||||
| Ethanol extract | Propionibacterium acnes | 19 | — | Clindamycin (1%) | [66] |
| S. epidermidis | 11 | ||||
| S. aureus | 3 | ||||
| Aqueous extract | S. aureus, S. saprophyticus, S. epidermidis, S. pneumonia, S. progenies, S. agalactiae, S. mutans, and E. feacalis | 26–32 | — | Ciprofloxacin | [67] |
| S. typhimurum | 35 | ||||
| S. typhi | 34 | ||||
| K. pneumoniae | 17 | ||||
| Methanol extract of FMCSS | P. aeruginosa | 8–14 | (150–600 mg/mL) | Ciprofloxacin (30 µg/ml) | [24] |
| S. typhi | 0–10 | ||||
| E. coli | 7–14 | ||||
| Klebsiella pneumonia | 8–13 | ||||
| S. aureus | 9–16 | ||||
| Methanol extract of DMCSS | P. aeruginosa | 10–16 | (150–600 mg/mL) | ||
| S. typhi | 7–12 | ||||
| E. coli | 9–15 | ||||
| Klebsiella pneumonia | 11–16 | ||||
| S. aureus | 12–20 | ||||
| Microwave assisted extraction (MAE) | S. aureus | 20 | — | Piperacillin-tazobactam (TZP) | [68] |
| Enterococcus | 25 | ||||
| E. coli | 19 | ||||
| Acenobacter baumanni | 23 | ||||
| Ethanol extract | S. aureus | — | 12.5 (mg/mL) | Quercetin | [29] |
| S. epidermidis | 12.5 (mg/mL) | ||||
| E. coli | 50 (mg/mL) | ||||
| P. aeruginosa | 25 (mg/mL) |
Antifungal properties of different CS extracts
| Type of extract | Fungal species | Minimum fungicidal concentration (MFC)/MIC | Positive control | References |
|---|---|---|---|---|
| Ethanol extract | A. niger | 1.8 mg/20 mL (MFC) | — | [62] |
| A. flavus | 2 mg/20 mL (MFC) | |||
| A. brasiliensis | 1.6 mg/20 mL (MFC) | |||
| MAE | Aspergillus nidulans | 1.4 cm (colony diameter) | Axicon | [68] |
| A. flavus | 1.5 cm (colony diameter) | |||
| Aspergillus fumigatus | 1.9 cm (colony diameter) | |||
| UAE | C. albicans | 125 (µg/mL) (MIC) | Fluconazole and Nystatin | [16] |
| Candida parapslosis | 250 (µg/mL) (MIC) | |||
| Candida krusei | 125 (µg/mL) (MIC) | |||
| Candida tropicalis | 500 (µg/mL) (MIC) | |||
| Cryptococcus neoformans | 125 (µg/mL) (MIC) | |||
| Cryptococcus gatti | 125 (µg/mL) (MIC) | |||
| Candida dubliniensis | 250 (µg/mL) (MIC) | |||
| Candida glabrata | 500 (µg/mL) (MIC) | |||
| Saccharomyces cerevisiae | 250 (µg/mL) (MIC) | |||
| Pet-ether, chloroform and methanol extract | C. albicans | 0 | Gentamycin (50 µg/mL) | [64] |
| Ethanol extract | C. albicans | 125 mg/mL (MIC) | Andrographis paniculata | [63] |
| Ethanol, chloroform methanol extract | C. albicans | 0 | Streptomycin | [63,23] |
According to Abirami et al. [62], the ethanol extract of CS was checked against (UTI) Urinary Tract Infection causing bacteria and some isolated fungi. The inhibitory action of the extract was observed against all UTI-isolated bacteria, where the highest inhibition zone was found against Proteus mirabilis (15 mm) followed by K. pneumoniae (12 mm), E. coli (10 mm), and S. aureus (9 mm), whereas the lowest minimum bactericidal concentration value was found to be 700 μg against K. pneumoniae and P. mirabilis. Further, the antifungal activity of ethanolic CS extract was tested against three isolated fungi and the result showed that the percentage of inhibition of mycelial growth was highest in Aspergillus brasiliensis, followed by A. niger, and A. flavus, at MFC 1.6 mg/20 mL, 1.8 mg/20 mL, and 2 mg/20 mL respectively. In another study by Feng et al. [63], an ethanolic extract of CS was tested against Bacillus cereus, P. aeruginosa, Bacillus coli, S. aureus, and C. albicans in the presence of Andrographis paniculata as a standard antibiotic. The result showed that the maximum antibacterial activity of the silk extract was found against B. cereus with the inhibition zone of (28 mm) and MIC of 62.5 mg/mL, followed by S. aureus with inhibition zone of (19 mm) and MIC of 500 mg/mL. Whereas, the extract was unable to inhibit B. coli and P. aeruginosa even at the maximum concentration of (500 mg/mL). However, it showed a good antifungal activity against C. albicans with the inhibition zone of (21 mm) and MIC of (250 mg/mL). These findings state that the ethanolic extract of CS is quite effective against several UTI bacteria and Fungi and it can be used against some specific class of microorganisms.
Various organic solvent extracts of CS were evaluated for their antimicrobial properties in a study by Nessa et al. [64]. All the different extracts (pet-ether, chloroform, and methanol) were evaluated for their antimicrobial activity against four gram-positive bacteria (S. aureus, B. subtilis, P. aeruginosa, B. cereus), eight gram-negative bacteria (E. aerogenes, S. typhi, S. paratyphi, E. coli, Shigella sonnei, S. flexneri, P. vulgaris, P. mirabilis) and one yeast (C. albicans). The gentamicin was taken as a reference antibiotic. The result revealed that the pet-ether extract (25 mg/mL) and the methanolic extract of CS (25 mg/mL) were effective against eleven out of twelve bacterial strains as shown in Table 2, whereas the chloroform extract (25 mg/mL) was only sensitive against the five bacterial strains and none of the extracts showed any sensitivity against the yeast (C. albicans). Similarly, Morshed and Islam [23] have also published a study in which they have determined the antimicrobial action of diverse microorganisms with ethanol, methanol, and chloroform extract of CS. They tested the four gram-positive bacteria (B. subtilis, S. aureus, B. cereus, P. aeruginosa), eight gram-negative bacteria (S. sonnei, S. flexneri, P. vulgaris, P. mirabilis, E. aerogenes, Salmonella typhi, S. paratyphi, and E. coli) and one yeast strain of C. albicans by taking streptomycin as a positive control. Methanol and ethanol extracts showed a sensitive response against all the bacteria except E. coli. While, chloroform extract showed sensitive response only against five (B. cereus, B. subtilis, S. aureus, S. sonnei, and E. aerogenes) bacterial strains. However, C. albicans did not show any response in all the three CS extracts. Different types of CS extracts have different impact on the outcomes and alcohol-based extracts are more effective than the others like pet-ether and chloroform.
A recent study put forward by Boeira et al. [31] showed the antibacterial and antifungal activity of CS extract by using UAE. Twenty-three microorganisms were collected and amongst them, five were gram positive, ten were gram negative bacteria and nine were clinically isolated yeasts. Chloramphenicol and ampicillin were used as a standard for bacterial analysis, whereas fluconazole and nystatin were used to control the sensitivity of the antifungal test. The UAE CS extract displayed a significant inhibition action against both the gram-negative and gram-positive bacteria, with MIC range between (62.5 and 250 µg/mL) as shown in Table 2. It also showed the antifungal potential by inhibiting the growth of fungi, with MIC values between (125 and 500 µg/mL) as shown in Table 3. The study also revealed that UAE CS extract showed better bactericidal potential than the positive control for B. cereus, B. subtilis, Enterococcus fecalis, E. coli, S. sonnei, S. typhimurium, and Morganella morganii bacteria at MIC of (62.5 µg/mL). Another study performed by Carvalho et al. [65] with Z. mays silk hexane extract against S. aureus, E. coli, and P. aeruginosas strains was done to determine the minimum inhibitory concentration. The result showed that the extract did not present a significant antibacterial effect even at the MIC values (≥1,024 µg/mL). However, the association of the hexane extract was then done with certain antibiotics and this modulation apparently decreased the MIC value for the antibiotics.
Another study was conducted by Nurani et al. [66] to assess the antibacterial effect of CS’s ethanolic extract against three acne-related bacteria S. aureus, S. epidermidis, and Propionibacterium acnes at increasing concentrations (10 to 100%). Clindamycin was taken as a standard antibiotic and its effectiveness was also taken into account. The result showed that the antibacterial activity of P. acnes and S. epidermidis was getting increased at the higher concentrations and that for S. aureus it was getting decreased (>70%). The diameter of the inhibition zone for P. acnes, S. epidermidis, and S. aureus was found to be 19.6 ± 0.09, 11.4 ± 0.4, and 2.8 ± 2.8 mm respectively at 100% concentration. The antimicrobial activity of aqueous extract of CS was estimated against different bacterial isolates in a study investigated by Saleh et al. [67]. The result showed that the extract was able to inhibit the growth of 11 gram-negative and 8 gram-positive bacterial pathogens according to the inhibition zone and the antibiotic ciprofloxacin was taken as standard. For gram-positive bacteria such as S. aureus, S. saprophyticus, S. epidermidis, S. pneumonia, S. progenies, S. agalactia, S. mutants, and E. faecalis, the inhibition zone ranged between (26 and 32 mm). Whereas, for gram-negative bacteria, the extract showed the maximum inhibition zone against S. typhimurum (35 mm), followed by S. typhi (34 mm) and the lowest value was shown by K. pneumonia (17 mm). From the above-mentioned results of different studies, it is identified that CS extracts are more effective against gram-positive bacteria than gram-negative bacteria. This variable susceptibility could be attributed to the difference in the structural composition of the cell walls of both bacteria.
Many studies have shown that the phytochemical composition of CS changes with the different maturity stages as well as with the different processing techniques. Later, Emmanuel et al. [24] recapitulated a study in which the antimicrobial activity of the methanolic extract of fresh matured CS and dried matured CS was done against bacteria by taking ciprofloxacin as a positive control. The result showed a positive effect of CS extract against E. coli, P. aeruginosa, K. pneumoniae, S. typhi, and S. aureus with the inhibition zone ranging between (10–16 ± 0.50 mm) for a fresh sample and (12–20 ± 2.50 mm) for the dried samples respectively. Qaiser et al. [68] evaluated the antagonist action of CS extract toward various fungal and bacterial pathogens. The MAE of CS was tested against the two gram-negative (Acenobacter baumanni and E. coli) and two gram-positive (Entarococcus and S. aureus) bacteria by taking piperacillin-tazobactam (TZP) as a positive control. The result showed that the maximum inhibition zone (20 and 25 mm) was obtained against the gram-positive bacteria followed by the gram-negative bacteria (19 and 23 mm). Alternatively, the (MAE) CS extract was also tested against fungus (Aspergillus nidulans, A. flavus, and Aspergillus fumigatus) by taking axicon as a positive control. The results revealed that the extract was able to reduce the growth of fungal pathogens. The antimicrobial activity of CS ethanolic extract was evaluated using four bacteria strains in a study directed by Azevedo et al. [29]. A more significant bactericidal effect was shown against gram-positive bacteria (S. aureus and S. epidermidis) with a minimum inhibition concentration of (12.5 mg/mL each) than in gram-negative bacteria (E. coli and P. aeruginosa) having MIC of (50 mg/mL) and (25 mg/mL) respectively. Quercetin was taken as a positive control and the analysis was done using the minimal bactericidal concentration of CS extract at (50 mg/mL).
6 Future prospective
CS can become one of the natural, cheap, and safe ingredients to be used for the formulation of new food products. It can be used as a natural antimicrobial and antioxidant ingredient in the food industry. Once thought of as an agricultural byproduct, maize silk is becoming recognized as an important, natural, and affordable ingredient with a wide range of uses in the culinary and medicinal sectors. The antibacterial and antioxidant qualities of this natural resource make it extremely promising. CS can act as a natural antibacterial agent in the food industry, preventing the growth of pathogenic microbes. Furthermore, because of its strong antioxidant properties, it is an ideal component of bioactive packaging materials that increase food products’ shelf-life by protecting them from oxidative damage. CS’s strong antioxidant and free radical-scavenging properties also make it an appropriate choice for use in animal feeds and nutritional supplements, especially in conditions with high oxidation-prone fat and lipid concentrations. CS’s high antioxidant activity is advantageous for both improving health and preserving food. It might boost the immune system as an immunostimulatory agent, adding protection against dangerous microbial diseases like coronavirus disease 2019 and monkeypox. Numerous studies on different CS extracts have shown how therapeutically they can be used. Its characteristics have been discovered to have potential use in the treatment of several chronic disorders, including diabetes, liver diseases, cancer, kidney diseases, hypertension, and parasite infections. This wide range of possible uses highlights CS’s potential as a natural therapeutic agent. Furthermore, the chemical components of CS extract have the potential to be used in the development of innovative pharmaceuticals. These include antifungal lotions for skin infections, novel antibiotic medications that can fight resistant bacterial strains, and other medicinal formulations. Despite its exceptional properties, more study is required to fully understand and utilize the potential of CS ingredients in application in various food and medical industries.
7 Conclusion
Microbial diseases are becoming more predominant in India due to antibiotic resistance. To resolve this problem, some alternative chemotherapeutic agents are being searched. This study has shown that CS is a good source of antimicrobial agents that can prevent the extension of microbial infections. CS contains many different bioactive compounds such as phenols, flavonoids, tannins, saponins, and terpenes which not only provide therapeutic effects but also act as a good antimicrobial agent. The antibacterial and antifungal effect of CS is majorly dependent on its variety, phytochemical composition, extraction solvent, and method of extraction. CS has proven to be an effective natural compound because, in the above-mentioned studies, it has shown good antimicrobial activity under lower values of minimum inhibition concentrations. It could be used for the formulation of new antimicrobial drugs which will eventually decrease the burden of antibiotic resistance. Thus, CS is worthy of further research which will elucidate its impact on human health.
Acknowledgements
The authors would like to thanks Lovely Professional University for providing faciltiies and databases to write the manuscript.
-
Funding information: The authors wish to thank the research center at the College of Pharmacy and Deanship of Scientific Research at King Saud University Riyadh Saudi Arabia for financial support.
-
Author contributions: All the authors have made a considerable contribution in collecting, analyzing, and interpreting the data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. TM: conceptualization and writing – original draft; PR: investigation, supervision, and validation; SK: investigation, supervision, and validation; Amanjyoti: formal analysis, methodology, and validation; SE: formal analysis, investigation, and writing – review and editing; AA: formal analysis, investigation, and writing – review and editing; RC: formal analysis, investigation, and writing – review and editing; RU: formal analysis, methodology, and validation; ASA: formal analysis, methodology, and validation and JS: conceptualization and writing – original draft.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All the data generated and analyzed are included within this review article and will be made available on request.
References
[1] Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. 2019 Feb;18:241–72. 10.1007/s11101-018-9591-z.Suche in Google Scholar
[2] Vashist H, Jindal A. Antimicrobial activities of medicinal plants–review. Int J Res Pharm Biomed Sci. 2012;3(1):222–30.Suche in Google Scholar
[3] Bhuvaneshwari K, Sivakami S. Analysis of nutrients and phytochemicals content in corn silk (Zea mays). Int J Sci Res. 2015;78(96):2319–7064.Suche in Google Scholar
[4] Limmatvapirat C, Nateesathittarn C, Dechasathian K, Moohummad T, Chinajitphan P, Limmatvapirat S. Phytochemical analysis of baby corn silk extracts. J Ayurveda Integr Med. 2020 Jul;11(3):344–51. 10.1016/j.jaim.2019.10.005.Suche in Google Scholar PubMed PubMed Central
[5] Shi S, Li S, Li W, Xu H. Corn silk tea for hypertension: a systematic review and meta-analysis of randomized controlled trials. Evidence-Based Complementary Altern Med. 2019 Jan;2019:2915498. 10.1155/2019/2915498.Suche in Google Scholar PubMed PubMed Central
[6] Mada SB, Sani L, Chechet GD. Corn silk from waste material to potential therapeutic agent: a mini review. Fuw Trends Sci Technol J. 2020;5(3):816–20.Suche in Google Scholar
[7] Elbossaty WF. Medical healthy care of Stigma maydis: Pharmacological review. Sci Food Sc Nutr. 2020;6(1):1–3.Suche in Google Scholar
[8] Vijitha TP, Saranya D. Corn Silk-A medicinal boon. Intern J Chem Tech Res. 2017;10(10):129–37.Suche in Google Scholar
[9] Saikaew K, Lertrat K, Ketthaisong D, Meenune M, Tangwongchai R. Influence of variety and maturity on bioactive compounds and antioxidant activity of purple waxy corn (Zea mays L. var. ceratina). Int Food Res J. 2018 Sep;25(5):1985–95.Suche in Google Scholar
[10] Somavat P, Kumar D, Singh V. Techno-economic feasibility analysis of blue and purple corn processing for anthocyanin extraction and ethanol production using modified dry grind process. Ind Crop products. 2018 May;115:78–87. 10.1016/j.indcrop.2018.02.015.Suche in Google Scholar
[11] Naeem MM. Protective effect of Corn silk (Zea mays L.) on kidney and liver functions of rats. Bull Natl Nutr Inst Arab Repub Egypt. 2022 Dec;60(2):122–53. 10.21608/bnni.2022.273591.Suche in Google Scholar
[12] Odelola OS, Oyetayo VO, Ogundare AO, Omoya FO, Ajayi OE. Antibacterial activity of zea mays silks and husks crude extract on biofilm producing multi-drug resistant bacteria from urinary catheters. Int J Pathog Res. 2023;12(2):37–51. 10.9734/ijpr/2023/v12i2224.Suche in Google Scholar
[13] Li P, Ren G, Sun Y, Jiang D, Liu C. Extraction optimization, preliminary identification, and bioactivities in corn silk. Evidence-based Complementary Altern Med. 2023;2023(1):5685174. 10.1155/2023/5685174.Suche in Google Scholar PubMed PubMed Central
[14] Kaur P, Singh J, Kaur M, Rasane P, Kaur S, Kaur J, et al. Corn silk as an agricultural waste: A comprehensive review on its nutritional composition and bioactive potential. Waste Biomass Valoriz. 2023 May;14(5):1413–32. 10.3390/agronomy12040777.Suche in Google Scholar
[15] Al-Janabi AS, Khadim OM. Mouth gargles and antimicrobial activities of extracts of corn silk. Int J Drug Deliv Technol. 2022;12(3):1025–7.10.25258/ijddt.12.3.18Suche in Google Scholar
[16] Boeira CP, Alves JD, Flores DC, de Moura MR, Melo PT, da Rosa CS. Antioxidant and antimicrobial effect of an innovative active film containing corn stigma residue extract for refrigerated meat conservation. J Food Process Preservation. 2021 Sep;45(9):e15721. 10.1111/jfpp.15721.Suche in Google Scholar
[17] Bernhoft A, Siem H, Bjertness E, Meltzer M, Flaten T, Holmsen E. Bioactive compounds in plants–benefits and risks for man and animals. Proceedings from a symposium held at The Norwegian Academy of Science and Letters, Oslo; 2010. p. 1–255.Suche in Google Scholar
[18] da Hora NR, Santana LF, da Silva VD, Costa SL, Zambotti-Villela L, Colepicolo P, et al. Identification of bioactive metabolites from corn silk extracts by a combination of metabolite profiling, univariate statistical analysis and chemometrics. Food Chem. 2021 Dec;365:130479. 10.1016/j.foodchem.2021.130479.Suche in Google Scholar PubMed
[19] Mulat M, Khan F, Muluneh G, Pandita A. Phytochemical profile and antimicrobial effects of different medicinal plant: current knowledge and future perspectives. Curr Tradit Med. 2020 Mar;6(1):24–42. 10.2174/2215083805666190730151118.Suche in Google Scholar
[20] Wang B, Xiao T, Ruan J, Liu W. Beneficial effects of corn silk on metabolic syndrome. Curr Pharm Des. 2017 Sep;23(34):5097–103. 10.2174/1381612823666170926152425.Suche in Google Scholar PubMed
[21] Logan-del Castillo EJ, Azares GF, Almonte CJ, Pascua KM, Santiago IB, So EG, et al. Effects of incorporation of microwave: Dried corn silk (Stigma maydis) powder on the quality and stability of Beef Patties. In Journal of Physics: Conference Series. Vol. 1529, Issue 3, IOP Publishing; 2020 Apr. p. 032064. 10.1088/1742-6596/1529/3/032064.Suche in Google Scholar
[22] Nawaz H, Aslam M, Muntaha ST. Effect of solvent polarity and extraction method on phytochemical composition and antioxidant potential of corn silk. Free Radic Antioxid. 2019 Apr;9(1):5–11. 10.5530/fra.2019.1.2.Suche in Google Scholar
[23] Morshed S, Islam SM. Antimicrobial activity and phytochemical properties of corn (Zea mays L.) silk. SKUAST J Res. 2015;17(1):8–14.Suche in Google Scholar
[24] Emmanuel SA, Olajide O, Abubakar S, Akiode SO, Etuk-Udo G. Chemical evaluation, free radical scavenging activities and antimicrobial evaluation of the methanolic extracts of corn silk (Zea mays. J Adv Med Pharm Sci. 2016 Jan;9(4):1–8. 10.9734/JAMPS/2016/28530.Suche in Google Scholar
[25] Solihah MA, Rosli WW, Nurhanan AR. Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts. Int Food Res J. 2012 Oct;19(4):1533.Suche in Google Scholar
[26] Sarepoua E, Tangwongchai R, Suriharn B, Lertrat K. Influence of variety and harvest maturity on phytochemical content in corn silk. Food Chem. 2015 Feb;169:424–9. 10.1016/j.foodchem.2014.07.136.Suche in Google Scholar PubMed
[27] Žilić S, Janković M, Basić Z, Vančetović J, Maksimović V. Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): Comparison with medicinal herbs. J Cereal Sci. 2016 May;69:363–70. 10.1016/j.jcs.2016.05.003.Suche in Google Scholar
[28] Ammor K, Amarti FE, Lagzizir R, Mahjoubi F, Bousta D, Chaqroune A. Study of antioxidant, anti-inflammatory, antinociceptive activities and toxicity of stigmata of zea mays extracts. Phytothérapie. 2021 Aug;19(4):216. 10.3166/phyto-2019-0209.Suche in Google Scholar
[29] Azevedo AS, Seibert JB, Amparo TR, Antunes AD, Sousa LR, Souza GH, et al. Chemical constituents, antioxidant potential, antibacterial study and photoprotective activity of Brazilian corn silk extract. Food Sci Technol. 2022 Aug;42:e98421. 10.1590/fst.98421.Suche in Google Scholar
[30] Tian S, Sun Y, Chen Z. Extraction of flavonoids from corn silk and biological activities in vitro. J Food Qual. 2021 Mar;2021:1–9. 10.1155/2021/7390425.Suche in Google Scholar
[31] Boeira CP, Flores DC, Lucas BN, Santos D, Flores EM, Reis FL, et al. Extraction of antioxidant and antimicrobial phytochemicals from corn stigma: a promising alternative to valorization of agricultural residues. Ciência Rural. 2022 Apr;52:e20210535. 10.1590/0103-8478cr20210535.Suche in Google Scholar
[32] Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms. 2021 Sep;9(10):2041. 10.3390/microorganisms9102041.Suche in Google Scholar PubMed PubMed Central
[33] Monte J, Abreu AC, Borges A, Simões LC, Simões M. Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens. 2014 Jun;3(2):473–98. 10.3390/pathogens3020473.Suche in Google Scholar PubMed PubMed Central
[34] Mickymaray S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics. 2019 Dec;8(4):257. 10.3390/antibiotics8040257.Suche in Google Scholar PubMed PubMed Central
[35] Sadeek AM, Abdallah EM. Phytochemical compounds as antibacterial agents a mini review. Saudi Arabia Glob J Pharm Sci. 2019;53(4):1–6. 10.19080/GJPPS.2019.07.555720.Suche in Google Scholar
[36] Efenberger-Szmechtyk M, Nowak A, Czyzowska A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit Rev Food Sci Nutr. 2021 Jan;61(1):149–78. 10.1080/10408398.2020.1722060.Suche in Google Scholar PubMed
[37] Negri M, Salci TP, Shinobu-Mesquita CS, Capoci IR, Svidzinski TI, Seki Kioshima E. Early state research on antifungal natural products. Molecules. 2014 Mar;19(3):2925–56. 10.3390/molecules19032925.Suche in Google Scholar PubMed PubMed Central
[38] Kapoor R, Sharma B, Kanwar SS. Antiviral phytochemicals: an overview. Biochem Physiol. 2017;6(2):7. 10.4172/2168-9652.1000220.Suche in Google Scholar
[39] Borges A, J Saavedra M, Simoes M. Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr Med Chem. 2015 Jul;22(21):2590–614.10.2174/0929867322666150530210522Suche in Google Scholar PubMed
[40] Hochma E, Yarmolinsky L, Khalfin B, Nisnevitch M, Ben-Shabat S, Nakonechny F. Antimicrobial effect of phytochemicals from edible plants. Processes. 2021 Nov;9(11):2089. 10.3390/pr9112089.Suche in Google Scholar
[41] Rahman MM, Rahaman MS, Islam MR, Hossain ME, Mannan Mithi F, Ahmed M, et al. Multifunctional therapeutic potential of phytocomplexes and natural extracts for antimicrobial properties. Antibiotics. 2021 Sep;10(9):1076. 10.3390/antibiotics10091076.Suche in Google Scholar PubMed PubMed Central
[42] Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sánchez E, Nabavi SF, et al. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol Res. 2017 Mar;196:44–68. 10.1016/j.micres.2016.12.003.Suche in Google Scholar PubMed
[43] Cheke RS, Patel HM, Patil VM, Ansari IA, Ambhore JP, Shinde SD, et al. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics. 2022 Apr;11(5):566. 10.3390/antibiotics11050566.Suche in Google Scholar PubMed PubMed Central
[44] Al-Majedy YK, Kadhum AA, Al-Amiery AA, Mohamad AB. Coumarins: The Antimicrobial agents. Syst Rev Pharm. 2017 Jan;8(1):62–70.10.5530/srp.2017.1.11Suche in Google Scholar
[45] Farha AK, Yang QQ, Kim G, Li HB, Zhu F, Liu HY, et al. Tannins as an alternative to antibiotics. Food Biosci. 2020 Dec;38:100751. 10.1016/j.fbio.2020.100751.Suche in Google Scholar
[46] Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—a review. Plants. 2017 Apr;6(2):16. 10.3390/plants6020016.Suche in Google Scholar PubMed PubMed Central
[47] Huang W, Wang Y, Tian W, Cui X, Tu P, Li J, et al. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics. 2022 Oct;11(10):1380. 10.3390/antibiotics11101380.Suche in Google Scholar PubMed PubMed Central
[48] Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013 Dec;6(12):1451–74. 10.3390/ph6121451.Suche in Google Scholar PubMed PubMed Central
[49] AlSheikh HM, Sultan I, Kumar V, Rather IA, Al-Sheikh H, Tasleem Jan A, et al. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics. 2020 Aug;9(8):480. 10.3390/antibiotics9080480.Suche in Google Scholar PubMed PubMed Central
[50] Omojate Godstime C, Enwa Felix O, Jewo Augustina O, Eze Christopher O. Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens–a review. J Pharm Chem Biol Sci. 2014 Aug;2(2):77–85.Suche in Google Scholar
[51] Redondo-Blanco S, Fernández J, López-Ibáñez S, Miguélez EM, Villar CJ, Lombó F. Plant phytochemicals in food preservation: Antifungal bioactivity: A review. J Food Prot. 2020 Jan;83(1):163–71. 10.4315/0362-028X.JFP-19-163.Suche in Google Scholar PubMed
[52] Ghildiyal R, Prakash V, Chaudhary VK, Gupta V, Gabrani R. Phytochemicals as antiviral agents: recent updates. Plant-derived Bioactives: Production, Prop Ther Appl. 2020;279–95. 10.1007/978-981-15-1761-7_12.Suche in Google Scholar
[53] Guil-Guerrero JL, Ramos L, Moreno C, Zúñiga-Paredes JC, Carlosama-Yepez M, Ruales P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest Sci. 2016 Jul;189:32–49. 10.1016/j.livsci.2016.04.021.Suche in Google Scholar
[54] Sharma P, Tyagi A, Bhansali P, Pareek S, Singh V, Ilyas A, et al. Saponins: Extraction, bio-medicinal properties and way forward to anti-viral representatives. Food Chem Toxicol. 2021 Apr;150:112075. 10.1016/j.fct.2021.112075.Suche in Google Scholar PubMed
[55] Sampedro J, Valdivia ER. New antimicrobial agents of plant origin. In: Antimicrobial compounds: current strategies and new alternatives. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013 Oct. p. 83–114. 10.1007/978-3-642-40444-3_4.Suche in Google Scholar
[56] Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM. Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem. 2013 Mar;20(7):932–52. 10.2174/092986713805219136.Suche in Google Scholar
[57] Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 2019 Dec;8:1–28. 10.1186/s13756-019-0559-6.Suche in Google Scholar PubMed PubMed Central
[58] Peng L, Kang S, Yin Z, Jia R, Song X, Li L, et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int J Clin Exp Pathol. 2015;8(5):5217.Suche in Google Scholar
[59] Cushnie TT, Cushnie B, Lamb AJ. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents. 2014 Nov;44(5):377–86. 10.1016/j.ijantimicag.2014.06.001.Suche in Google Scholar PubMed
[60] Prakash B, Kumar A, Singh PP, Songachan LS. Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. Functional and Preservative Properties of Phytochemicals. Academic Press; 2020. p. 1–45. 10.1016/B978-0-12-818593-3.00001-4.Suche in Google Scholar
[61] Patra AK. An overview of antimicrobial properties of different classes of phytochemicals. Dietary Phytochemicals and Microbes. Dordrecht: Springer; 2012. p. 1–32. 10.1007/978-94-007-3926-0_1.Suche in Google Scholar
[62] Abirami S, Priyalakshmi M, Soundariya A, Samrot AV, Saigeetha S, Emilin RR, et al. Antimicrobial activity, antiproliferative activity, amylase inhibitory activity and phytochemical analysis of ethanol extract of corn (Zea mays L.) silk. Curr Res Green SustaChem. 2021 Jan;4:100089. 10.1016/j.crgsc.2021.100089.Suche in Google Scholar
[63] Feng X, Wang L, Tao ML, Zhou Q, Zhong ZH. Studies on antimicrobial activity of ethanolic extract of maize silk. Afr J Microbiol Res. 2012 Jan;6(2):335–8. 10.5897/AJMR11.974.Suche in Google Scholar
[64] Nessa F, Ismail Z, Mohamed N. Antimicrobial activities of extracts and flavonoid glycosides of corn silk (Zea mays L). Int J Biotechnol Wellness Industries. 2012 Jun;1(2):115. 10.6000/1927-3037/2012.01.02.02.Suche in Google Scholar
[65] Carvalho AB, Cruz CA, Freitas CL, Aguiar JJ, Nunes PL, Lima VM, et al. Chemical profile, antibacterial activity and antibiotic-modulating effect of the hexanic zea Mays L. Silk extract (Poaceae). Antibiotics. 2019 Mar;8(1):22. 10.3390/antibiotics8010022.Suche in Google Scholar PubMed PubMed Central
[66] Nurani FA, Rejeki NR, Setyoputri T, Wardani PK, Ridwan FB, Suparmi S, et al. The potency of ethanolic extract from corn silk as natural antibiotics for acne-related bacteria: A preliminary study. Bangladesh J Med Sci. 2022 Jan;21(1):84–9. 10.3329/bjms.v21i1.56331.Suche in Google Scholar
[67] Saleh RH, Hindi NK, Ali MR. Antibacterial activity of aquatic zea Mays L. Hairs extract against different bacteria in babylon province: An in-vitro study. J Glob Pharma Technol. 2017;0975–8542.Suche in Google Scholar
[68] Qaiser H, Batool S, Gohar M, Moqaddes S. Evaluation of antagonistic action of corn silk extract towards various fungal and bacterial pathogens. Lahore Garrison Univ J Life Sci. 2020;3(4):224–30. 10.54692/lgujls.2019.030467.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal
Artikel in diesem Heft
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal

