Abstract
Graphene oxide (GO) is a versatile material with inherent unique properties that can be used in a wide range of applications. GO is produced from graphitic materials including graphite, and its properties can depend on the nature of stacking in the graphene structures. In this study, GO was prepared from pitch-based graphite fibers via the modified Hummer’s method and subsequently incorporated into an epoxy matrix to obtain graphene-loaded nanocomposites (EP/GO). Presented experimental results revealed that the addition of 0.6 wt% GO yielded an ∼110% increase in the fracture toughness. The corresponding fracture energies as well as the flexural strengths and flexural modulus exhibited similar trends to the fracture toughness. The thermophysical properties of the EP/GO, to further demonstrate the reinforcing effectiveness of GO, were also observed. Collectively, these results indicate that GO investigated in the study can be a viable reinforcement candidate to develop next-generation nanocomposites with multifunctional properties.
1 Introduction
Graphene-based materials, which contain a two-dimensional (2-D) single layer of carbon atoms arranged in a hexagonal lattice, have been widely investigated and commercialized because they are highly ordered, and they offer strong chemical resistance, large specific surface area (2,630 m2 g−1), high Young’s modulus (1 TPa), high thermal conductivity (5,000 W m−1 K−1), and high electron mobility (2.5 × 105 cm2 V−1 s−1) [1,2,3,4,5,6,7].
Many academic and industrial researchers are struggling in manufacturing graphene with high quality and large quantities, which is divided into two categories: one is the bottom-up method that synthesizes from hydrocarbon sources using chemical vapor deposition for single or a few layers with the minimum defect area [8,9]. This method cannot be used for most applications that require graphene in large quantities. The other is the top-down method that applies chemical and/or mechanical exfoliation of graphite, which is much advantageous to produce 2-D graphene oxide (GO) in a scalable manner. In particular, GO produced by the top-down method has attracted increasing attention as a nanofiller for polymer-based nanocomposites [10,11,12].
Until now, it was considered that graphite (synthetic or natural) is the primary source for mass production of GO or reduced GO using the top-down method. Jiao et al. reported that GOs synthesized from different types of natural graphite show a remarkable difference in the crystalline morphologies, chemical properties, etc. because of different graphitization degree [13]. Understanding the characteristics of precursors and controlling parameters such as medium and temperature during the synthesis process is crucial to obtain GO with desirable properties for use in a wide range of applications [14,15,16,17].
Along with these, other sp2-carbon sources are widely used for GO production, such as graphite fibers (GFs) [18], carbon nanotubes [19], coal [20], and biomass [21]. Among them, GFs have abundant crystalline graphite with a high carbon content of more than 99%. This is because they are manufactured by the graphitization process at approximately 3,000°C. GFs are thus extensively used in applications that require high strength and high modulus, including applications in the railways and the automotive, aerospace, drilling, and consumer product industries [22,23,24,25,26,27]. GFs with considerable amounts of 2-D conjugated sp2 domains are acceptable, which can be converted to graphene and/or graphene derivatives for mass production [18,28,29].
In this study, GO was prepared from pitch-based GFs using a typical oxidation and subsequent exfoliation process, called the Hummer’s method, and the chemical and morphological properties of GO were investigated. Moreover, GO-incorporated epoxy (EP/GO) nanocomposites as a function of GO loading amount were fabricated to investigate the interfacial interactions between GO and epoxy matrix. The present study aimed concretely at evaluating the effects of GO on the interfacial properties and mechanical properties (e.g., surface free energy, interfacial adhesion, fracture toughness, flexural strength, and flexural modulus) of epoxy nanocomposites. Furthermore, we demonstrate that GO derived from pitch-based GFs can be an effective reinforcement as an alternative 2-D material for polymeric matrix, and present perspective to meet the requirements of light weight and strong mechanical properties for automotive and aerospace applications [30,31].
2 Experimental
2.1 Materials
The pitch-based GFs (labeled as GF, model: XN-90-60S) were provided by Nippon Graphite Fiber Co., Ltd. Epoxy resin (EP, diglycidyl ether of bisphenol-A), with an epoxide equivalent weight of 185–190 g eq−1 at room temperature, was purchased from Kukdo Chemical Co., Korea. 4,4′-Diaminodiphenylmethane (DDM) was supplied by TCI Co., Japan. Potassium permanganate (KMnO4) was purchased from Daejung Co., Ltd. Sulfuric acid (H2SO4, 98%), phosphoric acid (H3PO4, 85%), and hydrogen peroxide (H2O2, 30%) solution were purchased from Duksan Pure Chemicals Co., Korea.
2.2 Preparation of GF-derived GO
The synthesis procedure is shown in Figure 1(a). The as-received GFs were cut into fragments and then ground in a ball mill at 250 rpm for 4 h to obtain GF particles (GFPs). Oxidation was performed using the modified Hummer’s method [32]. Then, 3 g of GFPs was added to a 500 mL three-necked round-bottom flask charged with a mixture of concentrated H2SO4 (360 mL) and H3PO4 (40 mL). Next, 21 g of KMnO4 was slowly added to the flask, and the temperature of the solution was maintained at 0–4°C for 6 h before increasing it to 60°C for 18 h. The solution was poured into 2,000 mL of ice water, followed by dropwise addition of H2O2 until no new bubbles emerged. The solution was treated by the following steps: static precipitation for 24 h, centrifugation to remove most of the acid solution, and infiltration to remove the remaining acids. The final GO sample was obtained using the freeze-drying process, and 4.8 g of GO was collected to be used as a reinforcement.
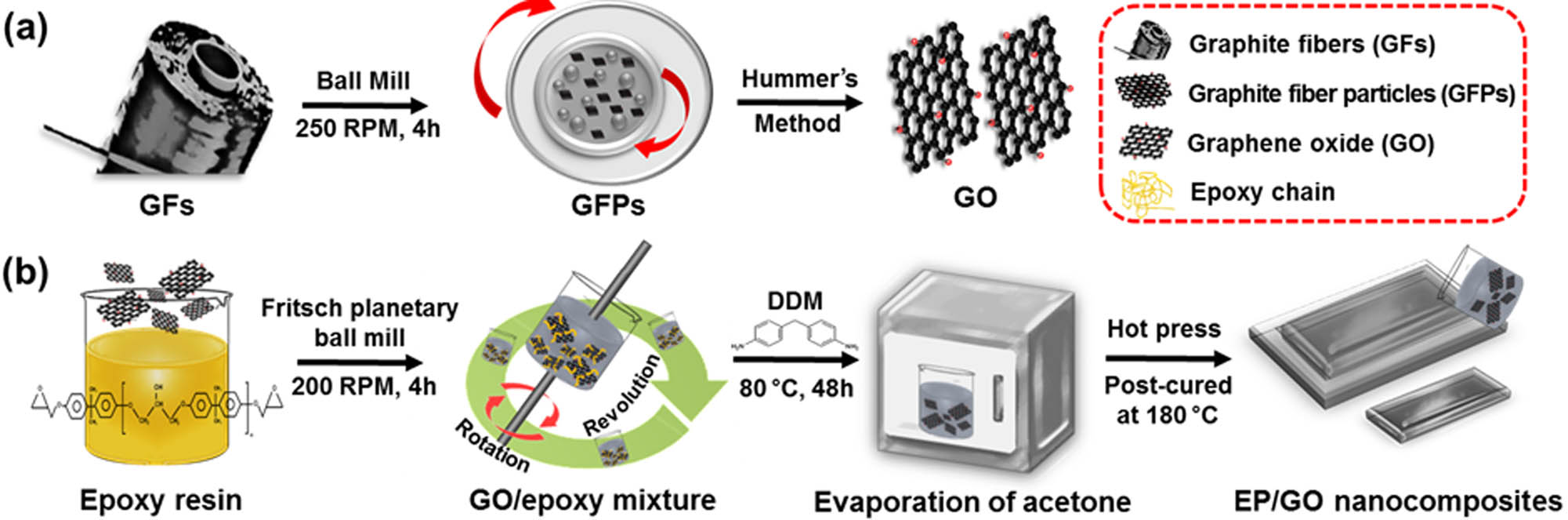
Schematic representation of EP/GO composites: (a) synthesis of GO derived from pitch-based GFs and (b) composite preparation process.
2.3 Fabrication of EP/GO nanocomposites
Figure 1(b) illustrates the fabrication process of EP/GO nanocomposites. A Fritsch planetary ball mill (Pulverisette 6, TMC) was employed to break up the agglomerates and enhance the dispersion of GO sheets. Without pretreatment of the samples, quantitative GO, EP, and a moderate amount of acetone were added to the grinding bowl and mixed at a rotational speed of 200 rpm for 4 h. Notably, the rotational speed should not exceed 250 rpm because the GO structure would be destroyed under the high shear stress [33]. The mixture was then degassed at 80°C for 48 h to remove the solvent completely. DDM (20 wt% of EP) was then slowly added to the solution and mixed with a planetary mixer, followed by further degassing at 60°C for 30 min. The prepared EP/GO samples were cured by the following three steps: 110°C for 1 h, 140°C for 2 h, and 170°C for 1 h.
2.4 Characterization
X-ray diffraction (XRD; D2 Phaser, Bruker, Germany) was used to investigate the structure of the GO in the 2θ range of 4–60°. Fourier-transform infrared spectroscopy (FT-IR; Jasco PS-4000, JASCO, Japan) was used to obtain the infrared spectra of the GO. The qualities of the fillers before and after thermal oxidation were analyzed by Raman spectroscopy (Raman; LabRAM Revolution, HORIBA, Japan) equipped with a charge-coupled device detector. X-ray photoelectron spectroscopy (XPS; ESCA LAB MK-II, VG Scientific, UK) was conducted to analyze the surface chemistry of the GO. High-resolution scanning electron microscopy (HR-SEM; SU 8010, Hitachi, Japan) was used to observe the cross-section morphology of the prepared nanocomposites. Field-emission transmission electron microscopy (FE-TEM; JEM-2100F, JEOL, Japan) was used to examine the structure of the GO. The dynamic mechanical properties were measured using rectangular specimens with dimensions of 20 mm × 5 mm × 2 mm, held in a dual-cantilever clamp, using a dynamic mechanical analyzer (DMA; Q800, TA Instruments, USA).
The total surface free energy, which is the sum of London dispersive (γ L) and specific components (γ SP), can be calculated using the following equation [34]:
where γ L and γ SP result from intermolecular interactions of London force of van der Waals attraction and the specific force, respectively.
According to Fowkes’ suggestion [34], the surface free energy of a solid material can be examined by measuring the contact angle using the following geometric mean, known as the work of adhesion, W A:
where θ is the contact angle; subscripts L and S represent liquid and solid, respectively; superscripts L and SP represent London force and specific force, respectively. Equations (5–6) can then be transformed into the following equation:
According to this equation, a plot of
The critical stress intensity factor (K IC) of the EP/GO nanocomposites was examined via a three-point flexural test, which was performed using a universal test machine according to the standard ASTM E-399. A span-to-depth ratio of 4:1 and a crosshead speed of 1 mm min−1 were considered. The K IC value of the cured epoxy and its nanocomposites was calculated using the following equations:
where a, P, d, L, and b represent the crack length, critical load for crack propagation, thickness of specimen, length of the span, and the width of specimen, respectively.
The critical strain energy release rate (G IC) of the epoxy and its nanocomposites was calculated using the following equation:
where E is the Young’s modulus obtained from the fracture test and ν is the Poisson’s ratio for the nanocomposites (0.3). The flexural properties were tested with an Instron 1125 mechanical tester according to the standard ASTM D-790. To determine the mechanical properties, five experimental measurements were conducted for each specimen and the average value was obtained.
3 Results and discussion
3.1 Characterization of the GO
HR-SEM images of the GFs used in the study were observed to verify the presence of lots of stacked 2-D carbon sheets along with the long axis of the GF, as shown in Figure 2. Figure 3 displays the FT-IR spectra of the GF and GO derived from the GF. As shown in Figure 3(a), the FT-IR spectra of the GF demonstrated a small absorption peak at 3,380 cm−1 corresponding to hydroxyl groups (−OH). For the GO, new peaks were observed at 1,732 cm−1, which can be attributed to stretching vibrations of the carboxylic groups (−COOH). The functional groups of the prepared GO are consistent with those reported in previous studies, demonstrating the feasibility of oxidation [36,37,38]. Photographs of GO suspension in water are shown in the inset of Figure 3(b). GO was homogeneously dispersed to generate a deep yellow-brown suspension. Figure 3(b) shows the TEM image of GO, exhibiting a typical 2-D nanosheet morphology with a particle size of approximately 2 µm. The grid can be clearly observed because of the superior transparency of the prepared GO. The XRD patterns of the GF and GO are shown in Figure 3(c). The pattern of the GF shows a typical graphite peak of (002) at 26.8°, corresponding to an interlayer d-spacing of 0.330 nm. In the case of GO, a new typical peak of (001) at 10.5° corresponding to a d-spacing of 0.84 nm appeared, indicating that a large degree of exfoliation occurred in the graphitic layers. This result confirmed that the stacked graphitic layers were completely exfoliated and converted into GO, which is in good agreement with the previous results of a typical structure of GO.
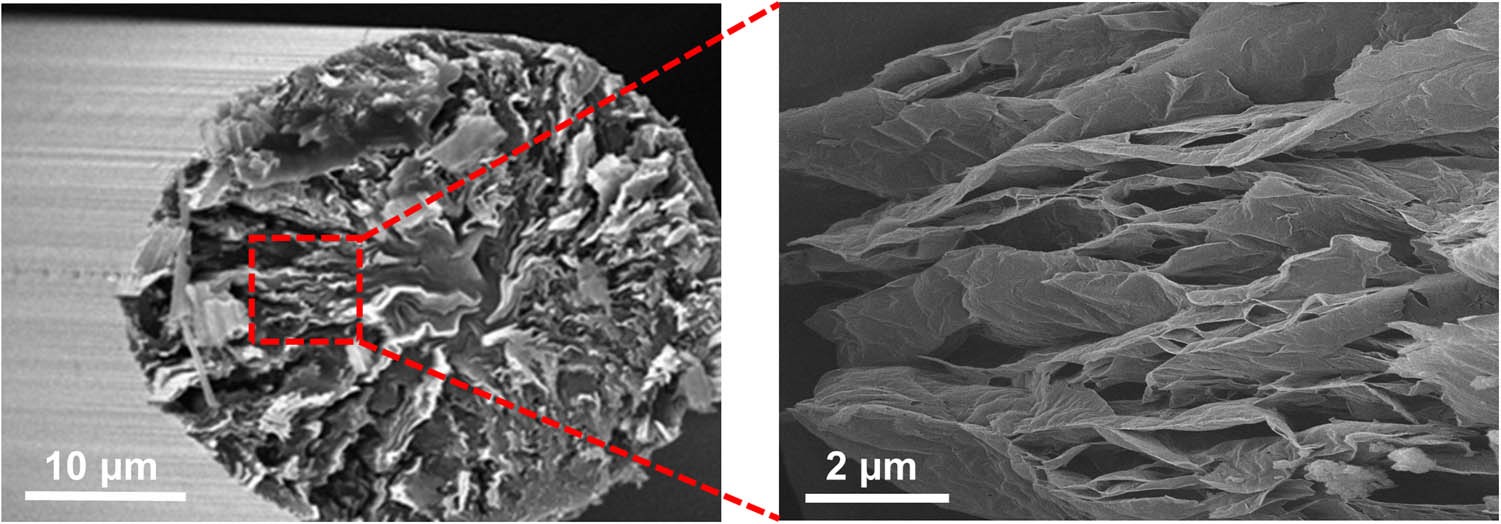
SEM images of the 2-D graphene nanosheets in the pitch-based GFs).
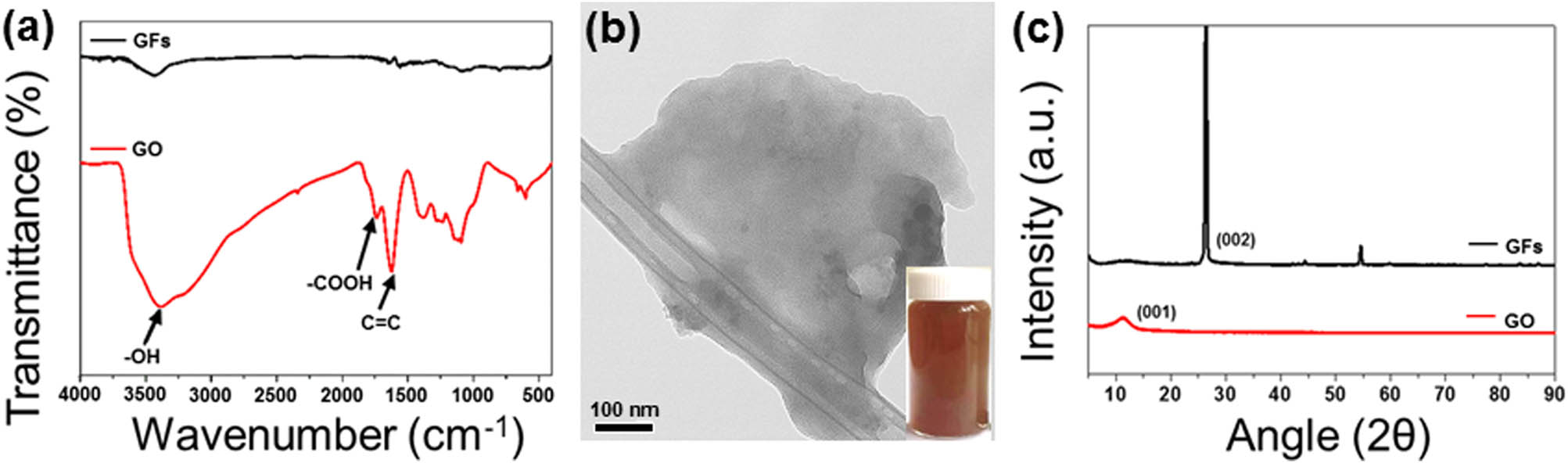
Characterization of GO: (a) FT-IR spectra of the GF and GO, (b) TEM image of GO (the inset: photographs of GO suspension in the water), and (c) XRD patterns of GF and GO.
We employed Raman spectroscopy to observe the microstructural characteristics of the GF and GO. Basically, the Raman spectra of carbon nanomaterials present two peaks corresponding to the G- and D-bands at approximately 1,590 and 1,350 cm−1 due to the presence of sp2 crystalline and sp3 amorphous hybridized carbons, respectively [32,39]. As expected, two characteristic peaks in the GFs and GO were observed at 1,584 and 1,352 cm−1, as shown in Figure 4(a). To investigate the degree of amorphous and crystalline phases, the integrated intensity ratio (
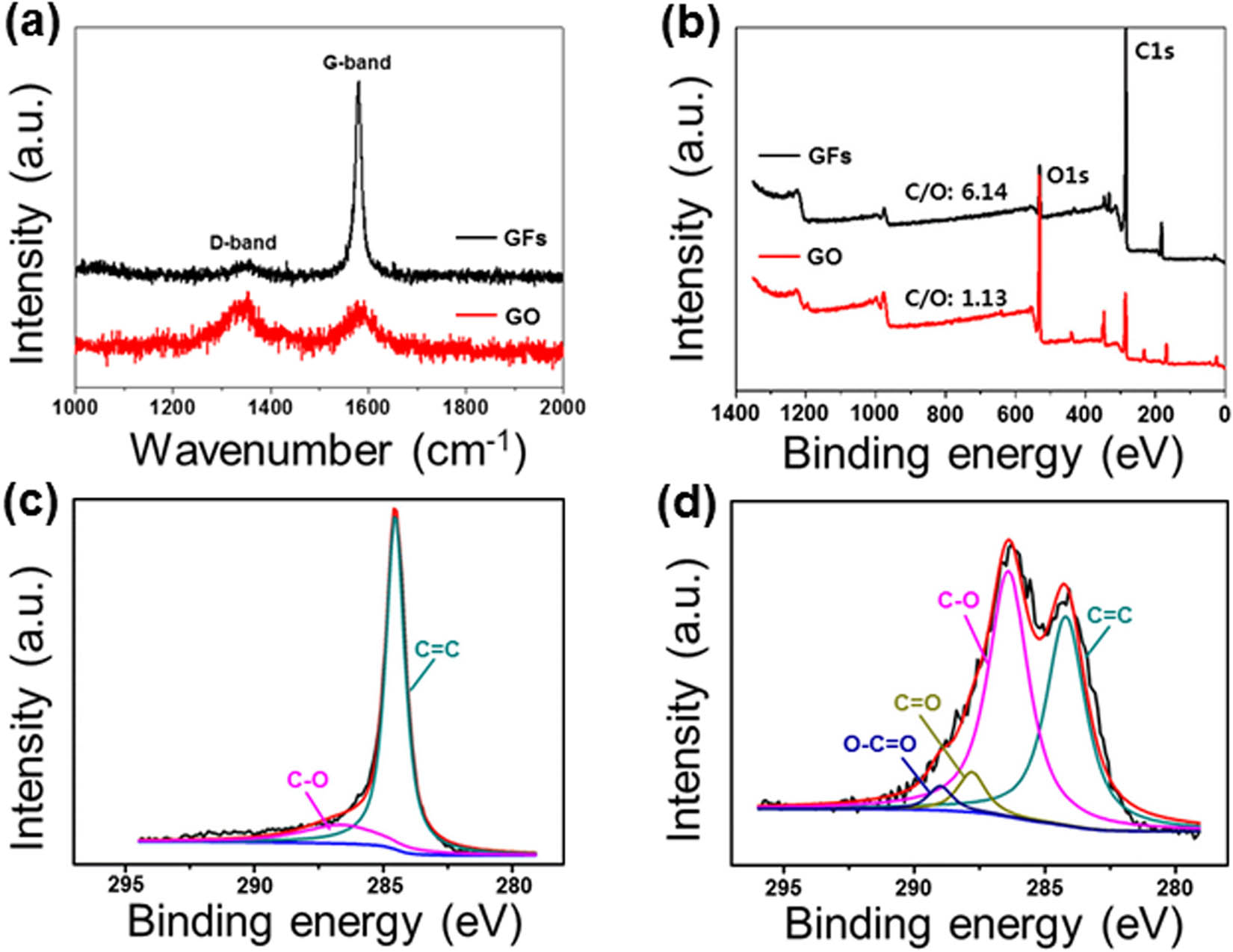
Characterization of GO: (a) Raman spectra of the GF and GO, (b) XPS survey spectra of the GF and GO, (c) C1s spectra of the GF, and (d) C1s spectra of the GO.
XPS analysis was conducted to determine the surface chemical compositions and functional groups of the GO. In Figure 4(b), the two predominant characteristic peaks observed at 285.0 and 531.0 eV can be attributed to C1s and O1s core-level emissions, respectively. The carbon/oxygen (C/O) ratios were also calculated. The C/O ratio of the GFs was 6.14, which is mostly composed of carbon in the pristine GF, while the GO showed a much lower value of C/O ratio (1.13). To further confirm the surface functional groups of the GFs and GO, their high-resolution single-scan C1s spectra were deconvoluted. In Figure 4(c and d), the peaks of the GFs observed at 284.4 and 286.6 eV correspond to the C–C and C–O groups, respectively, indicating the presence of majorly conjugated sp2 carbons and little oxygen. For the GO spectra, a broad peak was observed at 284.4 eV owing to an increase of full width at half maximum, resulting from the introduction of oxygen-functional groups. Moreover, the prominent peak at 289.2 eV corresponding to the carboxylic group (–COOH) was observed, indicating successful oxidation.
3.2 Interfacial properties of EP/GO nanocomposites
Surface free energy (γ) can strongly affect the wettability, adhesion, adsorption, and morphology of the components of composites. The London dispersion force, specific component force, and surface tension of the liquids are given in Table S1. The contact angles of the composites are shown in Table S2. Using the data provided in Tables S1 and S2, we calculated the surface free energies of the EP/GO, as plotted in Figure 5(a). The surface free energies of the EP/GO nanocomposites increased with increasing GO loading fractions. The EP/GO0.8 nanocomposites exhibited a surface free energy of 47.3 mJ m−2, indicating that GO is compatible with EPs. In addition, the calculated values of W A were gradually increased with an increase of the GO content (Figure 5(b)). Here, all the EP/GO nanocomposites exhibit good linearity for the relationship between the surface free energies and work adhesion, which clearly demonstrates enhanced interfacial adhesion [40,41]. We studied the wetting behavior of distilled water (carried out at 27°C for 10 min) acting on the nanocomposites in more detail. It can be seen from Figure 5(c) that the wetting behavior of the EP/GO0.0 nanocomposites was maintained at roughly 60.4° exhibited no obvious changes. By contrast, the wetting behavior of the EP/GO0.8 nanocomposite is seen to decrease rapidly and become saturated at around 38.6°. This decrease is due to the large number of carboxylic groups on the GO surface, from which water droplets can rapidly diffuse, thus providing an enhanced wettability for the nanocomposites.
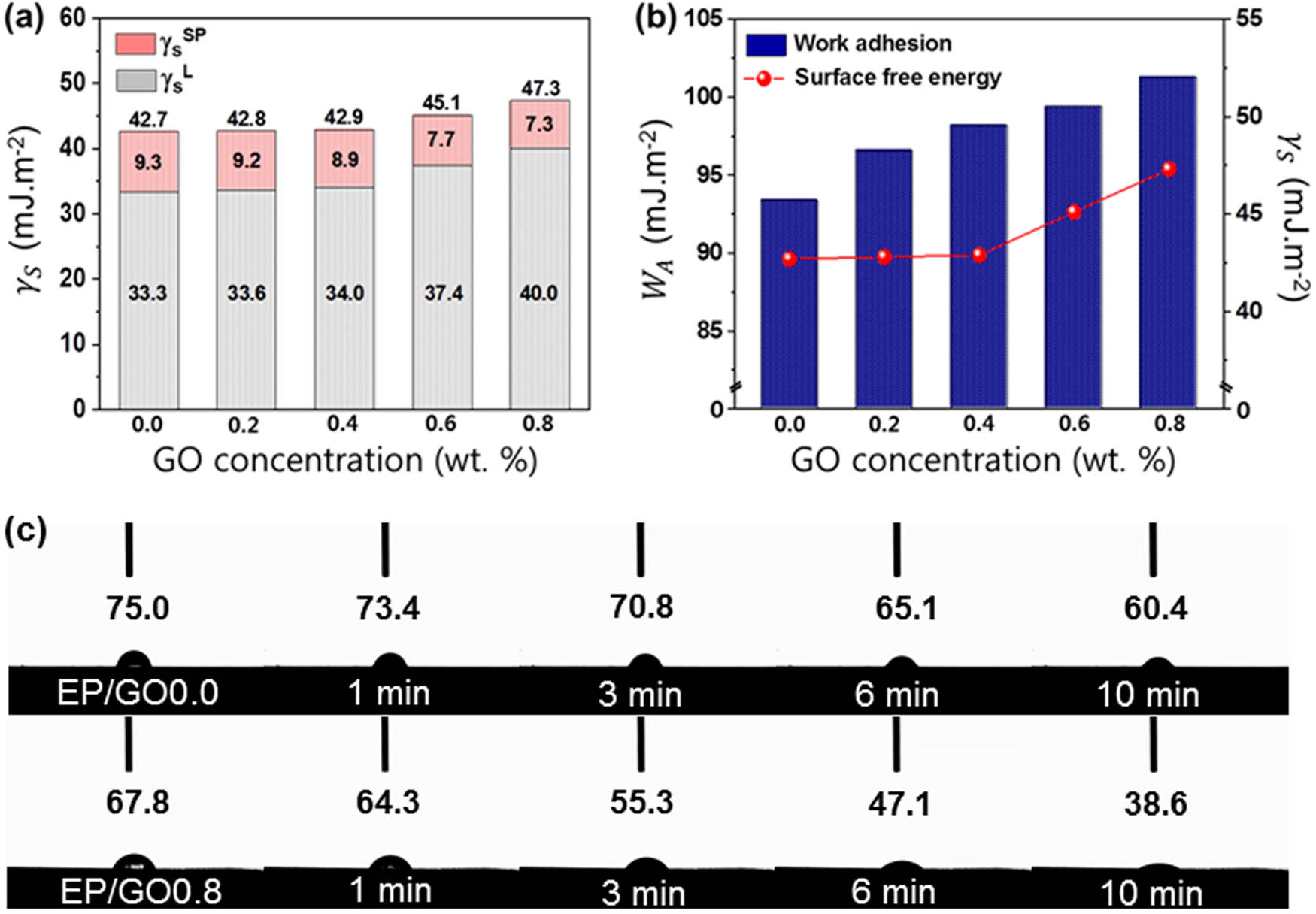
Interfacial behaviors of prepared EP/GO nanocomposites: (a) surface free energy, (b) work of adhesion, and (c) optical images of the wetting behavior of distilled water over time.
3.3 Mechanical properties of EP/GO nanocomposites
The bending strength of the EP/GO nanocomposites increased up to 0.6 wt% by weight and then decreased at a higher GO content (Figure 6(a)). The bending strength of the epoxy nanocomposites reached 55.7 MPa in EP/GO0.6 nanocomposites, which is twice that of EP/GO0.0 nanocomposites (28.5 MPa). In addition, the Young’s modulus (Figure 6(b)) showed a similar trend to the bending strength.
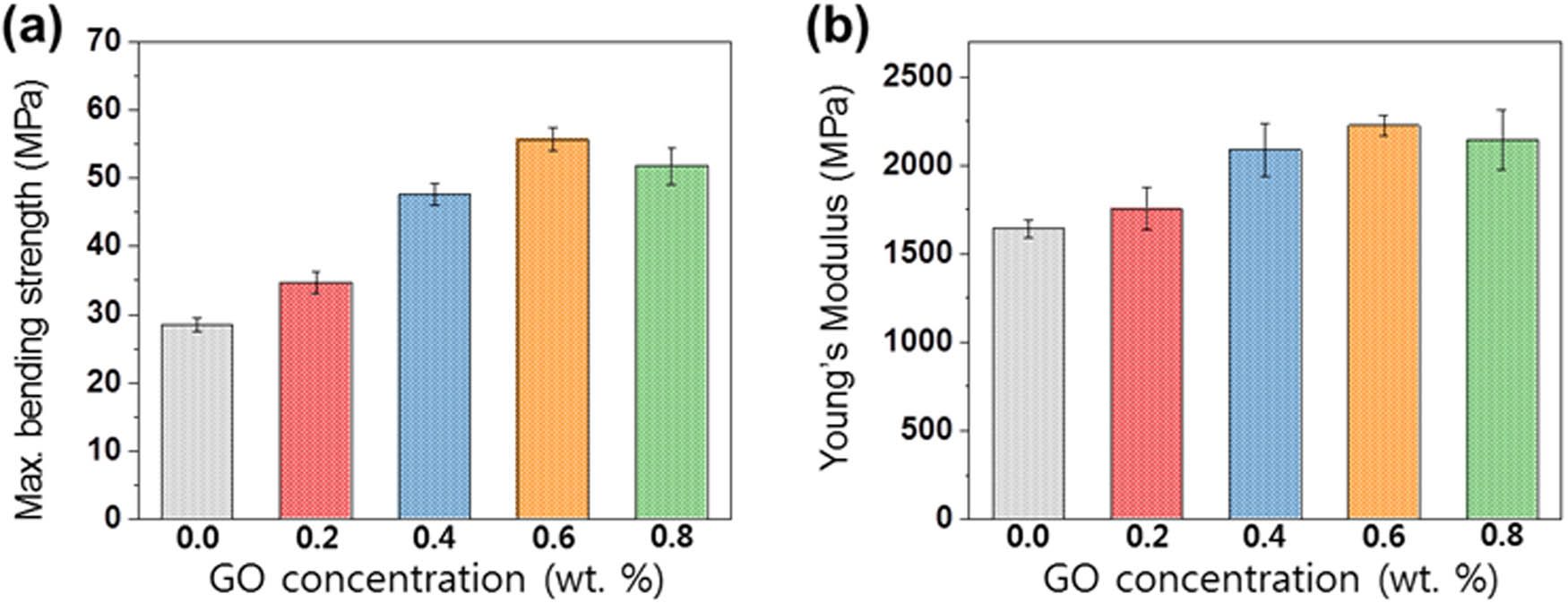
Mechanical properties of the EP/GO: (a) bending strength and (b) Young’s modulus.
Normally, filler dispersion, interfacial interactions between the filler and polymer matrix, and the intrinsic characteristics of the filler are known to be three dominant factors affecting the performance of nanocomposites. In this work, since all the specimens were prepared through the ball-milling process, thereby ensuring good dispersion of the filler in the polymer matrix, the dispersion factor alone cannot account for enhanced mechanical properties. Meanwhile, the EP/GO nanocomposites exhibited superior interfacial interactions because abundant oxygen-functional groups were introduced onto the GO surface, as shown in Figure 5, which have strong affinity with the epoxy matrix. Thus, these results revealed that the perfect interfacial interaction between GO and the epoxy occurred, resulting in enhanced mechanical properties of the nanocomposites [42,43]. To further demonstrate the interfacial properties between the filler and the matrix, the flexural strength (σ) and flexural modulus (E b) of the EP/GO nanocomposites with different GO loading amounts were also calculated, using the following equations (8) and (9) [44,45]:
where P is the applied peak load, b is the sample width, d is the specimen thickness, L is the support span,
Figure 7(a and b) shows the flexural strength and flexural modulus of EP/GO nanocomposites. With increasing GO loading fractions, the flexural strength gradually increased from 107 to 140 MPa. When the GO concentration exceeded 0.6% by weight, the flexural strength decreased slightly. When the GO concentration exceeded 0.6% by weight, the flexural strength decreased slightly. The fracture toughness values were determined using the three-point bending tests, as shown in Figure 7(c and d). The results demonstrate that GO enhances both the fracture toughness (K IC) and fracture energy (G IC) of the prepared nanocomposites. The K IC value of EP/GO0.6 nanocomposites increased by 109.3%; this enhancement can be attributed to the good dispersion of GO and thus the enhanced interfacial interactions within the epoxy matrix. Meanwhile, the EP/GO nanocomposites at higher fraction of GO (0.8% by weight) exhibited decreased K IC value, probably due to the formation of aggregates. Correspondingly, the EP/GO nanocomposites exhibited the highest G IC value in the EP/GO0.6 nanocomposites as well, demonstrating the best mechanical performances among the nanocomposites studied in the work.
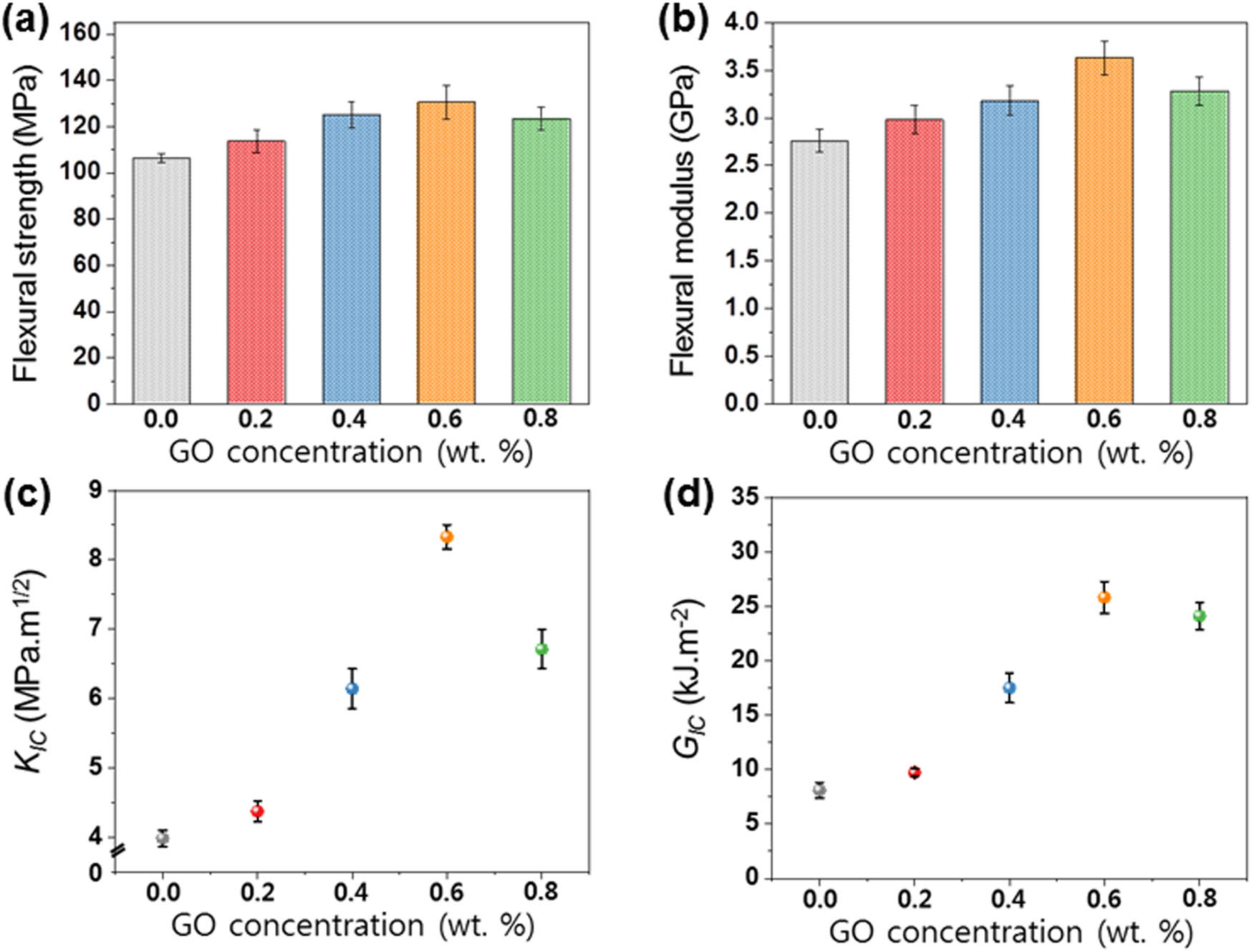
Mechanical properties of EP/GO nanocomposites: (a) flexural strength, (b) flexural modulus, (c) K IC, and (d) G IC.
It is well known that failure behaviors of nanocomposites are related to their structural integrity at both microscopic and macroscopic levels under external forces [44,46]. To reveal the failure behavior of the EP/GO nanocomposites, we observed their fracture surfaces using HR-SEM; the results are shown in Figure 8(a–d). With increasing GO loading fractions, the number of fatigue cracks increased and the surfaces became rougher. The equally distributed fatigue cracks on the fracture surfaces demonstrated homogeneously dispersed GO in an epoxy matrix. In EP/GO0.8 nanocomposites, the cracks were coarsened and pulled out, as shown in Figure 8(d), possibly due to the aggregation of the GO sheets. For the nanocomposites reinforced by a rigid additive, the plastic yielding of the epoxy matrix around the particles, subsequent void formations, and the interference of the rigid particles during crack propagation, including crack pinning and/or deflection, are considered as the main toughening mechanisms. In case of the EP/GO nanocomposites, crack deflection could act as the main toughening mechanism due to the increased number of cracks in the EP/GO nanocomposites [47].
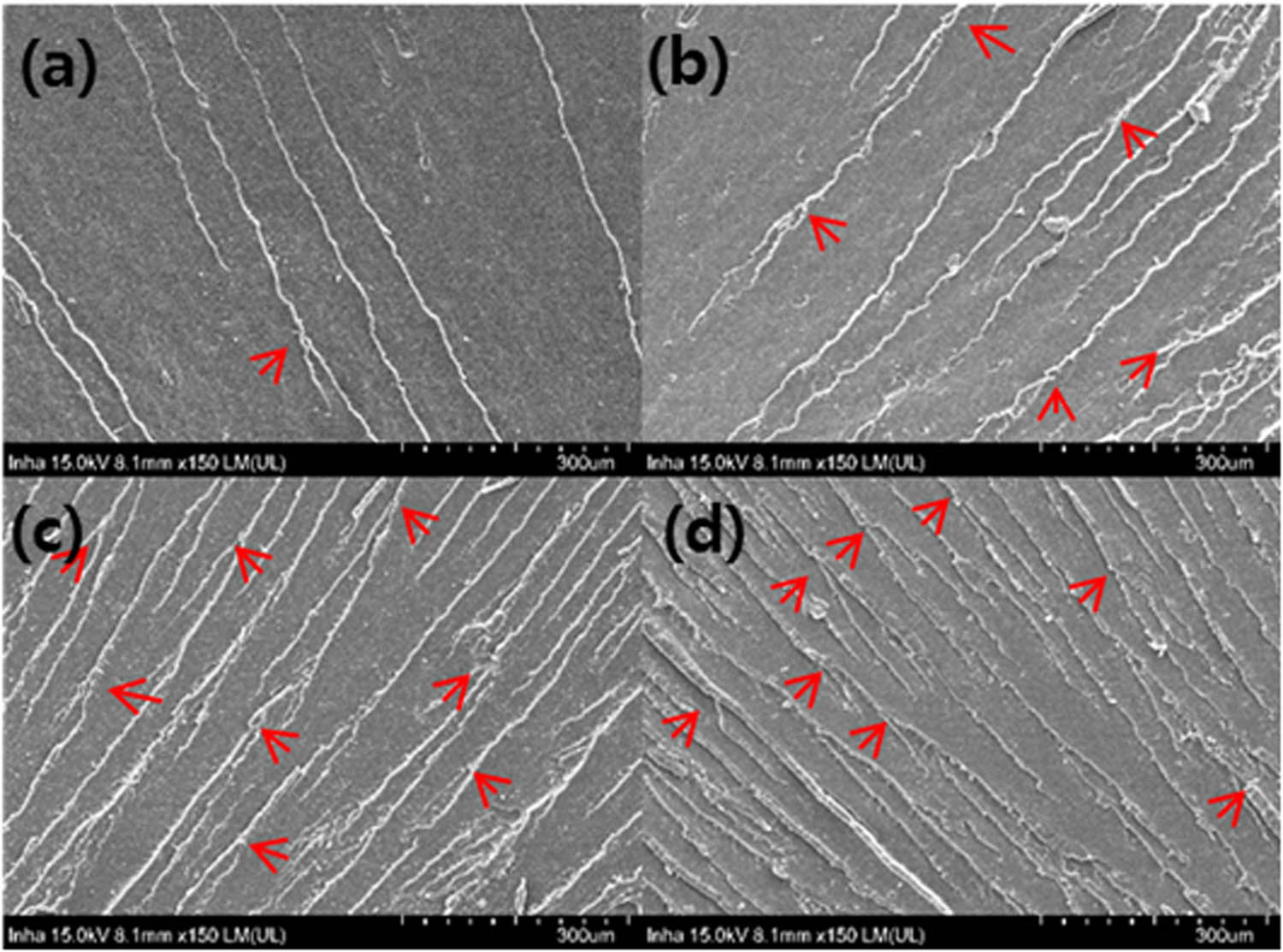
SEM images of the fracture surfaces: (a) EP/GO0.2, (b) EP/GO0.4, (c) EP/GO0.6, and (d) EP/GO0.8 nanocomposites.
In addition, the improvements in K IC achieved by the addition of GO derived from pitch-based GFs in this study are compared with previously reported values for epoxy nanocomposites containing GO, as shown in Figure 9. It can be demonstrated that the prepared GO derived from pitch-based GFs plays an effective reinforcement for the K IC improvements, resulting from enhanced interfacial interaction between the GO and epoxy matrix [48,49,50,51,52,53,54,55,56,57,58,59].
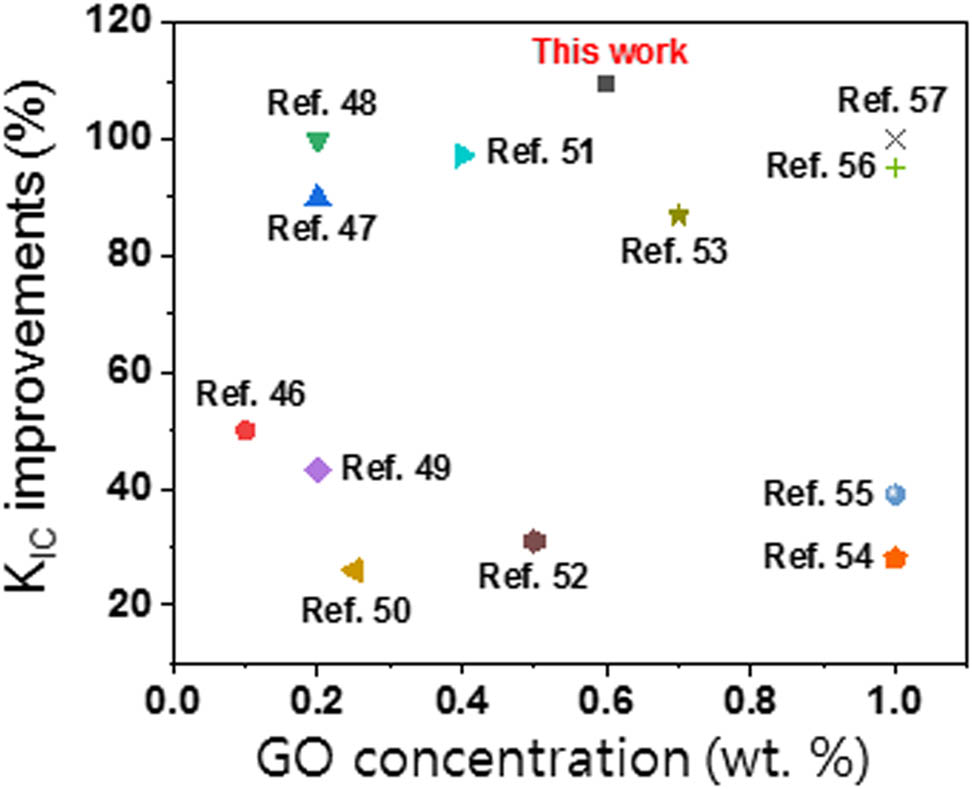
Comparison of the K IC improvements of GO-loaded epoxy nanocomposites.
3.4 Thermophysical properties of EP/GO nanocomposites
DMA is used not only to determine the amount of elastic energy stored in the prepared composites and the energy dissipated during strain, but also to examine the glass transition temperature (T g), which is the temperature at which sufficient vibration energy was accumulated to rearrange the crosslinked polymer chains inside the polymeric matrix. T g is also known to be related to relaxation and is sensitive to structural transformations. The corresponding data of the temperature-dependent storage modulus and loss factor of the EP/GO nanocomposites are listed in Table 1. These data indicate that the homogenous dispersion of GO led to improved interfacial interaction between the GO sheets and the epoxy matrix, thereby substantially restricting the segmental movement of the epoxy chains and resulting in an enhanced storage modulus. The EP/GO nanocomposites exhibited a similar level of the dynamic storage moduli at the rubbery plateau area at the same temperature. We speculate that the presence of GO could play an important role in enhancing the dynamic storage modulus of the nanocomposites owing to the interfacial interaction between GO and the epoxy matrix. Moreover, the EP/GO nanocomposites exhibited higher T g values than that of EP nanocomposites. The GO sheets served as anchor points among polymer chains, which restricted their mobility and increased the T g. The T g values increased with increasing GO loading fractions, demonstrating the enhanced interfacial interaction. The increase in the T g value was not significant because the addition of a filler may reduce the cross-linking density of the epoxy, thus decreasing T g [60].
Dynamic mechanical properties of neat epoxy and the EP/GO at 25°C
| Specimen | Storage modulus (MPa) | T g (°C) |
|---|---|---|
| EP | 2.16 | 87.76 |
| EP/GO0.2 | 2.19 | 88.33 |
| EP/GO0.4 | 2.20 | 89.97 |
| EP/GO0.6 | 2.23 | 90.38 |
| EP/GO0.8 | 2.30 | 90.09 |
4 Conclusion
In this study, GO was successfully synthesized using GFs as a precursor. The EP/GO nanocomposites at loadings less than 1% by weight were fabricated via ball-milling process. Presented detailed investigation of the mechanical and thermophysical properties of the EP/GO nanocomposites revealed that the values depend on the loading level of GO. In particular, when the GO loading was 0.6 wt%, the fracture toughness of the EP/GO nanocomposites exhibited a significant improvement of ∼82% compared to that of EP nanocomposites. The fracture energies as well as the flexural strength and flexural modulus showed similar trends to the fracture toughness, demonstrating the effective toughness of GO. Moreover, the presence of GO improved the thermophysical properties of the epoxy matrix. These results can be attributed to the enhanced interfacial adhesion between the GO and the epoxy matrix, affecting the surface free energy and work adhesion. Collectively, these enhancements can be attributed to the good dispersion of GO, thereby enhancing the interfacial interactions between the GO and the epoxy matrix. Thus, the authors believe that the GF-derived GO in this work can be a promising candidate as a reinforcing material for improving the mechanical properties of nanocomposites in a wide range of applications.
-
Funding information: This work was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program – Development of Technology on materials and components) (20010106, Adhesives with low water permeability and low outgassing) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and supported by the Technological Innovation R&D Program (S2849653) funded by the Small and Medium Business Administration (SMBA, Korea).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available in the Supplementary material on the journal website.
References
[1] Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, Stach EA, et al. Graphene-based composite materials. Nature. 2006;442:282–6.10.1038/nature04969Suche in Google Scholar PubMed
[2] Geim AK. Graphene: status and prospects. Science. 2009;324:1530–4.10.1126/science.1158877Suche in Google Scholar PubMed
[3] Suk JW, Piner RD, An J, Ruoff RS. Mechanical properties of monolayer graphene oxide. ACS Nano. 2010;4:6557–64.10.1021/nn101781vSuche in Google Scholar PubMed
[4] Meyer JC, Geim AK, Katsnelson MI, Novoselov KS, Booth TJ, Roth S. The structure of suspended graphene sheets. Nature. 2007;446:60–3.10.1038/nature05545Suche in Google Scholar PubMed
[5] Zhang Y, Heo YJ, Son YR, In I, An KH, Kim BJ, et al. Recent advanced thermal interfacial materials: a review of conducting mechanisms and parameters of carbon materials. Carbon. 2019;142:445–60.10.1016/j.carbon.2018.10.077Suche in Google Scholar
[6] Yu T, Soomro SA, Huang F, Wei W, Wang B, Zhou Z, et al. Naturally or artificially constructed nanocellulose architectures for epoxy composites: a review. Nanotechnol Rev. 2020;9:1643–59.10.1515/ntrev-2020-0116Suche in Google Scholar
[7] Liu C, Huang X, Wu YY, Deng X, Liu J, Zheng Z, et al. Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide. Nanotechnol Rev. 2020;9:155–69.10.1515/ntrev-2020-0014Suche in Google Scholar
[8] Muñoz R, Gómez‐Aleixandre C. Review of CVD synthesis of graphene. Chem Vap Depos. 2013;19:297–322.10.1002/cvde.201300051Suche in Google Scholar
[9] Lee HC, Liu WW, Chai SP, Mohamed AR, Aziz A, Khe CS, et al. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017;7:15644–93.10.1039/C7RA00392GSuche in Google Scholar
[10] Kim SH, Rhee KY, Park SJ. Amine-terminated chain-grafted nanodiamond/epoxy nanocomposites as interfacial materials: Thermal conductivity and fracture resistance. Compos Pt B-Eng. 2020;192:107983.10.1016/j.compositesb.2020.107983Suche in Google Scholar
[11] Chen G, Xu W, Zhu D. Recent advances in organic polymer thermoelectric composites. J Mater Chem C. 2017;5(18):4350–60.10.1039/C6TC05488ASuche in Google Scholar
[12] Dey A, Bajpai OP, Sikder AK, Chattopadhyay S, Khan MAS. Recent advances in CNT/graphene based thermoelectric polymer nanocomposite: a proficient move towards waste energy harvesting. Renew Sust Energ Rev. 2016;53:653–71.10.1016/j.rser.2015.09.004Suche in Google Scholar
[13] Jiao X, Qiu Y, Zhang L, Zhang X. Comparison of the characteristic properties of reduced graphene oxides synthesized from natural graphites with different graphitization degrees. RSC Adv. 2017;7(82):52337–44.10.1039/C7RA10809ESuche in Google Scholar
[14] Qiao Q, Liu C, Gao W, Huang L. Graphene oxide model with desirable structural and chemical properties. Carbon. 2019;143:566–77.10.1016/j.carbon.2018.11.063Suche in Google Scholar
[15] Ikram R, Jan BM, Ahmad W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J Mater Res Technol-JMRT. 2020;9:11587–610.10.1016/j.jmrt.2020.08.050Suche in Google Scholar
[16] Botas C, Álvarez P, Blanco C, Santamaría R, Granda M, Ares P, et al. The effect of the parent graphite on the structure of graphene oxide. Carbon. 2012;50:275–82.10.1016/j.carbon.2011.08.045Suche in Google Scholar
[17] Brisebois PP, Siaj M. Harvesting graphene oxide–years 1859 to 2019: a review of its structure, synthesis, properties and exfoliation. J Mater Chem C. 2020;8:1517–47.10.1039/C9TC03251GSuche in Google Scholar
[18] Lee M, Lee J, Park SY, Min B, Kim B, In I. Production of graphene oxide from pitch-based carbon fiber. Sci Rep. 2015;5:1–10.10.1038/srep11707Suche in Google Scholar PubMed PubMed Central
[19] Abdolkarimi-Mahabadi M, Manteghian M. Chemical oxidation of multi-walled carbon nanotube by sodium hypochlorite for production of graphene oxide nanosheets. Fuller Nanotub Carbon Nanostruct. 2015;23:860–4.10.1080/1536383X.2015.1016608Suche in Google Scholar
[20] Powell C, Beall GW. Graphene oxide and graphene from low grade coal: Synthesis, characterization and applications. Curr Opin Colloid Interface Sci. 2015;20:362–6.10.1016/j.cocis.2015.11.003Suche in Google Scholar
[21] Das VK, Shifrina ZB, Bronstein LM. Graphene and graphene-like materials in biomass conversion: paving the way to the future. J Mater Chem A. 2017;5:25131–43.10.1039/C7TA09418CSuche in Google Scholar
[22] Kim SH, Park SJ. Effect of graphene oxide/graphitic nanofiber nanohybrids on interfacial properties and fracture toughness of carbon fibers-reinforced epoxy matrix composites. Compos Pt B-Eng. 2021;227:109387.10.1016/j.compositesb.2021.109387Suche in Google Scholar
[23] Huang X. Fabrication and properties of carbon fibers. Materials. 2009;2:2369–403.10.3390/ma2042369Suche in Google Scholar
[24] Figueiredo JL, Bernardo CA, Baker RTK, Hüttinger KJ. Carbon fibers filaments and composites. Springer Sci Bus Media. 2013;177:1–236.10.1007/978-94-015-6847-0Suche in Google Scholar
[25] Park SJ. History and structure of carbon fibers. Vol. 210. Singapore: Springer; 2018. p. 1–30.10.1007/978-981-13-0538-2Suche in Google Scholar
[26] Yao SS, Jin FL, Rhee KY, Hui D, Park SJ. Recent advances in carbon-fiber-reinforced thermoplastic composites: a review. Compos Pt B-Eng. 2018;142:241–50.10.1016/j.compositesb.2017.12.007Suche in Google Scholar
[27] Kim SH, Park SJ, Rhee KY, Park SJ. Effects of ozonized carbon black on fracture and post-cracking toughness of carbon fiber-reinforced epoxy composites. Compos Pt B-Eng. 2019;177:107379.10.1016/j.compositesb.2019.107379Suche in Google Scholar
[28] Peng J, Gao W, Gupta BK, Liu Z, Romero-Aburto R, Ge L, et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012;12:844–9.10.1021/nl2038979Suche in Google Scholar PubMed
[29] Luo J, Cote LJ, Tung VC, Tan AT, Goins PE, Wu J, et al. Graphene oxide nanocolloids. J Am Chem Soc. 2010;132:17667–9.10.1021/ja1078943Suche in Google Scholar PubMed
[30] Yang X, Fan S, Li Y, Guo Y, Li Y, Ruan K, et al. Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Compos Pt A-Appl Sci Manuf. 2020;128:105670.10.1016/j.compositesa.2019.105670Suche in Google Scholar
[31] Song P, Liang C, Wang L, Qiu H, Gu H, Kong J, et al. Obviously improved electromagnetic interference shielding performances for epoxy composites via constructing honeycomb structural reduced graphene oxide. Compos Sci Technol. 2019;181:107698.10.1016/j.compscitech.2019.107698Suche in Google Scholar
[32] Lee SY, Singh P, Mahajan RL. Role of oxygen functional groups for improved performance of graphene-silicone composites as a thermal interface material. Carbon. 2019;145:131–9.10.1016/j.carbon.2018.12.054Suche in Google Scholar
[33] Wan YJ, Tang LC, Yan D, Zhao L, Li YB, Wu LB, et al. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos Sci Technol. 2013;82:60–8.10.1016/j.compscitech.2013.04.009Suche in Google Scholar
[34] Fowkes FM. Determination of interfacial tensions, contact angles, and dispersion forces in surfaces by assuming additivity of intermolecular interactions in surfaces. J Phys Chem. 1962;66:382–2.10.1021/j100808a524Suche in Google Scholar
[35] Park SJ, Cho MS, Lee JR. Studies on the surface free energy of carbon–carbon composites: effect of filler addition on the ILSS of composites. J Colloid Interface Sci. 2000;226:60–4.10.1006/jcis.2000.6787Suche in Google Scholar PubMed
[36] Johra FT, Lee JW, Jung WG. Facile and safe graphene preparation on solution based platform. J Ind Eng Chem. 2014;20:2883–7.10.1016/j.jiec.2013.11.022Suche in Google Scholar
[37] Dreyer DR, Todd AD, Bielawski CW. Harnessing the chemistry of graphene oxide. Chem Soc Rev. 2014;43:5288–301.10.1039/C4CS00060ASuche in Google Scholar PubMed
[38] Lee SY, Moore RB, Mahajan RL. An Al-assisted GO/rGO Janus film: fabrication and hygroscopic properties. Carbon. 2021;171:585–96.10.1016/j.carbon.2020.09.002Suche in Google Scholar
[39] Malard LM, Pimenta MA, Dresselhaus G, Dresselhaus MS. Raman spectroscopy in graphene. Phys Rep. 2009;473:51–87.10.1016/j.physrep.2009.02.003Suche in Google Scholar
[40] Behdinan K, Moradi-Dastjerdi R, Safaei B, Qin Z, Chu F, Hui D. Graphene and CNT impact on heat transfer response of nanocomposite cylinders. Nanotechnol Rev. 2020;9(1):41–52.10.1515/ntrev-2020-0004Suche in Google Scholar
[41] Wu Q, Miao WS, Gao HJ, Hui D. Mechanical properties of nanomaterials: a review. Nanotechnol Rev. 2020;9(1):259–73.10.1515/ntrev-2020-0021Suche in Google Scholar
[42] Keyte J, Pancholi K, Njuguna J. Recent developments in graphene oxide/epoxy carbon fiber-reinforced composites. Front Mater. 2019;6:224.10.3389/fmats.2019.00224Suche in Google Scholar
[43] Borooj MB, Shoushtari AM, Haji A, Sabet EN. Optimization of plasma treatment variables for the improvement of carbon fibres/epoxy composite performance by response surface methodology. Compos Sci Technol. 2016;128:215–21.10.1016/j.compscitech.2016.03.020Suche in Google Scholar
[44] Zhang Y, Rhee KY, Park SJ. Nanodiamond nanocluster-decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos Pt B-Eng. 2017;114:111–20.10.1016/j.compositesb.2017.01.051Suche in Google Scholar
[45] Kim SH, Park SM, Park SJ. Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites. Nanotechnol Rev. 2021;107:10–718.10.1515/ntrev-2021-0048Suche in Google Scholar
[46] Zhang Y, Park SJ. Enhanced interfacial interaction by grafting carboxylated‐macromolecular chains on nanodiamond surfaces for epoxy‐based thermosets. J Polym Sci Pt B-Polym Phys. 2017;55:1890–8.10.1002/polb.24522Suche in Google Scholar
[47] Wang X, Jin J, Song M. An investigation of the mechanism of graphene toughening epoxy. Carbon. 2013;65:324–33.10.1016/j.carbon.2013.08.032Suche in Google Scholar
[48] Galpaya D, Wang M, George G, Motta N, Waclawik E, Yan C. Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties. J Appl Phys. 2014;116(5):053518.10.1063/1.4892089Suche in Google Scholar
[49] Wang TT, Huang P, Li YQ, Hu N, Fu SY. Epoxy nanocomposites significantly toughened by both poly (sulfone) and graphene oxide. Compos Commun. 2019;14:55–60.10.1016/j.coco.2019.05.007Suche in Google Scholar
[50] Chhetri S, Adak NC, Samanta P, Murmu NC, Kuila T. Functionalized reduced graphene oxide/epoxy composites with enhanced mechanical properties and thermal stability. Polym Test. 2017;63:1–11.10.1016/j.polymertesting.2017.08.005Suche in Google Scholar
[51] Li Z, Wang R, Young RJ, Deng L, Yang F, Hao L, et al. Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymer. 2013;54(23):6437–46.10.1016/j.polymer.2013.09.054Suche in Google Scholar
[52] Wan YJ, Tang LC, Gong LX, Yan D, Li YB, Wu LB, et al. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon. 2014;69:467–80.10.1016/j.carbon.2013.12.050Suche in Google Scholar
[53] Konnola R, Joji J, Parameswaranpillai J, Joseph K. Structure and thermo-mechanical properties of CTBN-grafted-GO modified epoxy/DDS composites. RSC Adv. 2015;5(76):61775–86.10.1039/C5RA10599DSuche in Google Scholar
[54] Katti P, Kundan KV, Kumar S, Bose S. Improved mechanical properties through engineering the interface by poly (ether ether ketone) grafted graphene oxide in epoxy based nanocomposites. Polymer. 2017;122:184–93.10.1016/j.polymer.2017.06.059Suche in Google Scholar
[55] Sahu M, Raichur AM. Toughening of high performance tetrafunctional epoxy with poly (allyl amine) grafted graphene oxide. Compos B-Eng. 2019;168:15–24.10.1016/j.compositesb.2018.12.030Suche in Google Scholar
[56] Wazalwar R, Tripathi N, Raichur AM. Mechanical and curing behavior of epoxy composites reinforced with polystyrene-graphene oxide (PS-GO) core-shell particles. Compos C Open Access. 2021;5:100128.10.1016/j.jcomc.2021.100128Suche in Google Scholar
[57] Shokrieh MM, Ghoreishi SM, Esmkhani M, Zhao Z. Effects of graphene nanoplatelets and graphene nanosheets on fracture toughness of epoxy nanocomposites. Fatigue Fract Eng Mater Struct. 2014;37(10):1116–23.10.1111/ffe.12191Suche in Google Scholar
[58] Kang WS, Rhee KY, Park SJ. Influence of surface energetics of graphene oxide on fracture toughness of epoxy nanocomposites. Compos B-Eng. 2017;114:175–83.10.1016/j.compositesb.2017.01.032Suche in Google Scholar
[59] Bortz DR, Heras EG, Martin-Gullon I. Impressive fatigue life and fracture toughness improvements in graphene oxide/epoxy composites. Macromolecules. 2012;45(1):238–45.10.1021/ma201563kSuche in Google Scholar
[60] Zhang Y, Wang Y, Yu J, Chen L, Zhu J, Hu Z. Tuning the interface of graphene platelets/epoxy composites by the covalent grafting of polybenzimidazole. Polymer. 2014;55:4990–5000.10.1016/j.polymer.2014.07.045Suche in Google Scholar
© 2021 Seong-Hwang Kim et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

