Abstract
C22H19NO6, monoclinic, P21/n (no. 14), a = 9.195(4) Å, b = 17.253(5) Å, c = 12.127(5) Å, β = 95.100(16)°, V = 1916.4(12) Å3, Z = 4, Rgt(F) = 0.0405, wRref(F2) = 0.1084, T = 296(2) K.
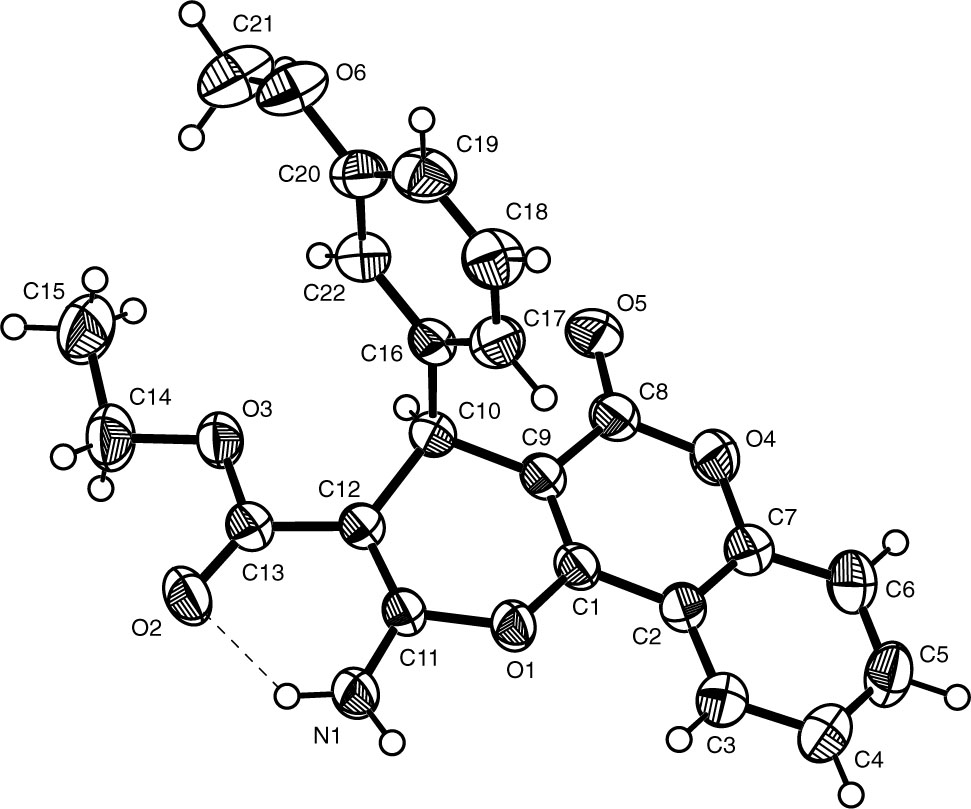
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.32 × 0.27 × 0.21 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9483, 3365, 0.045 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2537 |
| N(param)refined: | 263 |
| Programs: | Olex2 [9], SHELX [10], Bruker [11] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | 0.49560(16) | 0.37245(8) | 0.40727(11) | 0.0483(4) |
| H1A | 0.483575 | 0.418000 | 0.433799 | 0.058* |
| H1B | 0.454648 | 0.360356 | 0.343060 | 0.058* |
| O1 | 0.75159(14) | 0.40343(6) | 0.71414(9) | 0.0498(3) |
| O2 | 0.61551(16) | 0.46330(7) | 0.57665(10) | 0.0599(4) |
| O3 | 0.57946(12) | 0.25269(6) | 0.40508(9) | 0.0421(3) |
| O4 | 0.93243(14) | 0.13463(7) | 0.65864(9) | 0.0536(4) |
| O5 | 0.89809(14) | 0.09037(7) | 0.48794(9) | 0.0511(3) |
| O6 | 0.57235(15) | 0.23129(8) | 1.00681(10) | 0.0620(4) |
| C1 | 0.8455(3) | 0.46517(13) | 0.88042(17) | 0.0817(7) |
| H1C | 0.867432 | 0.514263 | 0.915277 | 0.123* |
| H1D | 0.775872 | 0.437907 | 0.920396 | 0.123* |
| H1E | 0.933327 | 0.435070 | 0.880484 | 0.123* |
| C2 | 0.7839(3) | 0.47789(11) | 0.76527(16) | 0.0636(6) |
| H2A | 0.695443 | 0.508595 | 0.764521 | 0.076* |
| H2B | 0.853418 | 0.505749 | 0.724477 | 0.076* |
| C3 | 0.66726(19) | 0.40382(9) | 0.61672(13) | 0.0405(4) |
| C4 | 0.65122(18) | 0.32779(9) | 0.56748(12) | 0.0356(4) |
| C5 | 0.57731(18) | 0.32087(9) | 0.46539(13) | 0.0358(4) |
| C6 | 0.68582(17) | 0.19993(9) | 0.43730(13) | 0.0362(4) |
| C7 | 0.75556(17) | 0.19973(9) | 0.53939(12) | 0.0362(4) |
| C8 | 0.72126(18) | 0.25723(9) | 0.62582(12) | 0.0356(4) |
| H8 | 0.813414 | 0.273607 | 0.665851 | 0.043* |
| C9 | 0.86593(19) | 0.14203(9) | 0.56841(13) | 0.0412(4) |
| C10 | 0.82475(19) | 0.09076(9) | 0.38317(13) | 0.0432(4) |
| C11 | 0.71586(18) | 0.14441(9) | 0.35368(13) | 0.0390(4) |
| C12 | 0.6459(2) | 0.14179(10) | 0.24681(14) | 0.0508(5) |
| H12 | 0.572176 | 0.177090 | 0.225675 | 0.061* |
| C13 | 0.6859(2) | 0.08683(11) | 0.17235(15) | 0.0600(5) |
| H13 | 0.639418 | 0.085091 | 0.101066 | 0.072* |
| C14 | 0.7948(2) | 0.03466(11) | 0.20428(16) | 0.0612(6) |
| H14 | 0.821873 | −0.001972 | 0.153675 | 0.073* |
| C15 | 0.8647(2) | 0.03555(11) | 0.30962(16) | 0.0566(5) |
| H15 | 0.937364 | −0.000385 | 0.330596 | 0.068* |
| C16 | 0.62571(18) | 0.22146(9) | 0.70925(12) | 0.0378(4) |
| C17 | 0.5164(2) | 0.16963(10) | 0.67677(14) | 0.0481(4) |
| H17 | 0.501334 | 0.154756 | 0.602927 | 0.058* |
| C18 | 0.4288(2) | 0.13959(11) | 0.75320(16) | 0.0570(5) |
| H18 | 0.355116 | 0.104679 | 0.730420 | 0.068* |
| C19 | 0.4498(2) | 0.16097(11) | 0.86284(15) | 0.0546(5) |
| H19 | 0.390564 | 0.140569 | 0.913951 | 0.065* |
| C20 | 0.5589(2) | 0.21266(10) | 0.89649(14) | 0.0460(4) |
| C21 | 0.64754(19) | 0.24259(10) | 0.82025(13) | 0.0435(4) |
| H21 | 0.721994 | 0.276966 | 0.843360 | 0.052* |
| C22 | 0.6966(3) | 0.27477(14) | 1.04759(16) | 0.0734(7) |
| H22A | 0.693073 | 0.283923 | 1.125394 | 0.110* |
| H22B | 0.783559 | 0.246354 | 1.035663 | 0.110* |
| H22C | 0.697183 | 0.323460 | 1.009323 | 0.110* |
Source of material
A mixture of 4-hydroxycoumarin (10 mmol), 3-methoxybenzaldehyde (10 mmol), ethyl cyanoacetate (10 mmol) and 4-(dimethylamino)pyridine (DMAP) (1 mmol) in ethanol (100 mL) was refluxed for 2–3 h and then cooled to room temperature. After filtering the precipitates, they were sequentially washed with ice-cooled water and ethanol and then dried under reduced pressure.
Experimental details
H atoms bonded to C and N atoms were positioned geometrically and refined using a riding model, with C—H = 0.96 Å and N—H = 0.86 Å with Uiso(H) = 1.2 times Ueq(C) and 1.2 times Ueq(N).
Comment
The progress achieved in the synthesis of heterocyclic compounds with biological potential is due to improvement of the methodological study of tested substances [1]. It is known that many coumarin derivatives have biological activity [2]. Ovarian cancer is a cancer that forms in or on an ovary. It results in abnormal cells that have the ability to invade or spread to other parts of the body. Symptoms become noticeable as the cancer progresses. These symptoms may include bloating, pelvic pain, abdominal swelling, and loss of appetite, among others. Common areas to which the cancer may spread include the lining of the abdomen, lymph nodes, lungs, and liver. Recent research indicated that the use of pyran-based heterocycles is an effective treatment to shrink ovarian cancer and control symptoms [3], [4], [5].
In the title crystal structure (Figure), the dihedral angle of the six-membered ring containing one O atom [C(4), C(5), O(3), C(6), C(7) and C(8)] and the adjacent six-membered carbon ring [C(16)—C(21)] is ∼83°. The bond distances of C—O, C—N and C—C are in their normal ranges and they can be compared with those in previously reported complexes. Comparable with the known similar structures in the reference [6], [7], [8], the most difference is that they own different functional groups. The one -OCH3 group or H atom in the title compound were replaced by other atoms or functional groups such as Cl atom and -CH3 group (Ref. 6), Cl atom (Ref. 7) and -NO2 group (Ref. 8).
References
Song, Y.; Li, N.; Gu, J.; Fu, S.; Peng, Z.; Zhao, C.; Zhang, Y.; Li, X.; Wang, Z.; Li, X.; Liu, G.: β-Hydroxybutyrate induces bovine hepatocyte apoptosis via an ROS-p38 signaling pathway. J. Dairy Sci. 99 (2016) 9184–9198.10.3168/jds.2016-11219Suche in Google Scholar PubMed
Sun, X.; Yuan, X.; Chen, L.; Wang, T.; Wang, Z.; Sun, G.; Li, X.; Li, X.; Liu, G.: Histamine induces bovine rumen epithelial cell inflammatory response via NF-κB pathway. Cell. Physiol. Biochem. 42 (2017) 1109–1119.10.1159/000478765Suche in Google Scholar PubMed
Li, J.; Meng, J.; Qu, D.; Zhang, Z.-D.; Li, F.; Yang, X.-H.; Li, J.-T.; Li, M.-K.: New biscoumarin and dihydropyran derivatives as antimicrobials. Res. Chem. Intermed. 41 (2015) 8257–8267.10.1007/s11164-014-1889-xSuche in Google Scholar
Wu, W.-X.; Zhang, Q.; Hu, X.: New 4-arylpolyhydroquinoline derivatives: inhibiting growth of oral tumor cells. Lat. Am. J. Pharm. 36 (2017) 816–819.Suche in Google Scholar
Shi, D.-Q.; Zhang, S.; Wang, X.-S.; Zhuang, Q.-Y.: Ethyl 2-amino-4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-benzo[b]pyran-3-carboxylate. Acta Crystallogr. E59 (2003) o1501–o1502.10.1107/S1600536803019834Suche in Google Scholar
Srinivasan, L.; Anandalwar, S. M.; Prasad, J. S.; Manvar, D.; Parecha, A.; Shah, A.: Synthesis and crystal structure of 2-amino-4-(3′-chlorophenyl)-3-ethoxycarbonyl-4H-pyrano-[3,2-c]-chromene-5-one. Anal. Sci. 22 (2006) X97–X98.10.2116/analscix.22.x97Suche in Google Scholar
Hu, X.; Song, L.: Ethyl 2-amino-4-(3-chlorophenyl)-5,10-dioxo-5,10-dihydro-4H-benzo[g]-chromene-3-carboxylate. Acta Crystallogr. E65 (2009) o1324–o1324.10.1107/S160053680901753XSuche in Google Scholar PubMed PubMed Central
Wang, X.-S.; Zeng, Z.-S.; Shi, D.-Q.; Chen, H.; Wei, X.-Y.; Zong, Z.-M.: Methyl 2-amino-4-(3-nitrophenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carboxylate dimethylformamide solvate. Acta Crystallogr. E60 (2004) o1877–o1879.10.1107/S160053680402358XSuche in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
Bruker. SAINT, APEX2 and SADABS. Bruker AXS Inc., Madison, WI, USA (2009).Suche in Google Scholar
©2018 Lei Shi et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn


