Abstract
C23H29Cl2N3OPd, monoclinic, P21/c (no. 14), a = 9.6053(2) Å, b = 17.4544(4) Å, c = 14.5156(3) Å, β = 104.6440(10)°, V = 2354.55(9) Å3, Z = 4, Rgt(F) = 0.0184, wRref(F2) = 0.0455, T = 173(2) K.
Data collection and handling.
| Crystal: | Prismatic, yellow |
| Size: | 0.18 × 0.14 × 0.10 mm |
| Wavelength: | Mo Kα radiation (λ =0.71073 Å) |
| μ: | 1.035 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, Φ and ω |
| Θmax, completeness: | 26.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 40421, 4597, 0.0285 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 4336 |
| N(param)refined: | 280 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Pd1 | 0.44186(2) | 0.59504(2) | 0.29572(2) | 0.01842(6) |
| Cl1 | 0.32834(4) | 0.54006(2) | 0.40204(3) | 0.02876(10) |
| Cl2 | 0.56841(5) | 0.63652(3) | 0.19015(3) | 0.03942(12) |
| O1 | 0.89028(13) | 0.66326(8) | 0.51956(10) | 0.0367(3) |
| N1 | 0.16752(14) | 0.56973(8) | 0.14050(10) | 0.0224(3) |
| N2 | 0.20747(15) | 0.68840(8) | 0.17615(9) | 0.0217(3) |
| N3 | 0.63118(15) | 0.59150(8) | 0.40947(10) | 0.0217(3) |
| H3 | 0.612(2) | 0.5607(10) | 0.4470(13) | 0.029(5) |
| C1 | 0.26041(17) | 0.61708(9) | 0.19956(11) | 0.0202(3) |
| C2 | 0.08339(19) | 0.68556(10) | 0.10234(12) | 0.0276(4) |
| H2 | 0.0270 | 0.7279 | 0.0732 | 0.033 |
| C3 | 0.05872(19) | 0.61143(10) | 0.08013(13) | 0.0293(4) |
| H3A | −0.0187 | 0.5912 | 0.0321 | 0.035 |
| C4 | 0.17304(17) | 0.48724(9) | 0.14617(11) | 0.0227(3) |
| C5 | 0.25769(18) | 0.44817(10) | 0.09693(12) | 0.0243(3) |
| C6 | 0.26825(19) | 0.36889(10) | 0.10829(13) | 0.0295(4) |
| H6 | 0.3263 | 0.3405 | 0.0764 | 0.035 |
| C7 | 0.1951(2) | 0.33122(11) | 0.16551(14) | 0.0341(4) |
| H7 | 0.2058 | 0.2774 | 0.1744 | 0.041 |
| C8 | 0.1064(2) | 0.37145(11) | 0.20989(13) | 0.0315(4) |
| H8 | 0.0542 | 0.3446 | 0.2472 | 0.038 |
| C9 | 0.09252(18) | 0.45072(10) | 0.20068(12) | 0.0263(4) |
| C10 | −0.0065(2) | 0.49396(12) | 0.24746(14) | 0.0344(4) |
| H10A | 0.0483 | 0.5331 | 0.2900 | 0.052 |
| H10B | −0.0499 | 0.4583 | 0.2843 | 0.052 |
| H10C | −0.0825 | 0.5186 | 0.1985 | 0.052 |
| C11 | 0.3302(2) | 0.48919(11) | 0.03107(14) | 0.0335(4) |
| H11A | 0.2582 | 0.5172 | −0.0170 | 0.050 |
| H11B | 0.3788 | 0.4519 | −0.0005 | 0.050 |
| H11C | 0.4011 | 0.5253 | 0.0676 | 0.050 |
| C12 | 0.25905(18) | 0.75756(9) | 0.22851(11) | 0.0234(3) |
| C13 | 0.21991(19) | 0.76937(10) | 0.31388(12) | 0.0265(4) |
| C14 | 0.2689(2) | 0.83622(11) | 0.36419(14) | 0.0360(4) |
| H14 | 0.2466 | 0.8456 | 0.4233 | 0.043 |
| C15 | 0.3494(2) | 0.88902(12) | 0.32915(15) | 0.0401(5) |
| H15 | 0.3824 | 0.9341 | 0.3646 | 0.048 |
| C16 | 0.3822(2) | 0.87697(11) | 0.24367(15) | 0.0363(4) |
| H16 | 0.4363 | 0.9144 | 0.2201 | 0.044 |
| C17 | 0.3374(2) | 0.81065(10) | 0.19069(13) | 0.0298(4) |
| C18 | 0.3732(3) | 0.79944(13) | 0.09689(15) | 0.0470(5) |
| H18A | 0.4613 | 0.7689 | 0.1062 | 0.071 |
| H18B | 0.3878 | 0.8494 | 0.0701 | 0.071 |
| H18C | 0.2938 | 0.7726 | 0.0530 | 0.071 |
| C19 | 0.1239(2) | 0.71428(11) | 0.34830(14) | 0.0353(4) |
| H19A | 0.0417 | 0.7009 | 0.2955 | 0.053 |
| H19B | 0.0892 | 0.7382 | 0.3994 | 0.053 |
| H19C | 0.1782 | 0.6678 | 0.3725 | 0.053 |
| C20 | 0.77465(18) | 0.57412(11) | 0.39448(13) | 0.0287(4) |
| H20A | 0.7915 | 0.6069 | 0.3427 | 0.034 |
| H20B | 0.7770 | 0.5200 | 0.3744 | 0.034 |
| C21 | 0.8937(2) | 0.58740(11) | 0.48402(15) | 0.0341(4) |
| H21A | 0.8834 | 0.5503 | 0.5335 | 0.041 |
| H21B | 0.9879 | 0.5783 | 0.4700 | 0.041 |
| C22 | 0.7553(2) | 0.67698(12) | 0.54095(14) | 0.0364(4) |
| H22A | 0.7543 | 0.7293 | 0.5670 | 0.044 |
| H22B | 0.7421 | 0.6401 | 0.5899 | 0.044 |
| C23 | 0.63340(18) | 0.66859(10) | 0.45247(12) | 0.0266(4) |
| H23A | 0.5408 | 0.6777 | 0.4689 | 0.032 |
| H23B | 0.6437 | 0.7078 | 0.4053 | 0.032 |
Source of material
The title compound can be easily obtained in moderate yield according to the literature method [4, 5] . Crystals suitable for X-ray diffraction were grown in a mixture of ethyl acetate and petroleum ether.
Experimental details
All hydrogen atoms attached to C atoms were introduced using the HFIX command in the SHELXL program [3]. The C—H distances in CH3 were restrained to 0.96 Å with Uiso values to be 1.5Ueq(C). Vinylic and aromatic C—H distances were restrained to 0.93 Å with Uiso values to be 1.2Ueq(C).
Discussion
N-Heterocyclic carbene-palladium(II) complexes, which usually are air- and thermal-stable, have shown excellent catalytic activity in the cross-coupling reactions [6], [7], [8]. Recently it was found that besides the carbene, the anicillary ligands played important roles in the complexes [9], [10], [11], [12], [13]. N-Heterocyclic carbene-Pd(II) complexes using morpholine as the anicillary ligand were used in the coupling of aryl sulfomates with arylboronic acids [4].
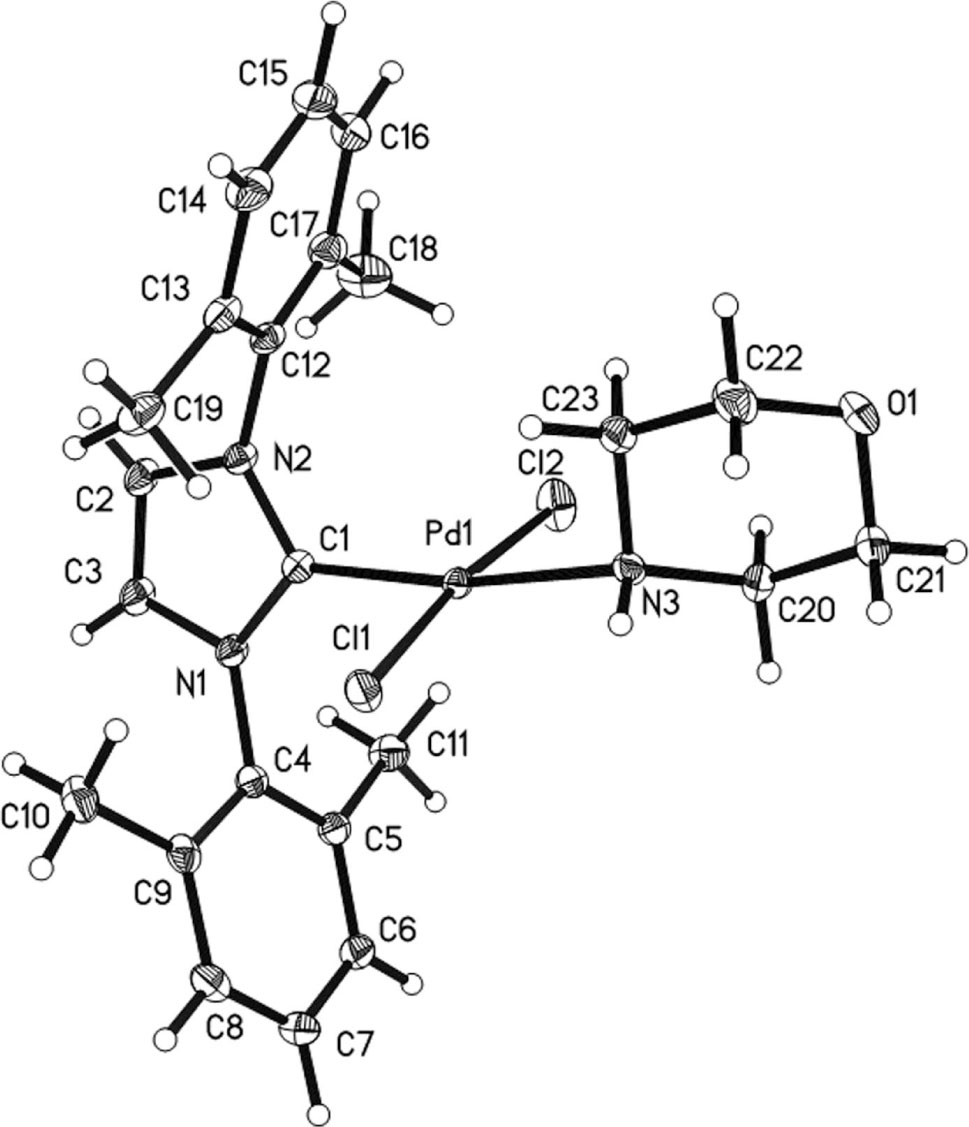
In the title compound, the Pd center is coordinated by four ligands: one carbene carbon atom, one N atom from morpholine, and two chlorine atoms, giving a slightly distorted square-planar geometry. It is noted here that the H atom on the N atom of morpholine kept untouched in this case. In such structure, the NHC and the N atom are trans to each other [C1—Pd1—N3 = 169.56(9)°, Cl1—Pd1—Cl2 = 173.55(3)°].
Acknowledgements
I acknowledge the financial support from Wenzhou University for the publication fee.
References
Bruker AXS Inc.: Bruker AXS SADABS, Version 2.03, SAINT, Version 6.02, Bruker AXS Inc. Madison, WI, USA (2002).Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
Wang, Z.-Y.; Ma, Q.-N.; Li, R.-H.; Shao, L.-X.: Palladium-catalyzed Suzuki-Miyaura coupling of aryl sulfamates with arylboronic acids. Org. Biomol. Chem. 11 (2013) 7899–7906.10.1039/c3ob41382aSearch in Google Scholar PubMed
Wang, Z.-Y.; Chen, G.-Q.; Shao, L.-X.: N-Heterocyclic carbene-palladium(II)-1-methylimidazole complex-catalyzed Suzuki-Miyaura coupling of aryl sulfonates with arylboronic acids. J. Org. Chem. 77 (2012) 6608–6614.10.1021/jo301270tSearch in Google Scholar PubMed
Froese, R. D. J.; Lombardi, C.; Pompeo, M.; Rucker, R. P.; Organ, M. G.: Designing Pd-N-heterocycic carbene complexes for high reactivity and selectivity for cross-coupling applications. Acc. Chem. Res. 50 (2017) 2244–2253.10.1021/acs.accounts.7b00249Search in Google Scholar PubMed
Valente, C.; Calimsiz, S.; Hoi, K. H.; Mallik, D.; Sayah, M.; Organ, M. G.: The Development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 51 (2012) 3314–3332.10.1002/anie.201106131Search in Google Scholar PubMed
Fortman, G. C.; Nolan, S. P.: N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem. Soc. Rev. 40 (2011) 5151–5169.10.1039/c1cs15088jSearch in Google Scholar PubMed
Zhu, L.; Gao, T.-T.; Shao, L.-X.: Well-defined NHC-Pd(II)-Im (NHC = N-heterocyclic carbene; Im = 1-methylimidazole) complexes catalyzed amination of aryl chlorides. Tetrahedron 67 (2011) 5150–5155.10.1016/j.tet.2011.05.057Search in Google Scholar
Huang, P.; Wang, Y.-X.; Yu, H.-F.; Lu, J.-M.: N-Heterocyclic carbene-palladium(II)-4,5-dihydrooxazole complexes: synthesis and catalytic activity toward amination of aryl chlorides. Organometallics 33 (2014) 1587–1593.10.1021/om401028dSearch in Google Scholar
Zhao, X.-Y.; Zhou, Q.; Lu, J.-M.: Synthesis and characterization of N-heterocyclic carbene-palladium(II) chlorides-1-methylindazole and -1-methylpyrazole complexes and their catalytic activity toward C–CN coupling of aryl chlorides. RSC Adv. 6 (2016) 24484–24490.10.1039/C6RA02556KSearch in Google Scholar
Liu, F.; Zhu, Y.-R.; Song, L.-G.; Lu, J.-M.: Synthesis of N-heterocyclic carbene–CPdCl2–C(iso)quinoline complexes and their application in arylamination at low catalyst loadings. Org. Biomol. Chem. 14 (2016) 2563–2571.10.1039/C6OB00013DSearch in Google Scholar
Zhang, Z.-M.; Gao, Y.-J.; Lu, J.-M.: Synthesis of N-heterocyclic carbene-Pd(II) complexes and their catalytic activity in the Buchwald-Hartwig amination of aryl chlorides. Tetrahedron 73 (2017) 7308–7314.10.1016/j.tet.2017.11.026Search in Google Scholar
©2018 Li Guo-Xing, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn

