Abstract
C16H21I3N8O4, monoclinic, P21/n (no. 14), a = 14.7257(7) Å, b = 10.5712(5) Å, c = 16.7501(8) Å, β = 114.408(2)°, V = 2374.4(2) Å3, Z = 4, Rgt(F) = 0.0254, wRref(F2) = 0.0760, T = 290(2) K.
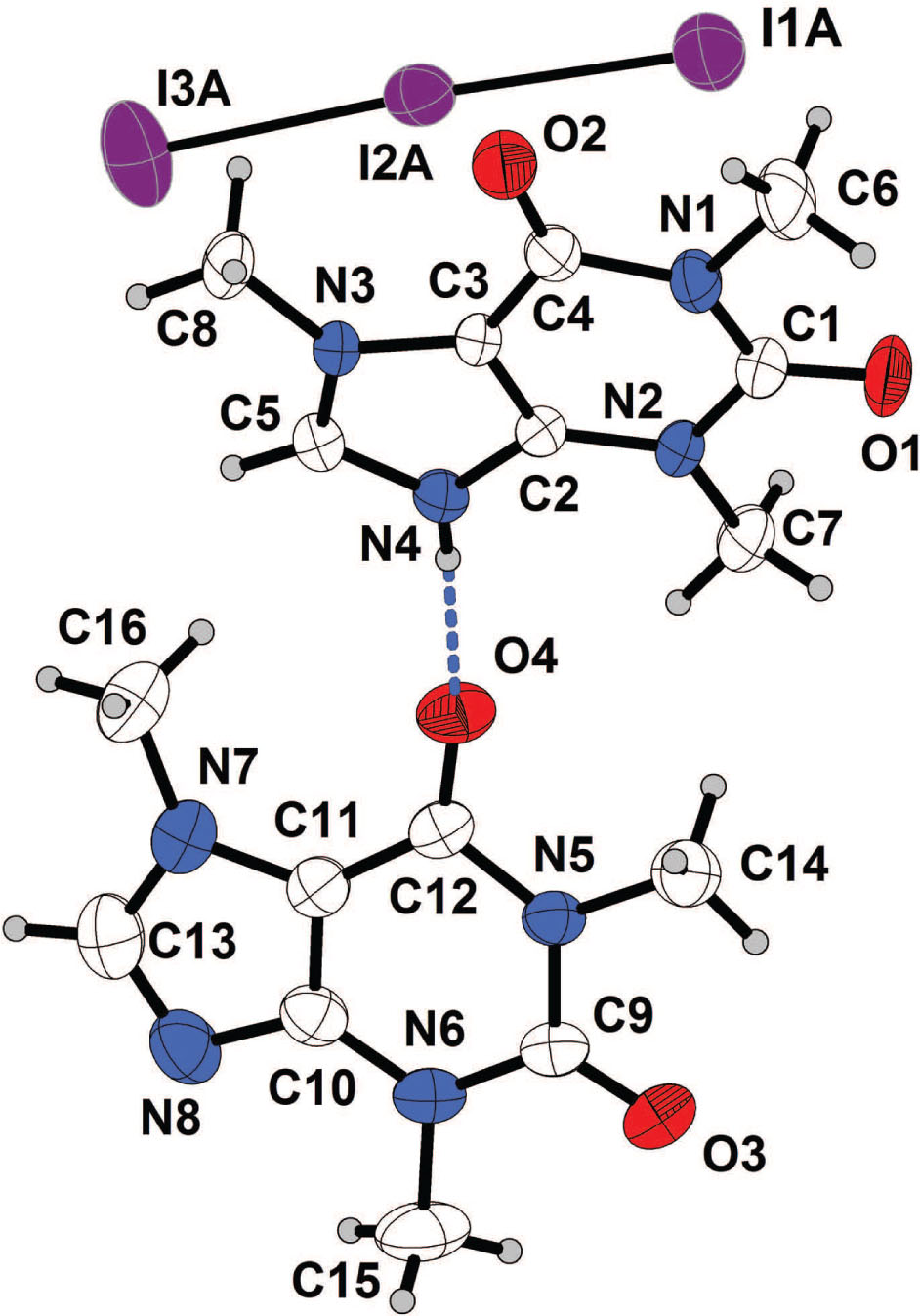
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange-brown block |
| Size: | 0.37 × 0.22 × 0.09 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.99 mm−1 |
| Diffractometer, scan mode: | APEX-II with ImS 2.0, φ and ω-scans |
| θmax, completeness: | 28.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 50390, 6007, 0.031 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5051 |
| N(param)refined: | 298 |
| Programs: | Bruker programs [1], SHELX [2], [3], |
| DIAMOND [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.49960(15) | 0.7740(2) | 0.92598(12) | 0.0515(5) |
| N1 | 0.34313(15) | 0.8568(2) | 0.86300(13) | 0.0355(4) |
| C1 | 0.42266(19) | 0.7887(2) | 0.86069(16) | 0.0357(5) |
| O2 | 0.19363(14) | 0.9609(2) | 0.79700(13) | 0.0476(5) |
| N2 | 0.41061(14) | 0.73470(19) | 0.78165(13) | 0.0323(4) |
| C2 | 0.32494(16) | 0.7579(2) | 0.71023(15) | 0.0283(4) |
| O3 | 0.57340(16) | 0.1957(2) | 0.58374(15) | 0.0543(5) |
| N3 | 0.17760(14) | 0.83646(19) | 0.62699(13) | 0.0319(4) |
| C3 | 0.25052(16) | 0.8314(2) | 0.71241(15) | 0.0291(4) |
| O4 | 0.38498(16) | 0.54937(19) | 0.55667(13) | 0.0465(5) |
| N4 | 0.29786(15) | 0.7182(2) | 0.62603(14) | 0.0330(4) |
| H4 | 0.324(2) | 0.669(3) | 0.608(2) | 0.048(10)* |
| C4 | 0.25565(18) | 0.8905(2) | 0.79041(16) | 0.0330(5) |
| N5 | 0.47505(16) | 0.36861(19) | 0.56947(14) | 0.0345(4) |
| C5 | 0.20813(18) | 0.7681(2) | 0.57761(16) | 0.0355(5) |
| H5 | 0.172661 | 0.755904 | 0.517635 | 0.043* |
| N6 | 0.45126(18) | 0.2348(2) | 0.44954(15) | 0.0405(5) |
| C6 | 0.3573(3) | 0.9101(3) | 0.94827(18) | 0.0546(8) |
| H6A | 0.394843 | 0.987184 | 0.958137 | 0.082* |
| H6B | 0.392836 | 0.850583 | 0.993804 | 0.082* |
| H6C | 0.293462 | 0.927362 | 0.948632 | 0.082* |
| N7 | 0.27222(17) | 0.4727(2) | 0.36275(16) | 0.0460(5) |
| C7 | 0.49322(19) | 0.6655(3) | 0.77591(19) | 0.0432(6) |
| H7A | 0.469432 | 0.617780 | 0.722367 | 0.065* |
| H7B | 0.521318 | 0.608904 | 0.824898 | 0.065* |
| H7C | 0.543352 | 0.724114 | 0.776676 | 0.065* |
| N8 | 0.3187(2) | 0.2992(3) | 0.31194(16) | 0.0532(6) |
| C8 | 0.0848(2) | 0.9092(3) | 0.5969(2) | 0.0469(7) |
| H8A | 0.100003 | 0.997970 | 0.603314 | 0.070* |
| H8B | 0.048781 | 0.887013 | 0.631335 | 0.070* |
| H8C | 0.044741 | 0.890454 | 0.536356 | 0.070* |
| C9 | 0.5049(2) | 0.2611(2) | 0.53707(18) | 0.0375(5) |
| C10 | 0.3755(2) | 0.3121(3) | 0.39890(18) | 0.0396(6) |
| C11 | 0.34984(19) | 0.4176(2) | 0.43303(18) | 0.0365(5) |
| C12 | 0.40038(18) | 0.4536(2) | 0.52121(17) | 0.0330(5) |
| C13 | 0.2569(3) | 0.3978(3) | 0.2937(2) | 0.0569(8) |
| H13 | 0.207597 | 0.413254 | 0.237982 | 0.068* |
| C14 | 0.5278(2) | 0.3913(3) | 0.66411(19) | 0.0472(7) |
| H14A | 0.558552 | 0.473290 | 0.673883 | 0.071* |
| H14B | 0.578080 | 0.327727 | 0.689671 | 0.071* |
| H14C | 0.481212 | 0.387781 | 0.690660 | 0.071* |
| C15 | 0.4778(3) | 0.1235(3) | 0.4119(3) | 0.0656(10) |
| H15A | 0.462902 | 0.139157 | 0.351268 | 0.098* |
| H15B | 0.440090 | 0.051959 | 0.416448 | 0.098* |
| H15C | 0.547695 | 0.106485 | 0.443116 | 0.098* |
| C16 | 0.2194(3) | 0.5896(3) | 0.3628(3) | 0.0618(9) |
| H16A | 0.188431 | 0.622432 | 0.304242 | 0.093* |
| H16B | 0.265727 | 0.650566 | 0.400257 | 0.093* |
| H16C | 0.169165 | 0.572475 | 0.384005 | 0.093* |
| I1Aa | 0.46702(3) | 1.10662(5) | 0.79487(3) | 0.05997(11) |
| I2Aa | 0.32749(2) | 1.09822(2) | 0.61021(2) | 0.04423(7) |
| I3Aa | 0.19370(2) | 1.07608(8) | 0.42627(3) | 0.07731(17) |
| I1Bb | 0.4784(10) | 1.0801(14) | 0.7790(9) | 0.05997(11) |
| I2Bb | 0.32749(2) | 1.09822(2) | 0.61021(2) | 0.04423(7) |
| I3Bb | 0.1870(8) | 1.137(2) | 0.4414(8) | 0.07731(17) |
a Occupancy: 0.967(2); b Occupancy: 0.033(2).
Source of material
The title compound caffeinium triiodide – caffeine (1/1) [systematic name: 1,3,7-trimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-9-ium triiodide – 1,3,7-trimethyl-3,7-dihydro-1H-purine-2,6-dione (1/1)] was obtained from a mixing solution of ethanol and equimolar amounts of caffeinium triiodide hydrate and caffeine. The crystals show an orange colour and are easily distinguishable from the dark caffeinium triiodide hydrate crystals.
Experimental details
The crystal used for the study was harvested directly from the mother liquor. Data collection followed the standard procedures of the Bruker APEX2 software [1]. Absorption corection was applied using the SADABS program implemented in the APEX2 program system [1]. Structure solution with the SHELXT [2] yielded all non-hydrogen atoms. During the latter stages of the refinement [3] a slight disorder of the triiodide anion was taken into account (not shown in the figure). The largest difference electron density peaks and holes are 0.99 and −0.88 e Å−3, respectively. The largest difference electron peaks (down to 0.51 e Å−3) are less than 0.84 Å away from iodine positions. Coordinates and Uiso of the H4 hydrogen atom (cf. the figure) were refined freely. All other hydrogen atoms were included using the standard riding models of the SHELX System (AFIX 43; 137) [3].
Comment
A report on the first triiodide (strychninium triiodide) dates back to the beginning of the 19th century [5]. A reinvestigation by combustion analysis in 1865 verified these findings [6]. In the course of the aforementioned study caffeinium triiodide monohydrate (caffHI3 ⋅ H2O) was identified. Further investigations in the 19th century on the caffeine/HI system followed with a view to analytical aspects [7], [8]. A preliminary structure determination of caffHI3 ⋅ H2O has been reported [9] and a reconsideration of this compound is in progress [10]. As part of a study on polyiodides trapped in hydrogen bonded surroundings [11], [12], [13], we have reinvestigated the system caffeine/HI/H2O/I2. In the course of this study, the new compound caffHI3 ⋅ caff was obtained. The appearance of this compound was expected, as we have already shown that depending on the pH value organic aminium halogenides co-crystals containing the organic aminium cation, the counter anion and one additional equivalent of the neutral organic amine can be easily obtained [11], [14], [15]. Even though caffeine has been known for many decades, studies on the medical and biological properties are still in progress [16]. Despite this interest in caffeine, we were surprised that structures of the N-protonated caffeine are still rare.
The asymmetric unit of the title crystal structure contains one N-protonated caffeinium cation, one triiodide counter anion and one caffeine molecule (cf. the figure). Bond lengths and angles in the title crystal structure are in the expected ranges. Caffeine is a weak base, a property which is expressed by its structural chemistry. Protonation at the 9-position of the purine moiety (in the title structure at N4) only takes place on reaction with strong acids [17], [18], [19], [20], [21]. Weak organic acids form co-crystals without any transfer of the proton from the organic acid to the caffeine molecule [22], [23]. In the title structure the protonated N atom of the caffeinium cation donates one hydrogen bond to the adjacent neutral caffeine molecule (cf. the figure; N—H = 0.78(3) Å; N⋯O = 2.722(3) Å). The caffeinium cation as well as the caffeine molecule are almost planar [rms deviation of fitted atoms (N1—N4; C1—C5) = 0.022 Å; rms deviation of fitted atoms (N5—N8; C9—C13) = 0.010 Å]. The dihedral angle is 21.37(9)°. In the packing scheme of the title crystal structure there is one triiodide anion above and another one below each caffeinium cation, which rules out any kind of π-π interactions. This feature of packing has been reported earlier [24]. The neutral caffeine molecules are pairwise arranged with an interplanar distance of 3.319(6) Å. The I—I distances in the triiodide anion are in the typical range for triiodide anions not involved in strong secondary interactions [11], [12], [13]. A detailed discussion of the geometric parameters of the anion based on the crystal structure is ruled out by its disorder. Furthermore, there are no intermolecular I⋯I halogen bonds as the shortest I⋯I distances are >4.3 Å.
To obtain more information about the geometry of the triiodide anion in the title structure a Raman spectrum was recorded (MultiRAM, Bruker Optics, range: 4000–50 cm−1). A strong Raman signal at ∼110 cm−1 was detected, which features a shoulder towards higher wavenumbers, and a weak signal at 216 cm−1. Both signals are in perfect accord with a roughly symmetrical I3− anion [25], [26], [27]. A significant asymmetry, which may be introduced by halogen [28], [29], [30] or hydrogen bonds [12] shifts the strongest Raman signals into the 150–165 cm−1 range.
This contribution forms part of the general interest on stacking properties of caffeine [31], [32], [33].
Acknowledgements
We gratefully acknowledge support by the Ministry of Innovation, Science and Research of North-Rhine Westphalia and the German Research Foundation (DFG) for financial support (Bruker diffractometer, APEX2-Dual Source; INST 208/589-1, project no. 208167569).
References
BRUKER. SAINT, APEX2 and SADABS. Bruker AXS Inc., Madison, WI, USA (2009).Suche in Google Scholar
Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Ver. 4.5.1. Crystal Impact, Bonn, Germany, 2018.Suche in Google Scholar
Pelletier, P.-J.; Caventou, J. B.: Sur un nouvel alcali végétal (la strychine) trouvé dans la fève de Saint-Ignace, la noix vomique, etc. Ann. Chim. Phys. 10 (1819) 142–177.Suche in Google Scholar
Tilden, W. A.: On the periodides of some of the organic bases. J. Chem. Soc. 18 (1865) 99–105.10.1039/JS8651800099Suche in Google Scholar
Gomberg, M.: On the action of Wagner’s reagent upon caffeine and a new method for the estimation of caffeine. J. Am. Chem. Soc. 18 (1896) 331–342.10.1021/ja02090a002Suche in Google Scholar
Gomberg, M.: Perhalides of caffeine. J. Am. Chem. Soc. 18 (1896) 347–377.10.1021/ja02090a004Suche in Google Scholar
Herbstein, F.; Kaftory, M.; Kapon, M.; Saenger, W.: Structures of three crystals containing approximately – linear chains of triiodide ions. Z. Kristallogr. 154 (1981) 11–30.10.1524/zkri.1981.154.1-2.11Suche in Google Scholar
Reiss, G. J.; Majewski, M. A.; Merkelbach, J.: Higher symmetry at lower temperatur: reinvestigation of the historic compound caffeinium triiodide monohydrate. 26th Annual Conference of the German Crystallographic Society, 2018, Essen, Germany. Z. Kristallogr. Suppl. 38 (2018) 93–94.Suche in Google Scholar
Reiss, G. J.: Two iodine-rich (dimethylphosphoryl)methanaminium iodides. Z. Kristallogr. CM 232 (2017) 789–795.10.1515/zkri-2017-2071Suche in Google Scholar
van Megen, M.; Reiss, G. J.: I62− anion composed of two asymmetric triiodide moieties: a competition between halogen and hydrogen bond. Inorganics 1 (2013) 3–13.10.3390/inorganics1010003Suche in Google Scholar
Reiss, G. J.; Leske, P. B.: The twinned crystal structure of bis((4-aminopyridin-1-ium) iodide triiodide, C20H28I8N8. Z. Kristallogr. NCS 229 (2014) 452–454.10.1515/ncrs-2014-0193Suche in Google Scholar
Reiss, G. J.: Constructor graphs as useful tools for the classification of hydrogen bonded solids: the case ctudy of the cationic (dimethylphosphoryl)methanaminium (dpmaH+) tecton. Crystals 6 (2016) 6.10.3390/cryst6010006Suche in Google Scholar
Buhl, D.; Gün, H.; Jablonka, A.; Reiss, G. J.: Synthesis, structure and spectroscopy of two structurally related hydrogen bonded compounds in the dpma/HClO4 system; dpma = (dimethylphosphoryl)methanamine. Crystals 3 (2013) 350–362.10.3390/cryst3020350Suche in Google Scholar
McLellan, T. M.; Caldwell, J. A.; Lieberman, H. R.: A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 71 (2016) 294–312.10.1016/j.neubiorev.2016.09.001Suche in Google Scholar
Mercer, A.; Trotter, J.: Crystal and molecular structure of 1,3,7-trimethyl-2,6-purinedione hydrochloride dihydrate (caffeninium hydrochloride dehydrate). Acta Crystallogr. B34 (1978) 450–453.10.1107/S0567740878003337Suche in Google Scholar
García-Raso, A.; Fiol, J. J.; Tasada, A.; Prieto, M. J.; Moreno, V.; Mata, I.; Molins, E.; Bunič, T.; Golobič, A.; Turel, I.: Ruthenium complexes with purine derivatives: Syntheses, structural characterization and preliminary studies with plasmidic DNA. Inorg. Chem. Commun. 8 (2005) 800–804.10.1016/j.inoche.2005.05.023Suche in Google Scholar
Chandramohan, A.; Gayathri, D.; Velmurugan, D.; Ravikumar, K.; Kandhaswamy, M. A.: 1,3,7-Trimethylxanthenium 2,4,6-trinitrophenolate. Acta Crystallogr. E63 (2007) o2495–o2496.10.1107/S1600536807017825Suche in Google Scholar
Jerin, C. V.; Athimoolam, S.: Caffeinium bisulfate monohydrate. Acta Crystallogr. E67 (2011) o2290.10.1107/S1600536811031540Suche in Google Scholar
Gurau, G.; Kelley, S. P.; Di Bona, K. R.; Smiglak, M.; Rogers, R. D.: Anhydrous caffeine hydrochloride and its hydration. Cryst. Growth Des. 12 (2012) 4658–4662.10.1021/cg300878jSuche in Google Scholar
Bucar, D.-K.; Day, G. M.; Halasz, I.; Zhang, G. G. Z.; Sander, J. R. G.; Reid, D. G.; MacGillivray, L. R.; Duer, M. J.; Jones, W.: The curious case of (caffeine)⋅(benzoic acid): how heteronuclear seeding allowed the formation of an elusive cocrystal. Chem. Sci. 4 (2013) 4417–4425.10.1039/c3sc51419fSuche in Google Scholar
Vangala, V. R.; Chow, P. S.; Schreyer, M.; Lau, G.; Tan, R. B. H.: Thermal and in situ X-ray diffraction analysis of a dimorphic co-crystal, 1:1 caffeine–glutaric acid. Cryst. Growth Des. 16 (2016) 578–586.10.1021/acs.cgd.5b00798Suche in Google Scholar
Cingi, M. B.; Lanfredi, A. M. M.; Tiripicchio, A.; Bandoli, G.; Clemente, D. A.: Crystal structure of 2:1 molecular complexes of caffeine with hexaaquamagnesium(II) bromide and hexaaquamanganese(II) triiodide iodide. Inorg. Chim. Acta 52 (1981) 237–243.10.1016/S0020-1693(00)88603-XSuche in Google Scholar
Batalov, V. I.; Dikhtiarenko, A.; Yushina, I. D.; Bartashevich, E. V.; Kim, D. G.; García-Granda, S.: Crystal structure of (E)-3-(1-iodoethylidene)-2,3-dihydro-[1, 4]thiazino-[2,3,4-ij]quinolin-4-ium triiodide, C13H11I4NS. Z. Kristallogr. NCS 229 (2014) 195–196.10.1515/ncrs-2014-0096Suche in Google Scholar
Marks, T. J.; Kalina, D. W.: Highly conductive halogenated low-dimensional materials. in Extended linear chain compounds; Miller, J. S., Ed.; Plenum Press, New York, USA, 1982; Vol. 1, pp. 197–331.10.1007/978-1-4613-3249-7_6Suche in Google Scholar
Otsuka, M.; Mori, H.; Kikuchi, H.; Takano, K.: Density functional theory calculations of iodine cluster anions: structures, chemical bonding nature, and vibrational spectra. Comput. Theor. Chem. 973 (2011) 69–75.10.1016/j.comptc.2011.07.002Suche in Google Scholar
Reiss, G. J.: I5− polymers with a layered arrangement: synthesis, spectroscopy, and structure of a new polyiodide salt in the nicotine/HI/I2 system. Naturforsch. B70 (2015) 735–739.10.1515/znb-2015-0092Suche in Google Scholar
Bartashevich, E. V.; Batalov, V. I.; Yushina, I. D.; Stash, A. I.; Chen, Y. S.: Nontypical iodine-halogen bonds in the crystal structure of (3E)-8-chloro-3-iodomethylidene-2,3-dihydro-1,4-oxazino[2,3,4-ij]quinolin-4-ium triiodide. Acta Crystallogr. C72 (2016) 341–345.10.1107/S2053229616003934Suche in Google Scholar PubMed
Bartashevich, E. V.; Stash, A. I.; Batalov, V. I.; Yushina, I. D.; Drebushchak, T. N.; Boldyreva, E. V.; Tsirelson, V. G.: The staple role of hydrogen and halogen bonds in crystalline (E)-8-((2,3-diiodo-4-(quinolin-8-ylthio)but-2-en-1-yl)thio)quinolin-1-ium triiodide. Struct. Chem. 27 (2016) 1553–1560.10.1007/s11224-016-0785-ySuche in Google Scholar
Ulanowska, K.; Piosik, J.; Gwizdek-Wiśniewska, A.; Wegrzyn, G.: Formation of stacking complexes between caffeine (1,2,3-trimethylxanthine) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine may attenuate biological effects of this neurotoxin. Bioorg. Chem. 33 (2005) 402–413.10.1016/j.bioorg.2005.07.004Suche in Google Scholar PubMed
Tavagnacco, L.; Gerelli, Y.; Cesáro, A.; Brady, J. W.: Stacking and branching in self-aggregation of caffeine in aqueous aolution: from the supramolecular to atomic scale clustering. J. Phys. Chem. B120 (2016) 9987–9996.10.1021/acs.jpcb.6b06980Suche in Google Scholar PubMed
Johnson, N. O.; Light, T. P.; MacDonald, G.; Zhang, Y.: Anion–caffeine interactions studied by 13C and 1H NMR and ATR–FTIR spectroscopy. J. Phys. Chem. B121 (2017) 1649–1659.10.1021/acs.jpcb.6b12150Suche in Google Scholar PubMed
©2018 Johannes Merkelbach et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn


