Abstract
Objectives
A high incidence of attention-deficit hyperactivity disorder (ADHD) has been reported in chronic pain (ChP) patients. Furthermore, an association between ChP and muscular dysregulation has been reported in adults with ADHD. The present study investigated whether ADHD was more prevalent among psychiatric outpatients with ChP than those without ChP, and if there was an association between ChP, muscular dysregulation and characteristics of pain in patients with ADHD.
Methods
One-hundred and twenty-one individuals remitted to an outpatient psychiatry unit took part in this naturalistic epidemiological cross-sectional study. They were assessed with a pain self-report form (localization, intensity, and onset) and a test of muscle dysregulation (the Motor Function Neurological Assessment). Prevalence of ADHD among patients with ChP, as well as the qualitative characteristics of ChP within the ADHDgroup are reported. Both ChP and pain intensity correlated with muscular dysregulation through Spearman’s rho analysis. Additionally, the relationship between various diagnostic categories (ADHD, affective disorders, anxiety, or personality disorders) and incidence of axial pain was evaluated in logistic regression.
Results
ADHD was significantly more prevalent in patients with ChP, than in patients without ChP. In the ADHD group, ChP and pain intensity was associated with muscular dysregulation, particularly with high muscle tone. ChP was more axial and widespread, than for the patients without ADHD, and started at an early age. ADHD diagnosis predicted axial pain, whereas affective-, anxiety-, or personality disorders did not.
Conclusions
The study suggests that ChP in ADHD is associated with muscular dysregulation and is qualitatively different from ChP in psychiatric patients without ADHD. These findings may lead to further understanding of potential mechanisms involved in ADHD and ChP, and in turn to new treatment strategies for both disorders.
1 Introduction
Chronic pain (ChP) disorders are a major public health problem, affecting about 20–40% of the adult population [1–3], and may seriously affect the quality of social and working life of those affected by the condition [1]. ChP without evidence of tissue damage, particularly in the form of low back pain, neck and shoulder pain, and chronic widespread pain presents a significant challenge in health care [4]. Patients with psychiatric problems often report ChP and physical discomfort, despite no medical condition. These comorbidities may negatively impact successful treatment of either conditions [5].
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by deficits in attention, activity regulation, and impulse control, significantly impairing personal, academic, and social functioning [6]. ADHD diagnosis occurs in about 5.9% of youth and 2.5% of adults [7], but is underdiagnosed in adults [8]. A strikingly high percentage of patients with ChP disorders have ADHD and vice versa. In one study, 72.5% of adults referred to a pain clinic for ChP and probable somatic syndrome disorder, met the ADHD diagnostic criteria [9], and 76.7% of adults with persistent low back pain exhibited ADHD symptoms [10]. Numerous studies have documented the prevalence of ADHD in patients with the ChP disorder fibromyalgia syndrome ranging from 24.5 to –44.72% [11–15]. A review article reports a high prevalence of ADHD also among youth with ChP, and conversely, a high prevalence of ChP in samples of youth with ADHD [16]. Very high prevalence of ChP symptoms and widespread pain in adult ADHD patients are reported by several, ranging from 39 to –80% [17–19]. Moreover, a population study found that high ADHD symptom scores were associated with increased pain likelihood [20].
The underlying mechanisms involved in ADHD and ChP comorbidity, are not well understood [21]. Genetic factors, increased exposure to trauma, HPA axis dysfunction, neuroinflammation, altered sensitivity to pain, and dysfunction of the dopaminergic system are proposed to be shared mechanisms that may influence the association between ADHD and ChP [21–24]. Additionally, long-lasting muscle contraction may also contribute to chronic muscle pain [25], and the dopamine system may be involved in both ADHD, ChP and modulation of muscle tone [26–29]. Motor dysregulation causing a persistently high muscle tone has been reported in close to 90% of both children and adults with ADHD [19,30], and has been suggested to be involved in the development of chronic muscle pain in ADHD. In a study on the experience and prevalence of ChP in adult ADHD patients compared to healthy controls, the ADHD group reported significantly more widespread pain and a higher pain intensity than the control subjects [19]. A highly significant correlation was found between the muscular regulation problems and pain intensity [19]. Axial pain was not explicitly investigated in that study, but the ADHD group displayed an increased tone in muscles in the neck, back, chest, shoulders, hips (i.e., axial and proximal stabilizing muscles), and legs. Such a heightened tone in these muscles was also found in children with ADHD [30]. These findings raise the question as whether there are distinctive characteristics of ChP in ADHD related to muscular dysregulation that is axial and widespread with an early onset. In the present study the objective was to investigate the prevalence of ADHD among psychiatric outpatients with ChP, and the association between ChP, muscular dysregulation and characteristic of pain in psychiatric outpatients. We hypothesized that:
Psychiatric patients with ChP would have a higher prevalence of ADHD, than would psychiatric patients without ChP.
ChP and intensity of pain in patients with ADHD are associated with muscular dysregulation.
ChP in patients with ADHD is characterized by widespread and axial pain, with an early onset. This “ADHD-pain” is qualitatively different from ChP in patients without ADHD.
2 Methods
2.1 Design
A naturalistic epidemiological cross-sectional study. Assessments regarding pain and muscular dysregulation were carried out before diagnostic assessment was completed.
2.2 Participants
Inclusion criteria: All individuals remitted for diagnostic assessment (or reassessment) to a public health general psychiatry outpatient clinic in a rural district of Norway over a period of 24 months were invited to participate. They received a written invitation to participate, with information about the project (focusing on ChP and muscular dysregulation in patients with and without ADHD) and a consent form. At their first appointment at the clinic, the patients were asked by the locally assigned therapist if they had consented to participate. Figure 1 shows the flow of inclusion of patients into the study.
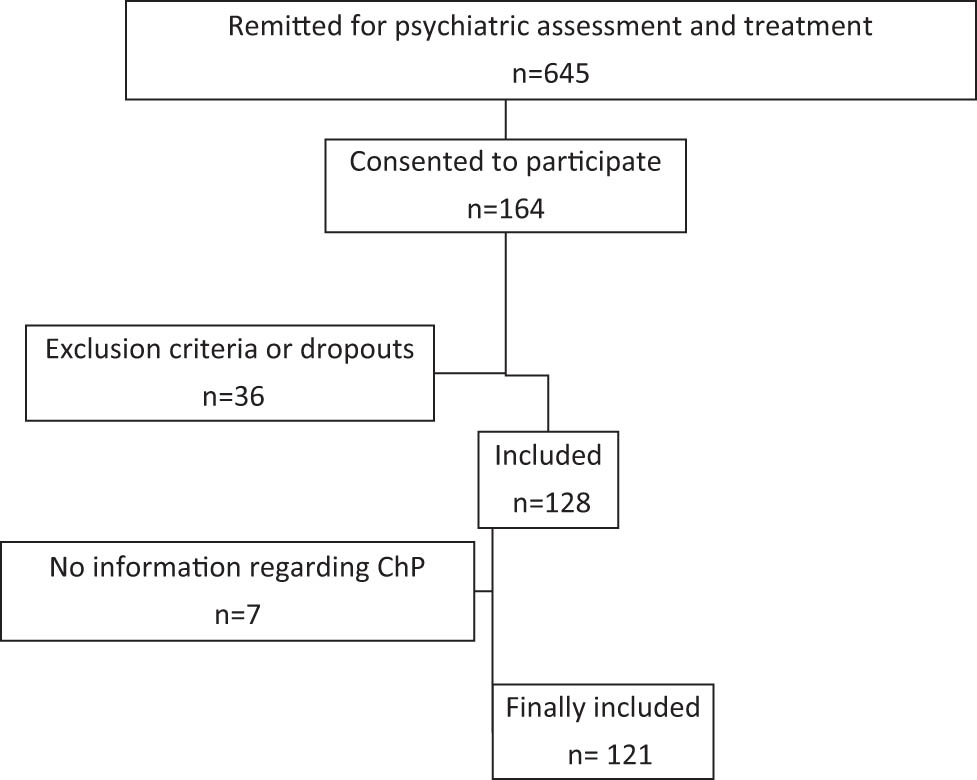
Flow chart of inclusion.
Exclusion criteria: Ongoing drug abuse, schizophrenia, other psychosis, rheumatic, orthopedic or neurological disorder, and drugs affecting muscular function.
2.3 Self-evaluation of pain
The pain self-report form included the questions: “Are you troubled by chronic pain/aches?” and “Approximately how old were you when the pain started?” The patient was also given a Pain Drawing, a schematic drawing of the outline of a human figure from ventral and dorsal perspectives with a horizontal line in the thoracolumbar region (TH12) for localization of pain [31] and a Visual Analog Scale (VAS) assessing pain intensity [32].
ChP was defined as “confirmed self-reported ChP” (answering yes to the question “Are you troubled by chronic pain/aches”), or a muscular ChP diagnosis reported in the psychiatric journal. No diagnostic assessment of ChP was performed.
Early onset of ChP was defined as the onset occurring at or before the age of 20.
Pain location: Painful areas of the body during the last 14 days were marked on the drawing. Pain location was categorized as “upper body” (marked above the horizontal line drawn at thoracic spine 12), “lower body” (marked below the horizontal line drawn at thoracic spine 12), “axial” (involving cervical spine or anterior chest or thoracic spine or low back pain) [33], and “widespread” (encompassing axial, upper body, and lower body pain).
Pain intensity: The VAS ranged pain from 0 = “No pain” to 100 = “Pain as bad as could be” during the last 14 days. A VAS score less than 5 was labeled as “No pain,” VAS scores from 5 to 44 as “Mild pain,” scores from 45 to 74 as “Moderate pain,” and scores 75 and higher as “Intense pain” [32].
2.4 Assessment of muscular dysregulation
The Motor Function Neurological Assessment battery (MFNU) was developed to evaluate clinically observed muscular regulation problems that were impacting daily activities in individuals with ADHD [30,34]. MFNU consists of 16 subtests, each scored according to three scoring categories (0–1–2), with a maximum problem score “MFNU Total Score” (MFNU-TS) being 32. The subtests were grouped into two sub-scores: “MFNU-Tone” (MFNU-T) and “MFNU-Inhibition” (MFNU-I). The MFNU-T comprises eight subtests primarily assessing muscle tone in axial and stabilizing muscles: the erector spinae, latissimus dorsi, and iliopsoas muscle, and in muscles of the calf, ankle, and foot. The eight remaining subtests mainly address inhibition problems. Four of these reveal progressive restricted movements of joints and increased muscle tone when active movements are repeated several times in succession. The scoring criteria for each subtest are described in detail in the MFNU manual [35]. A detailed video presentation of all subtests is accompanying the Norwegian MFNU manual [35]. For more information and videos refer Stray et al. [30].
2.5 Diagnostic assessment
Diagnostic assessments were carried out by a physician or a clinical psychologist, as part of the routine diagnostic assessment, which included anamnesis and the “PLUS” version of the MINI International neuropsychiatric interview (MINI-Plus) [36]. The Norwegian version of MINI-Plus [37] was used. The MINI-Plus is a widely used structured psychiatric interview for the major psychiatric disorders and have shown good reliability and validity for depression and anxiety disorders. Although the ADHD module of the MINI-Plus demonstrates excellent specificity for ADHD, its sensitivity is lower, and it only offers moderate inter-rater reliability [36]. When appropriate, MINI-Plus was supplied with the Structured Clinical Interview for DSM-IV Axis II Personality Disorders: SCID-II [38] or/and the Diagnostic Interview for assessment of ADHD in Adults 2.0 (DIVA-2) [39].
2.6 Diagnoses included
ADHD according to the MINI-Plus and/or DIVA-2.0 criteria. Both the combined and the predominantly inattentive type were included (the hyperactive subtype was very rarely diagnosed in the clinic, and was not diagnosed in our sample). Due to the lesser validity of the MINI-Plus for ADHD compared to other disorders, individuals suspected of ADHD based on referral or epicrisis but not diagnosed due to subthreshold ADHD symptoms were allocated to the NoADHD group.
Affective disorders according to the MINI-Plus criteria, including recurrent depression, ongoing depressive episode, dysthymia, and bipolar spectrum disorders.
Anxiety disorders according to the MINI-Plus criteria, including panic disorder, agoraphobia, social phobia, generalized anxiety disorder, post-traumatic stress disorder, and obsessive-compulsive disorder.
Personality disorders according to the SCID-II criteria.
2.7 Statistical analyses
The statistical analyses were carried out using IBM SPSS Statistics for Windows version 29 (SPSS Inc., Chicago, IL, USA). Two-sided alpha levels of p < 0.05 were considered statistically significant. Pearson Chi-Square test, Fisher exact test, Mann–Whitney U test, and correlation analyses (Spearman’s rho) were used as appropriate. Cramer’s V was used to calculate effect sizes (Cramer’s V: 0.1 = small, 0.3 = medium, 0.5 = large). Binary logistic regression was conducted to assess the impact of the diagnostic categories ADHD, affective disorders, anxiety disorders, and personality disorders, respectively, on axial pain. Due to the limited size of the sample, the regression analyses were performed with a model containing only one independent variable (diagnostic category). Some of the analyses were repeated without participants with subthreshold ADHD symptoms.
3 Results
3.1 Participants
A total of 121 patients were included in the study (Figure 1): 79 females (65.3%) and 42 males (34.7%). The age range was 18–66 years, with a mean age of 32.4 years (standard deviation: 10.8). Of the 121 participants, 109 underwent assessment using the MFNU. Three participants were already treated with central stimulants at the time of MFNU assessments. They did not use central stimulants on the day MFNU was performed.
3.2 Prevalence of ChP
Of the participants, 106 (87.6%) reported ChP, while 15 (12.4%) did not. There were no significant differences in age or gender between the patients with and without ChP.
3.3 Prevalence of ADHD in patients with ChP
There was a significantly higher prevalence of ADHD among participants with ChP compared to those without ChP (80.2% [n = 85] vs 40% [n = 6], χ² = 11.4, p < 0.001).
There were no significant differences between patients with and without ChP in the prevalence of affective-, anxiety-, or personality disorders (affective disorders: 34.0% vs 53.3%, anxiety disorders: 59.4% vs 40.0%, personality disorders: 17.0% vs 13.3%, respectively).
3.4 Prevalence of ADHD in the sample
Of the 121 participants, 91 patients (75.2%) met the criteria for ADHD (the “ADHD group”). The remaining 30 patients constituted the “NoADHD group.” Age and gender did not differ significantly between the ADHD and the NoADHD group. Comorbidity was frequent in both groups. Affective-, anxiety-, and personality disorders did not differ significantly between the ADHD and the NoADHD group (affective disorders: 31.9% vs 50.0%, anxiety disorders 52.7% vs 70.0%, personality disorders 17.6% vs 13.3%, respectively). In the NoADHD group, 16 patients reported considerable ADHD symptoms persisting from childhood, but diagnosis was unconfirmed due to subthreshold ADHD symptoms. Excluding these from the sample did not alter the differences between the ADHD and NoADHD groups regarding age, gender, affective-, anxiety-, and personality disorders.
3.5 ChP and muscular dysregulation within the ADHD group
Within the ADHD group, psychiatric patients with ChP exhibited significantly more muscular regulation problems than those without ChP, as indicated by the higher MFNU-TS (MWU = 358.5, p = 0.044). This difference was most pronounced in the MFNU-T (MWU = 379.0, p = 0.004) and not significant in the MFNU-I (both groups had a high MFNU-I).
3.6 ChP and muscular dysregulation within the NoADHD group
Within the “NoADHD group” there were no significant differences in MFNU scores between patients with and without ChP.
3.7 Correlations between muscular dysregulation and ChP
Correlations between muscular dysregulation and, respectively, ChP and pain intensity in patients with and without ADHD diagnosis are detailed in Table 1. In the ADHD group, muscular dysregulation was significantly correlated with ChP, in particular with pain intensity. These correlations were most pronounced regarding MFNU-T. No significant correlations were found within the NoADHD group.
Muscular dysregulation – correlation with chronic pain and pain intensity
| ADHD | noADHD | ||||
|---|---|---|---|---|---|
| Muscular dysregulation | ChP | Pain intensity | ChP | Pain intensity | |
| MFNU-TS | rho | 0.219 | 0.334 | 0.254 | 0.169 |
| sig | 0.043 | 0.002 | 0.241 | 0.452 | |
| n | 86 | 82 | 23 | 22 | |
| MFNU-T | rho | 0.258 | 0.353 | 0.315 | 0.157 |
| sig | 0.017 | 0.001 | 0.143 | 0.486 | |
| n | 86 | 82 | 23 | 22 | |
| MFNU-I | rho | 0.135 | 0.247 | 0.225 | 0.196 |
| sig | 0.217 | 0.025 | 0.301 | 0.383 | |
| n | 86 | 82 | 23 | 22 | |
NoADHD = patients not diagnosed with ADHD; MFNU = Motor Function Neurological Assessment battery; MFNU-TS = MFNU total score; MFNU-T = MFNU-Tone sub-score; MFNU-I = MFNU Inhibition sub-score.
3.8 Qualitative differences of ChP in patients with and without ADHD diagnosis
In the ADHD group, 85 (93.4%) reported ChP. Intensity of pain was significantly higher in the ADHD group as compared to the NoADHD group (χ 2 = 12.5, p = 0.004, V = 0.35). This difference increased when excluding those with subthreshold ADHD symptoms from the noADHD group (χ 2 = 20.4, p < 0.001, V = 0.53).
Pain and pain characteristics in patients with and without ADHD diagnosis are detailed in Table 2.
Pain characteristics in those with and without ADHD
| Pain | ADHD (n = 91) | NoADHD (n = 30) | NoADHD-ex1 (n = 15) | ADHD vs NoADHD (n = 121) | ADHD vs noADHD-ex1 (n = 105) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | χ 2 | p | V | χ 2 | p | V | |
| Chronic | 85 | 93.4 | 21 | 70.0 | 6 | 42.9 | 11.4 | <0.001 | 0.31 | 26.8 | <0.001 | 0.51 |
| Axial2 | 79 | 86.8 | 15 | 50.0 | 3 | 21.4 | 17.6 | <0.001 | 0.38 | 30.3 | <0.001 | 0.54 |
| Widespread3 | 70 | 76.9 | 15 | 50.0 | 3 | 21.4 | 7.8 | 0.005 | 0.25 | 17.6 | <0.001 | 0.41 |
| Early onset4 | 64 | 79.0 | 14 | 70.0 | 2 | 40.0 | 0.7 | 0.389 | 0.09 | 4.0 | 0.045 | 0.22 |
NoADHD = patients not diagnosed with ADHD. 1Excluding those with subthreshold ADHD symptoms from the ‘NoADHD’ group. 2Cervical spine or anterior chest or thoracic spine or low back pain. 3Axial, and both upper and lower part of the body. 4Before or equal to age 20 years (14 missing). χ 2 = Chi square or Fisher’s exact test. V = effect size.
Axial pain was predominantly observed in patients diagnosed with ADHD (86.8%). When excluding those with subthreshold ADHD symptoms from the sample, only 21.4% of the remaining NoADHD group reported axial pain. As Table 2 shows, this exclusion also resulted in a significant increase in differences between the two groups regarding ChP, widespread pain, and early onset of pain.
Logistic regression was performed to assess the impact of the diagnostic categories ADHD, affective disorders, anxiety disorders, and personality disorders, respectively, on experiencing axial pain. This analyses revealed that only ADHD diagnosis predicted axial pain (Table 3).
Effect of diagnostic category on reporting axial pain, binary logistic regression
| Independent variable | Dependent variable | n | Wald (df 1) | Odds ratio (95% confidence interval) | p | Nagelknerke R |
|---|---|---|---|---|---|---|
| ADHD | Axial pain | 121 | 15.49 | 6.58 (2.58–16.83) | <0.001 | 0.188 |
| ADHD-ex1 | Axial pain | 105 | 29.49 | 24.12 (5.87–99.23) | <0.001 | 0.324 |
| Affective disorders | Axial pain | 121 | 0.01 | 0.96 (0.40–2.34) | 0.934 | 0.000 |
| Anxiety disorders | Axial pain | 121 | 0.38 | 1.31 (0.56–3.09) | 0.538 | 0.005 |
| Personality disorders | Axial pain | 121 | 0.72 | 1.77 (0.48–6.55) | 0.385 | 0.010 |
1Excluding those with subthreshold ADHD symptoms from the sample. Axial pain = cervical spine or anterior chest or thoracic spine or low back pain.
4 Discussion
To our knowledge, this is the first study of the relationship between ChP and muscular regulation problems in patients with ADHD, and other psychiatric disorders. Consistent with our first hypothesis, psychiatric patients with ChP had a higher prevalence of ADHD than those without ChP. This is in accordance with the high occurrence of ADHD among ChP patients found in other studies [9,10]. Conversely, a large majority of the ADHD patients in our study suffered from ChP. A study using the same pain assessment as our study, found that 92% of adults with ADHD reported ChP – of whom 80% reported widespread pain, compared to 17.4% in the healthy control group [19]. Similarly, in a study of young women with ADHD, 75.9% reported ChP [18].
In our study, high pain intensity was reported both in the ADHD group and in those with subthreshold ADHD symptoms (but unconfirmed ADHD diagnosis). This is in line with findings of increased risk of experiencing high pain intensity among adults with higher ADHD symptoms [20].
Our findings provide support for our second hypothesis that ChP and intensity of pain in ADHD is associated with muscular dysregulation. ADHD patients with ChP demonstrated significantly more muscular regulation problems than those without ChP, particularly regarding high muscle tone (MFNU-T). Pain intensity was significantly correlated with both the MFNU-TS and the MFNU-T sub-score. The MFNU-I sub-score was not related to ChP. Our findings correspond to the notion that longstanding high muscle tone is thought to cause chronic muscle pain [25]. In our study, the majority of the ADHD patients with ChP reported experiencing pain from an early age. However, it is a matter of speculation whether a high muscle tone was concurrently present with ChP in childhood in these cases. Such an assumption is supported by earlier findings of heightened muscle tone in children with ADHD (84–92% of those within the ADHD group, compared to 0–15% of healthy controls) [30]. Furthermore, several studies have reported an association between ChP and ADHD in youths [16]. Taken together, these findings suggest that both ChP and pain intensity in ADHD are associated with muscular dysregulation, in particular high muscle tone.
Our third hypothesis was also supported. In addition to an early onset of pain, it predicted that ChP in patients diagnosed with ADHD would be characterized by being widespread and axial, qualitatively different from ChP in patients without ADHD. Our results showed that axial pain was predominantly present in patients diagnosed with ADHD (86.8% vs 50% in the NoADHD group). In this context, it is important to note that several of the patients with subthreshold ADHD symptoms in the NoADHD group demonstrated the same characteristics of ChP as the ADHD group, and that only 21.4% of the rest of the NoADHD group reported axial pain. In a study based on the same sample, we found that patients with subthreshold ADHD symptoms demonstrated the same muscular dysregulation as those with ADHD diagnosis (Udal et al., 2024 in preparation).
The present study shows that ADHD diagnosis was a predictor of “Axial pain,” whereas affective-, anxiety-, or personality disorders were not. The axial muscles, which include the muscles of the trunk and head, are involved in postural adjustment and control, which is essential to maintain the inherently unstable upright position of the human body. Thus, these muscles are continuously in use in the activities of daily life and will consequently be substantially affected by muscular dysregulation. This might explain the high muscle tone found in stabilizing muscles as the erector spinae, latissimus dorsi, and iliopsoas among others, which subsequently may lead to axial and widespread pain. Iliopsoas connects the lumbar spines to the lower limbs, and a tightened iliopsoas may exaggerate lumbar lordosis and lumbar pain [40]. This is in line with the association between ADHD symptoms and persistent chronic low back pain reported by Kasahara et al. [10].
The etiologies of ChP and ADHD are complex. Dopamine dysregulation is involved in the neurobiology of both ADHD [41], ChP and pain modulation [28,42]. In addition to the regulatory function of dopamine on behavioral self-regulation [43], the dopamine system also appears to be involved in modulation of skeletal muscle activity and muscle tone, as well as modulation of movements via higher-order motor centers in the brain [29]. Stimulants, which target the dopamine system, are the most effective medications for the treatment of core ADHD symptoms [44]. Stimulants also positively improve the regulation of muscle tone and muscular inhibition [45], and postural stability [46]. It has also been shown that stimulants may reduce ChP in patients with ADHD [9,17,47]. A prospective study showed that individuals with ADHD who had ongoing treatment with stimulants, reported a lower prevalence of chronic widespread pain than those not treated [17]. Treister et al. [24] found that adults with ADHD were more sensitive to pain compared to controls, and that stimulants partially reversed this altered pain response, indicating that MPH exert anti-nociceptive properties in these subjects. Taken together these findings indicate that the positive impact of stimulants on ChP may be a secondary effect of the normalization of muscle tone, and perhaps also a normalizing effect on pain sensitivity.
Because ADHD medication may improve both pain and ADHD symptoms [17,48], it is important to address ADHD in patients with ChP, especially those with an early onset axial pain. This is particularly important in pain clinics, where clinicians may not routinely screen for ADHD. An easily accessible and valid screening tool is the Adult ADHD Self-Report Scale which was developed in cooperation with the World Health Organization [49]. Moreover, the notably high prevalence of ADHD among psychiatric patients with ChP, highlights the importance of assessing pain in these patients. A deeper understanding of ADHD-induced pain, as demonstrated by our study, could enhance the precision of differential diagnosis and in some cases perhaps also prevent misdiagnosis and subsequent unnecessary or harmful treatment.
Longstanding, widespread and intense ChP would be expected to have a detrimental effect on the experienced quality of life [50]. However, one might consider whether the specific axial and widespread pain reported in this study in patients with ADHD also could have beneficial effects on daily activities and functioning in these people. Much of the physical restlessness and over-activity seen in ADHD might be understood as behavioral responses or adaptations to muscle stiffness and pain. By constantly changing positions, individuals with ADHD achieve short-term pain/discomfort relief and potentially enhance their alertness and attention to ongoing activities and tasks. From this viewpoint, stiffness and pain act as potent signals prompting the body to stretch, change position, and, if feasible, engage in movement, thereby promoting improved cognitive and adaptive functioning. In this context, the demands of modern urbanized life, which increasingly call for efficient, concentrated effort while limiting physical activity, are likely unsupportive of individuals with ADHD – or indeed anyone.
5 Limitations
The convenient and small sample size, and the low number of patients without ChP and without an ADHD diagnosis, limits generalizability of our findings. Nonetheless, the notable differences in pain prevalence and pain characteristics between those with and without ADHD, justify further studies with larger and more balance samples.
The high prevalence of patients with ChP and with ADHD in the sample was to be expected, as ChP and ADHD were the main topics of the study, probably attracting participants already experiencing pain and/or ADHD symptoms. Also, a disproportionately high prevalence of ADHD is reported among psychiatric patients in Northern Europe [51] especially within the catchment area of the study [52], which may have further biased our sample. The predominance of women in our sample is consistent with other adult psychiatric outpatient studies [53]. Besides, it is likely that more women than men would attend such studies because females are more likely to experience chronic and widespread pain [54]. Among our participants, however, there were no differences between men and women regarding characteristics of ChP.
One potential limitation of the MFNU is its reliance on subjective evaluation in the scoring procedures. However, the MFNU has been shown to be a reliable test, with high internal consistency and interrater agreement [30]. Efforts were also made to ensure the examiner was blinded to diagnoses as much as possible.
6 Conclusions
Our research indicates a high prevalence of ADHD among psychiatric patients experiencing ChP. Those diagnosed with ADHD often report axial and widespread pain with an early onset – symptoms less common in the other psychiatric conditions studied. Notably, ChP in patients with ADHD was strongly associated with muscular dysregulation, particularly a high muscle tone. These observations underscore the need for further research into the mechanisms linking ADHD with ChP, which could lead to more targeted treatment strategies for these conditions, either separately or in combination.
The notably high prevalence of ADHD among psychiatric patients with ChP, highlights the importance of assessing pain in psychiatric patients, and also to investigate ADHD symptoms in patients with ChP disorders, especially in those with longstanding axial pain.
-
Research ethics: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as amended in 2013), and has been approved by the Regional Ethics Committee and the Data protection controller of Sørlandet Hospital. Registration number and name of registry: The Norwegian Regional Ethics Committee 2014/1231. The study was approved by The Norwegian Data Inspectorate, The National Committee for Medical Research Ethics in Norway.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The dataset generated and analyzed during the current study are available upon request to the corresponding author.
-
Artificial intelligence/Machine learning tools: Not applicable.
References
[1] Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain (London, Engl). 2006;10(4):287–333.10.1016/j.ejpain.2005.06.009Search in Google Scholar PubMed
[2] Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157(1):55–64.10.1097/j.pain.0000000000000314Search in Google Scholar PubMed PubMed Central
[3] Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608.10.1001/jama.2013.13805Search in Google Scholar PubMed PubMed Central
[4] El-Tallawy SN, Nalamasu R, Salem GI, LeQuang JAK, Pergolizzi JV, Christo PJ. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther. 2021;10(1):181–209.10.1007/s40122-021-00235-2Search in Google Scholar PubMed PubMed Central
[5] Workman EA, Hubbard JR, Felker BL. Comorbid psychiatric disorders and predictors of pain management program success in patients with chronic pain. Prim Care Companion J Clin Psychiatry. 2002;4(4):137–40.10.4088/PCC.v04n0404Search in Google Scholar PubMed PubMed Central
[6] APA. Diagnostic and statistical manual of mental disorders: Fifth edition DSM-V. American Psychiatric Association Publishing; 2013.Search in Google Scholar
[7] Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789–818.10.1016/j.neubiorev.2021.01.022Search in Google Scholar PubMed PubMed Central
[8] Ginsberg Y, Quintero J, Anand E, Casillas M, Upadhyaya HP. Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature. Prim Care Companion CNS Disord. 2014;16(3):23591.10.4088/PCC.13r01600Search in Google Scholar PubMed PubMed Central
[9] Kasahara S, Niwa SI, Matsudaira K, Sato N, Oka H, Yamada Y. Attention-deficit/hyperactivity disorder and chronic pain. Psychosom Med. 2020;82(3):346–7.10.1097/PSY.0000000000000789Search in Google Scholar PubMed
[10] Kasahara S, Niwa SI, Matsudaira K, Sato N, Oka H, Fujii T, et al. High attention-deficit/hyperactivity disorder scale scores among patients with persistent chronic nonspecific low back pain. Pain Physician. 2021;24(3):E299–307.10.36076/ppj.2021/24/E299Search in Google Scholar
[11] van Rensburg R, Meyer HP, Hitchcock SA, Schuler CE. Screening for adult ADHD in patients with fibromyalgia syndrome. Pain Med (Malden, MA). 2018;19(9):1825–31.10.1093/pm/pnx275Search in Google Scholar PubMed
[12] Pallanti S, Porta F, Salerno L. Adult attention deficit hyperactivity disorder in patients with fibromyalgia syndrome: assessment and disabilities. J Psychiatr Res. 2021;136:537–42.10.1016/j.jpsychires.2020.10.027Search in Google Scholar PubMed
[13] Derksen MT, Vreeling MJ, Tchetverikov I. High frequency of adult attention deficit hyperactivity disorder among fibromyalgia patients in the Netherlands: should a systematic collaboration between rheumatologists and psychiatrists be sought? Clin Exp Rheumatol. 2015;33(1 Suppl 88):S141.Search in Google Scholar
[14] Yılmaz E, Tamam L. Attention-deficit hyperactivity disorder and impulsivity in female patients with fibromyalgia. Neuropsychiatr Dis Treat. 2018;14:1883–9.10.2147/NDT.S159312Search in Google Scholar PubMed PubMed Central
[15] Turkoglu G. Attention-deficit hyperactivity disorder symptoms and quality of life in female patients with fibromyalgia. Turk J Med Sci. 2021;51(4):1747–55.10.3906/sag-2010-29Search in Google Scholar PubMed PubMed Central
[16] Battison EAJ, Brown PCM, Holley AL, Wilson AC. Associations between chronic pain and attention-deficit hyperactivity disorder (ADHD) in youth: a scoping review. Children (Basel). 2023;10(1):142.10.3390/children10010142Search in Google Scholar PubMed PubMed Central
[17] Asztely K, Kopp S, Gillberg C, Waern M, Bergman S. Chronic pain and health-related quality of life in women with autism and/or ADHD: a prospective longitudinal study. J Pain Res. 2019;12:2925–32.10.2147/JPR.S212422Search in Google Scholar PubMed PubMed Central
[18] Mundal I, Schei J, Lydersen S, Thomsen PH, Nøvik TS, Kvitland LR. Prevalence of chronic and multisite pain in adolescents and young adults with ADHD: a comparative study between clinical and general population samples (the HUNT study). Eur Child Adolesc Psychiatry. 2023;33(5):1433–42.10.1007/s00787-023-02249-xSearch in Google Scholar PubMed PubMed Central
[19] Stray LL, Kristensen O, Lomeland M, Skorstad M, Stray T, Tonnessen FE. Motor regulation problems and pain in adults diagnosed with ADHD. Behav Brain Funct. 2013;9(1):18.10.1186/1744-9081-9-18Search in Google Scholar PubMed PubMed Central
[20] Stickley A, Koyanagi A, Takahashi H, Kamio Y. ADHD symptoms and pain among adults in England. Psychiatry Res. 2016;246:326–31.10.1016/j.psychres.2016.10.004Search in Google Scholar PubMed
[21] Johnston KJA, Huckins LM. Chronic pain and psychiatric conditions. Complex Psychiatry. 2023;9(1–4):24–43.10.1159/000527041Search in Google Scholar PubMed PubMed Central
[22] Bou Khalil R, Khoury E, Richa S. The comorbidity of fibromyalgia syndrome and attention deficit and hyperactivity disorder from a pathogenic perspective. Pain Med (Malden, MA). 2018;19(9):1705–9.10.1093/pm/pny142Search in Google Scholar PubMed
[23] Kerekes N, Sanchez-Perez AM, Landry M. Neuroinflammation as a possible link between attention-deficit/hyperactivity disorder (ADHD) and pain. Med Hypotheses. 2021;157:110717.10.1016/j.mehy.2021.110717Search in Google Scholar PubMed
[24] Treister R, Eisenberg E, Demeter N, Pud D. Alterations in pain response are partially reversed by methylphenidate (Ritalin) in adults with attention deficit hyperactivity disorder (ADHD). Pain Practice: Off J World Inst Pain. 2015;15(1):4–11.10.1111/papr.12129Search in Google Scholar PubMed
[25] Mense S, Masi AT. Increased muscle tone as a cause of muscle pain. In: Mense S, Gerwin RD, editors. Muscle pain: understanding the mechanisms. Berlin, Heidelberg: Springer; 2010. p. 207–49.10.1007/978-3-540-85021-2_6Search in Google Scholar
[26] Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol. 2019;39(1):31–59.10.1007/s10571-018-0632-3Search in Google Scholar PubMed
[27] Wood PB, Holman AJ. An elephant among us: the role of dopamine in the pathophysiology of fibromyalgia. J Rheumatol. 2009;36(2):221–4.10.3899/jrheum.080583Search in Google Scholar PubMed
[28] Li C, Liu S, Lu X, Tao F. Role of descending dopaminergic pathways in pain modulation. Curr Neuropharmacol. 2019;17(12):1176–82.10.2174/1570159X17666190430102531Search in Google Scholar PubMed PubMed Central
[29] Schwarz PB, Peever JH. Dopamine triggers skeletal muscle tone by activating D1-like receptors on somatic motoneurons. J Neurophysiol. 2011;106(3):1299–309.10.1152/jn.00230.2011Search in Google Scholar PubMed
[30] Stray LL, Stray T, Iversen S, Ruud A, Ellertsen B, Tonnessen FE. The motor function neurological assessment (MFNU) as an indicator of motor function problems in boys with ADHD. Behav Brain Funct: BBF. 2009;5:22.10.1186/1744-9081-5-22Search in Google Scholar PubMed PubMed Central
[31] Kvåle A, Ellertsen B, Skouen JS. Relationships between physical findings (GPE-78) and psychological profiles (MMPI-2) in patients with long-lasting musculoskeletal pain. Nordic J Psychiatry. 2001;55(3):177–84.10.1080/08039480152036056Search in Google Scholar PubMed
[32] Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain: Off J Am Pain Soc. 2003;4(7):407–14.10.1016/S1526-5900(03)00716-8Search in Google Scholar
[33] Wolfe F, Hauser W. Fibromyalgia diagnosis and diagnostic criteria. Ann Med. 2011;43(7):495–502.10.3109/07853890.2011.595734Search in Google Scholar PubMed
[34] Stray LL, Stray T, Kristensen O. Neuromuscular regulation problems in attention deficit hyperactivity disorder assessed by the motor function neurological assessment. J Nov Physiother. 2015;255(5):2.Search in Google Scholar
[35] Stray LL, Iversen S, Stray T, Ellertsen B, Ruud A. Motor function neurological examination user manual. Stavanger, Norway: University of Stavanger; 2006.10.1037/t38642-000Search in Google Scholar
[36] Palma-Álvarez RF, Barta C, Carpentier PJ, Carruthers S, Crunelle CL, Demetrovics Z, et al. Validity of the ADHD module of the mini international neuropsychiatric interview PLUS for screening of adult ADHD in treatment seeking substance use disorder patients: ADHD screening with MINI-Plus. Rev Psiquiatr Salud Ment (Barc.). 2020.Search in Google Scholar
[37] Leiknes KA, Leganger S, Malt EA, Malt U. Mini plus. Oslo: Psykosomatisk avdeling, Rikshospitalet; 1999.Search in Google Scholar
[38] First MB, Gibbon M. User’s guide for the structured clinical interview for DSM-IV axis II personality disorders: SCID-II. Washington DC: American Psychiatric Association; 1997.Search in Google Scholar
[39] Kooij JJS, Francken MH. Diagnostisch Interview voor ADHD-2 (DIVA): Kenniscentrum ADHD bij volwassenen PsyQ; 2009. http://www.psyq.nl/Programma/Kenniscentrum-ADHD-bij-volwassenen.Search in Google Scholar
[40] Lakkadsha TM, Qureshi MI, Kovela RK, Saifee SS, Lalwani SS. Efficacy of single stretching session of iliopsoas using proprioceptive neuromuscular facilitation versus muscle energy technique on low back pain in patients with lumbar hyper-lordosis. Cureus. 2022;14(8):e27916.10.7759/cureus.27916Search in Google Scholar PubMed PubMed Central
[41] Curatolo P, D’Agati E, Moavero R. The neurobiological basis of ADHD. Ital J Pediatr. 2010;36(1):79.10.1186/1824-7288-36-79Search in Google Scholar PubMed PubMed Central
[42] Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153(4):744–54.10.1016/j.pain.2012.01.002Search in Google Scholar PubMed PubMed Central
[43] Posner MI, Rothbart MK. Toward a physical basis of attention and self regulation. Phys Life Rev. 2009;6(2):103–20.10.1016/j.plrev.2009.02.001Search in Google Scholar PubMed PubMed Central
[44] Golmirzaei J, Mahboobi H, Yazdanparast M, Mushtaq G, Kamal MA, Hamzei E. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590–4.10.2174/1381612822666151124235816Search in Google Scholar PubMed
[45] Stray LL, Stray T, Iversen S, Ellertsen B, Ruud A. Methylphenidate improves motor functions in children diagnosed with hyperkinetic disorder. Behav Brain Funct. 2009;5:1–12.10.1186/1744-9081-5-21Search in Google Scholar PubMed PubMed Central
[46] Jacobi-Polishook T, Shorer Z, Melzer I. The effect of methylphenidate on postural stability under single and dual task conditions in children with attention deficit hyperactivity disorder – a double blind randomized control trial. J Neurol Sci. 2009;280(1–2):15–21.10.1016/j.jns.2009.01.007Search in Google Scholar PubMed
[47] Zain E, Sugimoto A, Egawa J, Someya T. Case report: methylphenidate improved chronic pain in an adult patient with attention deficit hyperactivity disorder. Front Psychiatry. 2023;14:1091399.10.3389/fpsyt.2023.1091399Search in Google Scholar PubMed PubMed Central
[48] Kasahara S, Kato Y, Takahashi M, Matsudaira K, Sato N, Niwa SI, et al. Case report: remission of chronic low back pain and oral dysesthesia comorbid with attention deficit/hyperactivity disorder by treatment with atomoxetine and pramipexole. Front Pain Res (Lausanne). 2023;4:1159134.10.3389/fpain.2023.1159134Search in Google Scholar PubMed PubMed Central
[49] Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35(2):245–56.10.1017/S0033291704002892Search in Google Scholar
[50] Mangerud WL, Bjerkeset O, Lydersen S, Indredavik MS. Chronic pain and pain-related disability across psychiatric disorders in a clinical adolescent sample. BMC Psychiatry. 2013;13:272.10.1186/1471-244X-13-272Search in Google Scholar PubMed PubMed Central
[51] Deberdt W, Thome J, Lebrec J, Kraemer S, Fregenal I, Ramos-Quiroga JA, et al. Prevalence of ADHD in nonpsychotic adult psychiatric care (ADPSYC): a multinational cross-sectional study in Europe. BMC Psychiatry. 2015;15:242.10.1186/s12888-015-0624-5Search in Google Scholar PubMed PubMed Central
[52] Suren P, Bakken IJ, Lie KK, Schjolberg S, Aase H, Reichborn-Kjennerud T, et al. Differences across counties in the registered prevalence of autism, ADHD, epilepsy and cerebral palsy in Norway. Tidsskr Laegeforen. 2013;133(18):1929–34.Search in Google Scholar
[53] Almeida Montes LG, Hernandez Garcia AO, Ricardo-Garcell J. ADHD prevalence in adult outpatients with nonpsychotic psychiatric illnesses. J Atten Disord. 2007;11(2):150–6.10.1177/1087054707304428Search in Google Scholar PubMed
[54] Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–83.10.1016/j.bja.2019.03.023Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)


