Abstract
Objective:
The aim of this study was to assess the associations between the characteristics of United States (US) adults (≥50 years) who used opioids and self-reported pain severity using a nationally representative dataset.
Methods:
This retrospective cross-sectional database study used 2019 Medical Expenditure Panel Survey data to identify US adults aged ≥50 years with self-reported pain within the past 4 weeks and ≥1 opioid prescription within the calendar year (n = 1,077). Weighted multivariable logistic regression analysis modeled associations between various characteristics and self-reported pain severity (quite a bit/extreme vs less/moderate pain).
Results:
The adjusted logistic regression model indicated that greater odds of reporting quite a bit/extreme pain was associated with the following: age 50–64 vs ≥65 (adjusted odds ratio [AOR] = 1.76; 95% confidence interval [CI] = 1.22–2.54), non-Hispanic vs Hispanic (AOR = 2.0; CI = 1.18–3.39), unemployed vs employed (AOR = 2.01; CI = 1.33–3.05), no health insurance vs private insurance (AOR = 6.80; CI = 1.43–32.26), fair/poor vs excellent/very good/good health (AOR = 3.10; CI = 2.19–4.39), fair/poor vs excellent/very good/good mental health (AOR = 2.16; CI = 1.39–3.38), non-smoker vs smoker (AOR = 1.80; CI = 1.19–2.71), and instrumental activity of daily living, yes vs no (AOR = 2.27; CI = 1.30–3.96).
Conclusion:
Understanding the several characteristics associated with pain severity in US adults ≥50 years who used an opioid may help transform healthcare approaches to prevention, education, and management of pain severity in later life.
1 Introduction
Pain is physiologically a tool for protection, but when its adaptive function is lost, it can develop into a pathologic state that negatively influences the quality of life [1]. The revised definition of pain by the International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [2]. Mechanisms underlying pain have a neural basis; however, being aware of pain is a perception that is subjective and cannot be measured directly and objectively [3].
Findings from the National Health Institute Survey in 2019 estimated that 20.4% of United States (US) adults experienced chronic pain and 7.4% of US adults experienced high-impact pain (pain interfering with life or work activities). This study also noted that pain increases with age and peaks in prevalence among adults age ≥65 years [4]. In 2008, the national cost of pain in the US was estimated to range from $560 to $635 billion and this was more than the annual cost of diabetes ($188 billion), cancer ($243 billion), and heart disease ($309 billion) [5]. Over time, it is conceivable that the financial burden of pain will increase.
Physical and behavioral therapies, opioids, and non-opioid analgesics are some of the treatment options for managing pain [6,7,8,9]. Opioids are important for the management of severe, transient pain during acute painful events and near the end of life [10]. However, the use of opioids in pain management is debatable owing to the potential harm associated with the long-term use of opioids such as high opioid misuse rates, addiction unacceptable fatality rates, and a significant burden on the societies affected [10]. Existing studies have also reported that a high risk of falls or fractures in older adults with osteoarthritis is associated with increased use of opioids [11,12]. Using data from Medicare and Medicaid Services, there has been an estimated greater than 3-fold increase in opioid use disorder among older US adults between 2013 and 2018 and pain has been identified as a risk factor for problematic opioid use [13,14].
Given the challenges associated with opioid use and pain management, it is pertinent to explore and understand the characteristics of the older population with pain who use opioids. Effective pain management requires a comprehensive assessment of pain [15]. In addition to assessing pain characteristics (pain intensity, frequency, severity, and duration) in the older population, individual characteristics may also be considered for a holistic approach. In previous studies, emphasis has been on pain characteristics and their effect on the quality of life and disability in the aging populace [16,17,18]. However, these pain characteristics in older adults are influenced by several factors, and understanding the individual characteristics can point to potential predictors of pain severity, frequency, and duration. It can also provide critical insight into traits that may be harnessed to enhance pain management techniques and inform individualized approaches or strategies for managing pain. The aim of this study is to identify individual characteristics that are linked to pain severity in older US adults ≥50 years who used opioids (Figure 1).
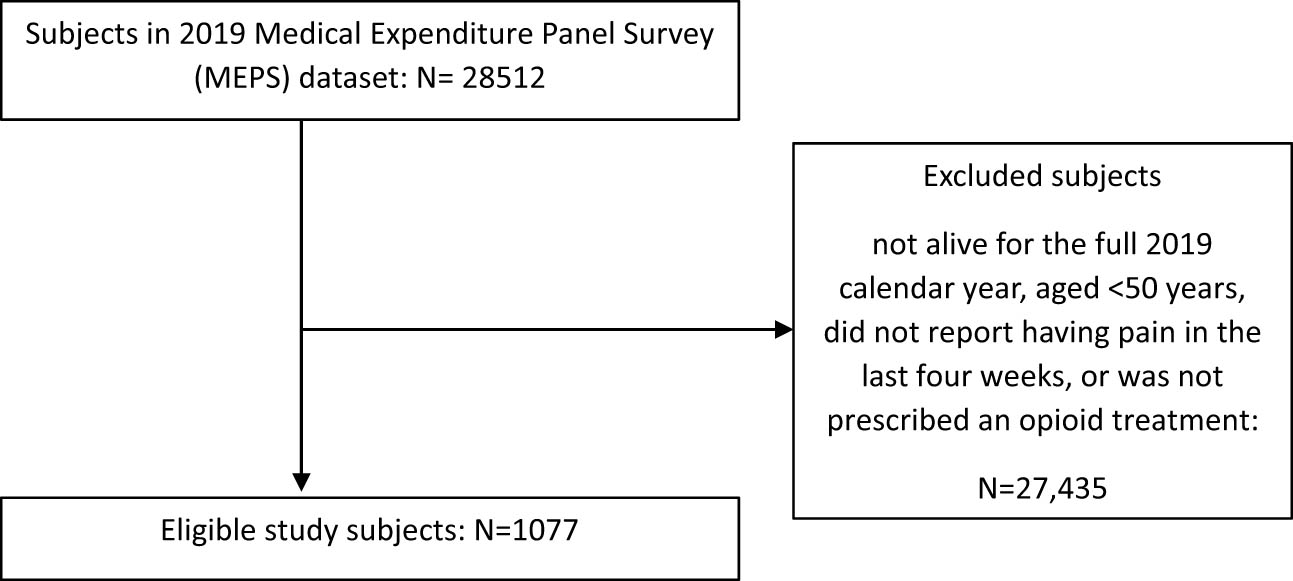
Study subject eligibility flowchart.
2 Methods
The Medical Expenditure Panel Survey (MEPS) data source consists of a set of large-scale surveys of families and individuals, their medical providers (doctors, hospitals, pharmacies, etc.), and employers across the US and it is conducted by the Agency for Healthcare Research and Quality [19]. The 2019 full-year consolidated data file consists of MEPS data obtained from Panel 23 (rounds 3–5) and Panel 24 (rounds 1–3). This file contains variables pertaining to survey administration, demographics, income, person-level conditions, health status, disability days, quality of care, employment, health insurance, and person-level medical care use and expenditures [20]. The 2019 prescribed medicine file provides detailed information on household-reported prescribed medicines for a nationally representative sample of the civilian noninstitutionalized population of the US [21].
Eligible participants for this retrospective cross-sectional study were US adults who were alive for the full calendar year, aged ≥50 years, with self-reported pain within the past 4 weeks, and ≥1 opioid prescription within the calendar year. The dependent variable (pain) was assessed based on the participant’s response (of a little bit/moderately; quite a bit/extremely) to the question, “During the past 4 weeks, pain interfered with normal work outside the home and housework” [22]. Therapeutic class codes 60 (narcotic analgesics) and 191 (narcotic analgesics combination) from the MEPS 2019 prescribed medicines file were used to identify participants who had ≥1 opioid prescription in 2019 [23].
The Behavioral Model of Health Services Use was used to group the independent variables [24]. These variables included:
Predisposing variables: age (50–64, ≥65 years); gender (male, female); race (white, other); ethnicity (Hispanic, non-Hispanic).
Enabling variables: education status (high school or less, more than high school); employment status (employed, unemployed); insurance status (private, public, none); marital status (married, other); and income level (poor/near poor/low income, middle/high income).
Need variables: activities of daily living (ADL) (ADL, no ADL); instrumental activity of daily living (IADL) (IADL, no IADL); chronic conditions (<2 vs ≥2); health status (excellent/very good/good vs fair/poor perceived health); and mental health status (excellent/very good/good vs fair/poor perceived mental health).
Personal health practice variables: regular exercise (yes, no) and smoker (yes, no).
External environmental variables: region (Northeast; Midwest; South; and West).
Chi-squared tests were used to compare the demographic characteristics of subjects stratified by their self-reported pain severity (little/moderate vs quite a bit/extreme). Multivariable logistic regression models were used to assess statistically significant associations between various characteristics and quite a bit/extreme self-reported pain severity, with little/moderate self-reported pain severity as the reference group. The odds ratio and 95% confidence limits for each variable were reported. The a priori alpha level was 0.05. The Taylor series linearization method was used to compute the variance of MEPS estimates and analyses were weighted to obtain nationally representative estimates. The cluster and strata analyses were used to maintain the effect of the sampling design on the MEPS estimates of variance and sampling errors while a domain analysis was done to differentiate the eligible population from the ineligible population. Analyses were conducted using SAS (v9.4, SAS institute Inc., Cary, NC, USA).
3 Results
The 2019 MEPS dataset contained data for 28,512 persons. There were 1,077 subjects who met the eligibility criteria of the study (low/moderate pain = 556, quite a bit/extreme pain = 521). The weighted population was 12,109,702 (low/moderate pain = 54.8% [95% confidence Interval (CI) = 51.2–58.4%], quite a bit/extreme pain = 45.2% [95% CI = 41.6–48.8%]).
Participants for both stratified levels of pain (low/moderate pain, quite a bit/extreme pain) most commonly had the following characteristics: ≥65 years of age, female, white, non-Hispanic, more than high school education completed, unemployed, private insurance, married, middle/high income, had no limitations (ADL or IADL), ≥2 chronic conditions, excellent/very good/good perceived health status, excellent/very good/good perceived mental health status, no regular exercise, and non-smokers. The most common region to reside in was the South region. The following variables had statistically significant differences (p < 0.05) between the groups: sex, race, education completed, employment status, insurance status, marital status, income level, ADL limitation, IADL limitation, perceived health status, perceived mental health status, and exercise status. For further details see Table 1.
Characteristics of US older adults (age ≥ 50 years) with self-reported pain in the past 4 weeks who used opioids stratified by self-reported pain severity (little/moderate vs quite a bit/extreme) in the 2019 MEPS (weighted N = 12,109,702)
| Variables | Little/moderate pain (weighted N = 6,640,276) Weighted% (95% CI) | Quite a bit/extreme pain (weighted N = 5,469,426) Weighted% (95% CI) | p |
|---|---|---|---|
| Age (years) | 0.0576 | ||
| 50–64 | 44.7 (39.7, 49.8) | 60.0 (46.5, 57.4) | |
| ≥ 65 | 55.3 (50.2, 60.3) | 48.0 (42.6, 53.5) | |
| Sex | 0.0458 | ||
| Male | 42.5 (38.0, 47.0) | 36.1 (31.9, 40.4) | |
| Female | 57.5 (53.0, 61.9) | 63.9 (59.6, 68.1) | |
| Race | 0.0331 | ||
| White | 85.2 (81.5, 89.0) | 79.5 (74.9, 84.1) | |
| Other | 14.8 (11.0, 18.5) | 20.5 (15.9, 25.1) | |
| Ethnicity | 0.8747 | ||
| Hispanic | 7.4 (5.0, 9.8) | 7.2 (4.7, 9.7) | |
| Non-Hispanic | 92.6 (90.2, 95.0) | 92.8 (90.3, 95.3) | |
| Education completed | 0.0001 | ||
| High school or less | 42.4 (37.6, 47.2) | 54.7 (49.9, 59.4) | |
| More than high school | 57.6 (52.8, 62.4) | 45.3 (40.6, 50.1) | |
| Employment status | <0.0001 | ||
| Employed | 41.0 (36.3, 45.6) | 19.5 (15.3, 23.6) | |
| Unemployed | 59.0 (54.4, 63.7) | 80.5 (76.4, 84.7) | |
| Insurance status | <0.0001 | ||
| Private | 63.2 (58.8, 67.6) | 38.9 (34.2, 43.6) | |
| Public | 36.3 (31.9, 40.6) | 58.7 (54.1, 63.3) | |
| None | 0.5 (0, 1.1) | 2.4 (0.1, 4.7) | |
| Marital status | 0.0282 | ||
| Married | 58.1 (53.0, 63.2) | 49.5 (43.8, 55.2) | |
| Other | 41.9 (36.8, 47.0) | 50.5 (44.8, 56.2) | |
| Income level | <0.0001 | ||
| Poor/near poor/low income | 29.4 (25.2, 33.5) | 43.7 (38.5, 48.9) | |
| Middle/high income | 70.6 (66.5, 74.8) | 56.3 (51.1, 61.5) | |
| ADL limitation | <0.0001 | ||
| Yes | 2.9 (1.3, 4.5) | 15.1 (11.4, 18.8) | |
| No | 97.1 (95.5, 98.7) | 84.9 (81.2, 88.6) | |
| IADL limitation | <0.0001 | ||
| Yes | 5.6 (3.5, 7.7) | 23.9 (19.7, 28.2) | |
| No | 94.4 (92.3, 96.5) | 76.1 (71.8, 80.3) | |
| Chronic conditions | 0.2366 | ||
| <2 | 13.2 (10.0, 16.4) | 10.3 (7.0, 13.7) | |
| ≥2 | 86.8 (83.6, 90.0) | 89.7 (86.3, 93.0) | |
| Perceived health status | <0.0001 | ||
| Excellent/very good/good | 77.2 (73.4, 80.9) | 40.8 (36.5, 45.2) | |
| Fair/poor | 22.8 (19.1, 26.6) | 59.2 (54.8, 63.5) | |
| Perceived mental health status | <0.0001 | ||
| Excellent/very good/good | 90.4 (87.8, 93.0) | 67.1 (62.5, 71.7) | |
| Fair/poor | 9.6 (7.0, 12.2) | 32.9 (28.3, 37.5) | |
| Exercise | <0.0001 | ||
| Yes | 45.6 (40.4, 50.9) | 28.4 (23.6, 33.2) | |
| No | 54.4 (49.1, 59.6) | 71.6 (66.8, 76.4) | |
| Smoker | 0.7601 | ||
| Yes | 23.3 (19.4, 27.2) | 24.2 (20.4, 27.9) | |
| No | 76.7 (72.8, 80.6) | 75.8 (72.1, 79.6) | |
| Region | 0.1198 | ||
| Northeast | 12.5 (8.4, 16.5) | 13.0 (7.3, 18.7) | |
| Midwest | 24.7 (20.3, 29.1) | 23.9 (19.6, 28.3) | |
| South | 36.4 (31.3, 41.5) | 43.4 (37.4, 49.4) | |
| West | 26.4 (21.2, 31.5) | 19.6 (15.4, 23.9) |
Analysis based on an unweighted sample n = 1,077 (little/moderate pain n = 556; quite a bit/extreme pain n = 521) of US adults alive during the calendar year 2019, age ≥ 50 years, with self-reported pain in the past 4 weeks who used at least one opioid. Statistically significant differences between groups based on chi-square tests. ADL = activities of daily living. IADL = instrumental activities of daily living.
In the fully adjusted logistic regression model, higher odds of reporting quite a bit/extreme pain were associated with the following: age (years) 50–64 vs ≥ 65 (adjusted odds ratio [AOR] = 1.76; 95% CI = 1.22–2.54) and IADL limitation, yes vs no (AOR = 2.27; 95% CI = 1.30–3.96). Lower odds of reporting quite a bit/extreme pain were associated with the following: Hispanic vs non-Hispanic (AOR = 0.50; 95% CI = 0.30–0.85), employed vs unemployed (AOR = 0.50; 95% CI = 0.33–0.75); private insurance vs no health insurance (AOR = 0.15; 95% CI = 0.03–0.70), excellent/very good/good health vs fair/poor (AOR = 0.32; 95% CI = 0.23–0.46), excellent/very good/good mental health vs fair/poor (AOR = 0.46; 95% CI = 0.30–0.72), and smoker vs non-smoker (AOR = 0.56; 95% CI = 0.37–0.84). For further details see Table 2.
Characteristics associated with quite a bit/extreme pain (vs little/moderate pain) among US older adults (age ≥50 years) with self-reported pain in the past 4 weeks who used opioids in the 2019 MEPS
| Factors | Model 1 AOR (95% confidence limits) | Model 2 AOR (95% confidence limits) | Model 3 AOR (95% confidence limits) | Model 4 AOR (95% confidence limits) | Model 5 AOR (95% confidence limits) |
|---|---|---|---|---|---|
| Age 50–64 vs ≥ 65 years | 1.34 (0.99–1.81) | 1.94 (1.41–2.69) | 1.61 (1.14–2.27) | 1.75 (1.22–2.52) | 1.76 (1.22–2.54) |
| Male vs female sex | 0.75 (0.58–0.98) | 0.83 (0.63–1.11) | 0.76 (0.55–1.05) | 0.81 (0.58–1.12) | 0.81 (0.58–1.12) |
| White vs other race | 0.69 (0.48–1.00) | 0.90 (0.59–1.35) | 0.91 (0.57–1.44) | 0.87 (0.55–1.39) | 0.88 (0.54–1.43) |
| Hispanic vs non-Hispanic ethnicity | 0.96 (0.59–1.54) | 0.68 (0.42–1.12) | 0.52 (0.31–0.87) | 0.48 (0.29–0.82) | 0.50 (0.30–0.85) |
| High school or less vs higher than high school education | 1.13 (0.85–1.50) | 1.04 (0.77–1.39) | 1.10 (0.83–1.47) | 1.07 (0.79–1.44) | |
| Employed vs unemployed | 0.36 (0.25–0.52) | 0.51 (0.34–0.76) | 0.49 (0.32–0.74) | 0.50 (0.33–0.75) | |
| Private vs no health insurance | 0.12 (0.02–0.83) | 0.14 (0.03–0.71) | 0.15 (0.03–0.71) | 0.15 (0.03–0.70) | |
| Public vs no health insurance | 0.25 (0.04–1.59) | 0.25 (0.05–1.22) | 0.26 (0.06–1.21) | 0.26 (0.06–1.20) | |
| Married vs other marital status | 0.89 (0.65–1.23) | 1.06 (0.74–1.52) | 1.00 (0.70–1.44) | 0.99 (0.69–1.42) | |
| Poor/near poor/low-income vs middle/high income | 1.03 (0.74–1.44) | 0.92 (0.65–1.32) | 0.94 (0.65–1.34) | 0.93 (0.65–1.32) | |
| ADL limitation vs no ADL limitation | 1.71 (0.77, 3.78) | 1.64 (0.77–3.49) | 1.70 (0.79–3.66) | ||
| IADL limitation vs no IADL limitation | 2.37 (1.36–4.15) | 2.32 (1.34–4.00) | 2.27 (1.30–3.96) | ||
| < 2 vs ≥2 chronic conditions | 0.96 (0.57–1.63) | 1.03 (0.62–1.72) | 1.04 (0.62–1.73) | ||
| Excellent/very good/good vs fair/poor perceived health | 0.32 (0.23–0.44) | 0.32 (0.23–0.46) | 0.32 (0.23–0.46) | ||
| Excellent/very good/good vs fair/poor perceived mental health | 0.49 (0.32–0.76) | 0.47 (0.30–0.73) | 0.46 (0.30–0.72) | ||
| Exercise vs no exercise | 0.75 (0.52–1.08) | 0.75 (0.52–1.08) | |||
| Smoker yes vs no | 0.57 (0.38–0.85) | 0.56 (0.37–0.84) | |||
| Northeast vs West region | 1.21 (0.70–2.08) | ||||
| Midwest vs West region | 1.40 (0.93–2.12) | ||||
| South vs West region | 1.33 (0.89–1.97) |
Analysis based on an unweighted sample n = 1,077 (little/moderate pain n = 556; quite a bit/extreme pain n = 521) of US adults alive during the calendar year 2019, age ≥ 50 years, with self-reported pain in the past 4 weeks who used at least one opioid. The reference group in the binomial logistic regression models was little/moderate pain. Model 1 had a c-statistic of 0.559. Model 2 had a c-statistic of 0.693. Model 3 had a c-statistic of 0.774. Model 4 had a c-statistic of 0.778. Model 5 had a c-statistic of 0.778. Bold indicates the characteristic has a significant association with pain severity. ADL = activities of daily living. IADL = instrumental activities of daily living.
Model 1 included predisposing variables (age, sex, race, ethnicity).
Model 2 included predisposing and enabling variables (employment status, education status, health insurance status, marital status, income status).
Model 3 included predisposing, enabling, and need variables (ADL limitation, IADL limitation, chronic conditions, health status, mental health status).
Model 4 included predisposing, enabling, need, and personal health practices variables (exercise, smoker).
Model 5 included predisposing, enabling, need, personal health practices, and external environmental variables (region).
4 Discussion
This study identified several characteristics associated with self-reported pain severity in US adults ≥50 years who have used an opioid. Age (50–64 years vs >65 years) and ethnicity (Hispanic vs non-Hispanic) were the predisposing variables that had the greatest association with quite a bit/extreme pain severity. Previous work by Dahlhamer et al. found that compared to other age groups, individuals aged 45–64 had a greater prevalence of both chronic pain and high-impact pain [25]. A possible explanation for this finding was that older people (over 65 years) are more likely to be retired and may be able to avoid activities that could cause pain at work or may be able to handle their health and pain better because they have more time [26]. Additionally, it has been reported that non-Hispanic white adults have a substantially greater age-adjusted prevalence of persistent pain than any other racial and ethnic subgroups [25]. This may explain why the proportion of Hispanic adults in the current study was lower than the proportion in the general population. It may also be the case that Hispanic adults use the healthcare system less (either due to lack of choice, or lack of access or health insurance).
Among the enabling variables, employment and health insurance status were the characteristics significantly associated with self-reported pain severity. Individuals who were employed had lower odds of reporting quite a bit/extreme pain, compared to those who were unemployed. The presence of chronic pain may result in functional limitations and disabilities that can adversely affect an individual’s ability to secure employment. This suggestion is supported by previous findings that the risk of developing limitations in activities or participatory restrictions, such as being unable to work for a livelihood, is highly correlated with pain [27]. Individuals with private health insurance had lower odds of reporting quite a bit/extreme pain, than those who had no insurance. It is not surprising that unemployment and lack of health insurance coverage are associated with increased pain severity. Low earnings and lack of insurance further restrict the ability to get medical care and may relegate people to less costly treatments that may be less suitable [28].
Among the need variables, IADL limitation, perceived health status, and perceived mental health status showed significant association with reporting quite a bit/extreme pain. These findings are similar to those of previous studies, which found that greater levels of self-reported pain severity were associated with greater odds of reporting a functional limitation [29]. Another study also found that functional limitations that are typically linked with aging appear in subjects with severe pain much earlier in life [30]. In another study, pain was negatively associated with health-related quality of life, and physical and mental health was found to have an inverse relationship with pain intensity [31]. Several studies have also found that mental health disorders such as depression are associated with pain. For example, a study on older adults in Germany found that the factors that best predicted depression in later life were multisite pain, frequency, and severity of pain [32,33,34,35].
Smoking status was the only personal health practice variable to be associated with self-reported pain severity. Smokers had a lower likelihood of reporting quite a bit/extreme pain compared to non-smokers. The association between pain and smoking status can be influenced by various factors. For example, in a previous study, after adjusting for depression in the multivariate linear regression analyses, smoking status was not associated with baseline pain severity [36]. In contrast to our findings, when pain intensity between smokers and non-smokers was compared, it was discovered that smokers reported considerably higher pain levels than non-smokers at the time of consultation [37]. This difference in findings may be due to the complex and bi-directional relationship between smoking and pain [37,38].
Previous research using adjusted logistic regression analysis in a similar cohort of US adults aged ≥50 years with pain who used opioids has recently been conducted. In one study, those aged 60–69 years (vs ≥80 years), those who reported they were in excellent/very good/good (vs fair/poor) health, those who reported they were normal/underweight and overweight (vs obese), and those who reported they had little (vs extreme) pain had higher odds of reporting doing frequent exercise [39]. In another study, those aged 50–64 (vs ≥65 years), Hispanic (vs non-Hispanic) ethnicity, employed (vs unemployed), and frequent exercise (vs none) were associated with lower odds of multimorbidity [40]. In a further study, those with extreme (vs little) and quite a bit of (vs little) pain, males (vs females), white race (vs not white race), high school or less (vs more than high school) education, and current smoker (vs not a current smoker) were associated with lower odds of reporting good health. Being employed (vs unemployed), having <2 chronic conditions (vs ≥2), and doing regular physical activity (vs not doing so) were associated with higher odds of reporting good health [41]. In one final study, having extreme, quite a bit, and moderate (vs little) pain, being unemployed (vs employed), unmarried (vs married), having poor (vs good) overall health, and residing in the Midwest (vs West) were all associated with greater odds of having any limitation [42]. Several of the characteristics observed in these studies were also observed in the current study. These findings demonstrate that there are some characteristics that are frequently associated with outcomes of interest among the older US population with pain who used opioids while others appear to depend on the outcome being studied.
The strengths of this study include the use of a nationally representative database of the US population which consists of different racial and ethnic minorities, and this makes the results more generalizable. That said, the proportion of white people in this study cohort was higher than the general population. Further research with a sample consisting of more diverse race and ethnicities is warranted to further investigate the effect of race. This study involved adults who were opioid users and MEPS provides data on prescribed medicine use and this facilitates research on prescribed medicine utilization. Despite these strengths of using MEPS, there are also potential biases and limitations; bias may arise from the MEPS response rate, recall bias may exist because MEPS data are self-reported and since the study design is cross-sectional, causal inference cannot be made.
The various characteristics associated with self-reported pain severity in this study provide critical insight that may be useful in developing and implementing targeted and innovative individualized pain management strategies in older adults. Future research may be geared toward integrating characteristics peculiar to an individual in the process of pain evaluation and in selecting interventions to manage pain, as it may impact response to treatment positively. Additionally, future studies can explore the challenges associated with integrated and comprehensive pain management programs in older adults.
Acknowledgements
None.
-
Research ethics: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as amended in 2013) and has been approved by the authors Institutional Review Board (IRB protocol 00002146).
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state that there is no conflict of interest.
-
Research funding: Dr. Axon reports grant funding from American Association of Colleges of Pharmacy, Arizona Department of Health, Merck & Co., National Council for Prescription Drug Programs, Pharmacy Quality Alliance, and Tabula Rasa HealthCare Group, outside of this study.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
[1] Raffaeli W, Arnaudo E. Pain as a disease: an overview. J Pain Res. 2017;10:2003–8. 10.2147/JPR.S138864.Search in Google Scholar PubMed PubMed Central
[2] Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–82. 10.1097/j.pain.0000000000001939.Search in Google Scholar PubMed PubMed Central
[3] Institute of Medicine (US). Committee on pain, disability, and chronic illness behavior. In: Sterweis M, Kleinman A, Mechanic D, editors. Pain and disability: Clinical, behavioral, and public policy perspectives. Washington (DC): National Academies Press; 1987. 10.17226/991.Search in Google Scholar PubMed
[4] Zelaya CE, Dahlhamer JM, Lucas JW, Connor EM. Chronic Pain and High-impact Chronic Pain Among U.S. Adults, 2019. Vol. 390, NCHS Data Brief.; 2020. p. 1–8.Search in Google Scholar
[5] Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–24. 10.1016/j.jpain.2012.03.009.Search in Google Scholar PubMed
[6] Axon DR, Patel MJ, Martin JR, Slack MK. Use of multidomain management strategies by community dwelling adults with chronic pain: evidence from a systematic review. Scand J Pain. 2019;19(1):9–23. 10.1515/sjpain-2018-0306.Search in Google Scholar PubMed
[7] Axon DR, Bhattacharjee S, Warholak TL, Slack MK. Xm(2) Scores for Estimating Total Exposure to Multimodal Strategies Identified by Pharmacists for Managing Pain: Validity Testing and Clinical Relevance. Pain Res Manag. 2018;2018:2530286. 10.1155/2018/2530286.Search in Google Scholar PubMed PubMed Central
[8] Karadag Arli S, Bakan AB, Varol E, Aslan G. Investigation of pain and life satisfaction in older adults. Geriatr Gerontol Int. 2018;18(1):5–11. 10.1111/ggi.13125v.13125.Search in Google Scholar
[9] Lints-Martindale AC, Hadjistavropoulos T, Lix LM, Thorpe L. A comparative investigation of observational pain assessment tools for older adults with dementia. Clin J Pain. 2012;28(3):226–37. 10.1097/AJP.0b013e3182290d90.Search in Google Scholar PubMed
[10] International Association for the Study of Pain. Opioid for pain management; 2018. https://www.iasp-pain.org/advocacy/iasp-statements/opioids-for-pain-management/Accessed 5 June 2023.Search in Google Scholar
[11] Rolita L, Spegman A, Tang X, Cronstein BN. Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc. 2013;61(3):335–40. 10.1111/jgs.12148.Search in Google Scholar PubMed PubMed Central
[12] Miller M, Sturmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59(3):430–8. 10.1111/j.1532-5415.2011.03318.x.Search in Google Scholar PubMed PubMed Central
[13] Shoff C, Yang TC, Shaw BA. Trends in opioid use disorder among older adults: Analyzing medicare data, 2013-2018. Am J Prev Med. 2021;60(6):850–5.10.1016/j.amepre.2021.01.010Search in Google Scholar PubMed PubMed Central
[14] Dufort A, Samaan Z. Problematic opioid use among older adults: epidemiology, adverse outcomes and treatment considerations. Drugs Aging. 2021;38(12):1043–53. 10.1007/s40266-021-00893-z.Search in Google Scholar PubMed PubMed Central
[15] Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther. 2013;35(11):1659–68. 10.1016/j.clinthera.2013.09.026.Search in Google Scholar PubMed
[16] Johansson MM, Barbero M, Peolsson A, Falla D, Cescon C, Folli A, et al. Pain Characteristics and Quality of Life in Older People at High Risk of Future Hospitalization. Int J Environ Res Public Health. 2021;18(3):958. 10.3390/ijerph18030958.Search in Google Scholar PubMed PubMed Central
[17] Makino K, Lee S, Bae S, Jung S, Shinkai Y, Chiba I, et al. Pain characteristics and incidence of functional disability among community-dwelling older adults. PLoS One. 2019;14(4):e0215467. 10.1371/journal.pone.0215467.Search in Google Scholar PubMed PubMed Central
[18] Mailis-Gagnon A, Nicholson K, Yegneswaran B, Zurowski M. Pain characteristics of adults 65 years of age and older referred to a tertiary care pain clinic. Pain Res Manag. 2008;13(5):389–94. 10.1155/2008/541963.Search in Google Scholar PubMed PubMed Central
[19] Agency for Healthcare Research and Quality. Survey Background. https://www.meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp Accessed 5 June 2023.Search in Google Scholar
[20] Agency for Healthcare Research and Quality. Survey Background. https://www.meps.ahrq.gov/data_stats/download_data/pufs/h216/h216doc.pdf Accessed 5 June 2023.Search in Google Scholar
[21] Agency for Healthcare Research and Quality. Survey Background. https://www.meps.ahrq.gov/data_stats/download_data/pufs/h213a/h213adoc.pdf Accessed on 5 June 2023.Search in Google Scholar
[22] Agency for Healthcare Research and Quality. MEPS HC-216 2019 Full Year Consolidated Data Codebook. https://meps.ahrq.gov/data_stats/download_data/pufs/h216/h216cb.pdf Accessed 5 June 2023.Search in Google Scholar
[23] Agency for Healthcare Research and Quality. MEPS HC-213A Codebook 2019 Prescribed Medicines File. https://meps.ahrq.gov/data_stats/download_data/pufs/h213a/h213acb.pdf Accessed 5 June 2023.Search in Google Scholar
[24] Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10. 10.2307/2137284.Search in Google Scholar
[25] Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of Chronic pain and high-impact chronic pain among adults – United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–6. 10.15585/mmwr.mm6736a2.Search in Google Scholar PubMed PubMed Central
[26] Axon DR, Le D. Predictors of pain severity among community-dwelling older adults with pain in the United States: Findings from a cross-sectional, retrospective study using 2017 Medical Expenditure Panel Survey. Medicine. 2021;100(20):e26011. 10.1097/MD.0000000000026011.Search in Google Scholar PubMed PubMed Central
[27] Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20(2):146–60. 10.1016/j.jpain.2018.07.006.Search in Google Scholar PubMed PubMed Central
[28] American Academy of Pain Medicine. Minimum Insurance Benefits for Patients with Chronic Pain; 2014. https://painmed.org/minimum-insurance-benefits-for-patients-with-chronic-pain/Accessed 5 June 2023.Search in Google Scholar
[29] Arku D, Axon DR. Pain severity is associated with self-reported functional limitations: Findings from the 2018 Medical Expenditure Panel Survey (MEPS). Pain Med. 2023;24(6):720–2. 10.1093/pm/pnac173.Search in Google Scholar PubMed
[30] Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009;57(9):1556–61. 10.1111/j.1532-5415.2009.02388.x.Search in Google Scholar PubMed PubMed Central
[31] Park SJ, Yoon DM, Yoon KB, Moon JA, Kim SH. Factors associated with higher reported pain levels in patients with chronic musculoskeletal pain: A cross-sectional, correlational analysis. PLoS One. 2016;11(9):e0163132. 10.1371/journal.pone.0163132.Search in Google Scholar PubMed PubMed Central
[32] Denkinger MD, Lukas A, Nikolaus T, Peter R, Franke S. Multisite pain, pain frequency and pain severity are associated with depression in older adults: results from the ActiFE Ulm study. Age Ageing. 2014;43(4):510–4. 10.1093/ageing/afu013.Search in Google Scholar PubMed
[33] Miki K, Nakae A, Shi K, Yasuda Y, Yamamori H, Fujimoto M, et al. Frequency of mental disorders among chronic pain patients with or without fibromyalgia in Japan. Neuropsychopharmacol Rep. 2018;38(4):167–74. 10.1002/npr2.12025.Search in Google Scholar PubMed PubMed Central
[34] Owen-Smith A, Stewart C, Sesay MM, Strasser SM, Yarborough BJ, Ahmedani B, et al. Chronic pain diagnoses and opioid dispensings among insured individuals with serious mental illness. BMC Psychiatry. 2020;20(1):40. 10.1186/s12888-020-2456-1.Search in Google Scholar PubMed PubMed Central
[35] Roughan WH, Campos AI, Garcia-Marin LM, Cuellar-Partida G, Lupton MK, Hickie IB, et al. Comorbid chronic pain and depression: shared risk factors and differential antidepressant effectiveness. Front Psychiatry. 2021;12:643609. 10.3389/fpsyt.2021.643609.Search in Google Scholar PubMed PubMed Central
[36] Hooten MW, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152(1):223–9. 10.1016/j.pain.2010.10.045.Search in Google Scholar PubMed PubMed Central
[37] Khan JS, Hah JM, Mackey SC. Effects of smoking on patients with chronic pain: a propensity-weighted analysis on the Collaborative Health Outcomes Information Registry. Pain. 2019;160(10):2374–9. 10.1097/j.pain.0000000000001631.Search in Google Scholar PubMed PubMed Central
[38] Zale EL, Maisto SA, Ditre JW. Anxiety and depression in bidirectional relations between pain and smoking: Implications for smoking cessation. Behav Modif. 2016;40(1–2):7–28. 10.1177/0145445515610744.Search in Google Scholar PubMed PubMed Central
[39] Axon DR, Quigg MD. Characteristics associated with self-reported exercise among US adults age ≥50 years with self-reported pain in the past four weeks who used an opioid. Healthcare. 2023;11(8):1129. 10.3390/healthcare11081129.Search in Google Scholar PubMed PubMed Central
[40] Axon DR, Grieser M. Characteristics associated with multimorbidity among older United States adult opioid users with pain. J Clin Med. 2023;12(20):6684. 10.3390/jcm12206684.Search in Google Scholar PubMed PubMed Central
[41] Axon DR, Maldonado T. Investigating the association of pain intensity and health status among older US adults with pain who used opioids in 2020 using the Medical Expenditure Panel Survey. Healthcare. 2023;11(14):2010. 10.3390/healthcare11142010.Search in Google Scholar PubMed PubMed Central
[42] Aqel O, Agu U, Almatruk Z, Axon DR. Association between pain burden and presence of any limitation among older adults ( ≥ 50 years of age) with pain who used opioids in the United States: Cross-sectional study using 2020 Medical Expenditure Panel Survey. Medicine. 2023;102(33):e34863. 10.1097/MD.0000000000034863.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)

