Abstract
Objective
The aim of this study was to validate the Neuropathic Pain for Post-Surgical Patients (NeuPPS) scale against clinically verified neuropathic pain (NP) by quantitative sensory testing (QST) as well as evaluation of other psychometric properties. The NeuPPS is a validated 5-item scale designed to evaluate NP in surgical populations.
Methods
Data from 537 women aged >18 years scheduled for primary breast cancer surgery enrolled in a previous study for assessing risk factors for persistent pain after breast cancer treatment were used. Exclusion criteria were any other breast surgery or relevant comorbidity. A total of 448 eligible questionnaires were available at 6 months and 455 at 12 months. At 12 months, 290 patients completed a clinical examination and QST. NeuPPS and PainDETECT were analyzed against patients with and without clinically verified NP. NP was assessed using a standardized QST protocol including a clinical assessment. Furthermore, the NeuPPS and PainDETECT scores were psychometrically tested with an item response theory method, the Rasch analysis, to assess construct validity. Primary outcomes were the diagnostic accuracy measures for the NeuPPS, and secondary measures were psychometric analyses of the NeuPPS after 6 and 12 months. PainDETECT was also compared to clinically verified NP as well as NeuPPS comparing the stability of the estimates.
Results
Comparing the NeuPPS scores with verified NP using a receiver operating characteristic curve, the NeuPPS had an area under the curve of 0.80. Using a cutoff of 1, the NeuPPS had a sensitivity of 88% and a specificity of 59%, and using a cutoff of 3, the values were 35 and 96%, respectively. Analysis of the PainDETECT indicated that the used cutoffs may be inappropriate in a surgical population.
Conclusion
The present study supports the validity of the NeuPPS as a screening tool for NP in a surgical population.
1 Introduction
Persistent pain is a well-known undesirable consequence of surgery [1]. Moderate-to-severe persistent pain after breast cancer surgery affects 10–20% of the patients depending on definition and treatment [2,3]. The condition may persist for several years [4] and negatively affect physical function and quality of life [5]. “Sensory disturbances” are frequently observed together with persistent pain after surgery [4,6] and may point toward a neuropathic origin. Among patients treated for primary breast cancer, the prevalence of neuropathic pain (NP) is estimated between 15 and 30% [7].
We have previously developed a simple 5-item NP questionnaire tool for postsurgical patients: the Neuropathic Pain for Post-Surgical Patients (NeuPPS) scale [8]. The items have been content validated [5] and construct validated using item response theory [8]. Also, it has convergent validity with the S-LANSS [8].
Using a previous prospective cohort of patients treated for primary invasive breast cancer [9,10], the aims of the present study were as follows: (1) to assess the concurrent validity of the NeuPPS scale by comparing it to “NP” verified with clinical examination including quantitative sensory testing (QST), (2) to assess sensitivity and specificity and develop cutoff scores for “probable NP” in the NeuPPS, and (3) to test the psychometric properties including the stability between 6 and 12 months follow-up using a longitudinal Rasch model. Finally, we included a comparison with the often-used PainDETECT scale [11].
2 Methods
2.1 Study and design
The present validation study was based on data from a previous prospective cohort study. Previously, we have published predictive factors for the development of persistent pain following the treatment for primary invasive breast cancer [9], and with the relationship between persistent pain and sensory loss 1 year postoperatively [10]. Here, we present analyses of questionnaire data vs clinical assessment with QST. Patients were recruited preoperatively from 1 November 2011 to 14 October 2013 at Rigshospitalet, Copenhagen, Denmark, and received a baseline questionnaire and follow-up questionnaires both 6 and 12 months postoperatively [9]. Furthermore, patients were invited to participate in a QST examination 1 year postoperatively [10]. Inclusion criteria for the study were women of 18 years or above undergoing surgery for stage I and II invasive breast cancer. Exclusion criteria were previous ipsilateral breast cancer, previous aesthetic breast surgery, primary or secondary reconstructive surgery, bilateral breast cancer, neoadjuvant treatment, pregnancy, diseases in the nervous system, psychiatric disease, unable to understand Danish, or unable to consent to the study. A total of 537 patients with baseline data were included, and 491 and 475 patients were still participating in the study 6 and 12 months after surgery, respectively. Patients from this cohort were invited for a QST examination 1 year postoperatively, of whom 290 participated [10]. Patients were treated according to the 2010 DBCG guidelines [12]. Data on surgical treatment were retrieved through medical records, and data on adjuvant treatment were retrieved through the DBCG database [13]. Surgical handling of the intercostobrachial nerve was recorded on a specially designed record sheet [14], registering whether the nerve was identified or not, and for identified nerves whether it was preserved, partially preserved, or completely sectioned. Detailed data on demographics and treatment have been published elsewhere [9,10].
2.2 NeuPPS and other questionnaires
The NeuPPS is a 5-item questionnaire where each item is dichotomous (yes/no) and gives 0 or 1 point and where the items are additive giving a total score between 0 and 5 (Figure 1). It was developed for postsurgical patients, asking for symptoms specifically located in the area of surgery [8].

The neuropathic pain for post-surgical patients (NeuPPS).
Patients also received questions regarding pain in the area of surgery with the following predefined anatomical localizations: the breast, side of the chest, axilla, and arm. Pain was measured with a numerical rating scale (NRS) ranging from 0 to 10. The frequency of pain was measured with a 5-point verbal scale: “constantly,” “daily,” “weekly,” “monthly,” and “more rarely.” Only pain experienced on a weekly basis or more frequently was considered. In the present study, only questions regarding pain at rest were used.
The study also included the PainDETECT questionnaire [11] consisting of three Likert items scored from zero to 10 (pain now, strongest, and on average, respectively), an item describing the course of pain (four categories with scores ranging from −1 to 1), an item asking about radiating pain (with a +2 score), and 7 items asking about different sensory disturbances symptoms with 6 possible answers (ranging from “never” to “very strongly” scored from 0 to 5 for each item) [11]. The score may obtain a total score of 38, with scores from 0 to 12 regarded as “unlikely” NP, scores from 13 to 18 as “ambiguous,” and scores from 19 to 38 as “likely” NP [11]. The PainDETECT questionnaire was originally developed to distinguish NP from non-NP among patients with back pain [11].
2.3 Clinical determination of “probable” NP diagnosis (QST)
One-year postoperative clinical examination of patients was performed using a predefined QST protocol as previously described in detail, including interview, clinical examination, localities, equipment, calibration, sequence, and the test–retest properties of the protocol [15]. The QST included cold and warm detection thresholds, heat pain threshold, mechanical detection threshold, mechanical pain threshold, dynamic mechanical allodynia, and pressure pain threshold [15]. We used the NeuPSIG guidelines [16] and grading system [17]. Because QST is considered a valid, but not recommended as a stand-alone test, we chose to define NP in the cohort as “probable” and not “definite.”
For the purpose of determining whether patients had “probable” NP, all patients were assessed for the presence of pain and affected sensory function in the area related to the surgery (breast, side of chest, axilla, and arm). NP was then defined in the following way: (1) pain reporting in the area of surgery as defined above at 12-month follow-up; (2) the pain should be at least on a weekly basis; (3) clinical examination with hypoesthesia (mechanical, warmth, and/or cold), hyperesthesia, allodynia, and/or hyperalgesia in the same area as the pain reporting; and (4) confirmed by QST testing of mechanical or thermal thresholds.
2.4 Statistics
The items in the NeuPPS were initially validated at both 6 and 12 months after surgery using an item response theory model, the Rasch model [18–20]. Rasch models enable assessment of whether the scale measures one trait only, whether scale scores increase with an increase of the trait, and whether items are independent of each other and of exogenous variables. Based on previous validations, five items had been chosen for the NeuPPS [8]. The overall fit of the data 6 and 12 months post-operative was assessed with the Andersen conditional likelihood ratio test (CLR) [21], and individual item fit by comparing observed and expected correlations between the separate item scores and the sum of the remaining items [22]. Item fit was also examined graphically with empirical and theoretical item characteristic curves [23]. The independence of item responses to exogenous variables and the absence of differential item functioning (DIF) were assessed using likelihood ratio tests in log-linear Rasch models [24]. DIF was tested for factors proposed to modify the risk for persistent pain after breast cancer surgery: chemotherapy (yes/no), age (<50, 50–59, 60–69, ≥70 years), radiotherapy (yes/no), axillary surgery (sentinel lymph node biopsy/axillary lymph node dissection), endocrine therapy (yes/no), and breast surgery (mastectomy/breast-conserving surgery). We then tested the NeuPPS in a longitudinal Rasch model [25,26], to test whether item parameters changed between 6- and 12-month follow-ups. Patients with missing data were excluded from the analyses.
The correlation between PainDETECT and the NeuPPS scores was tested at both 6- and 12-month follow-ups using the Spearman rank test. A correlation coefficient of <0.25 was considered small; 0.25–0.50 as moderate; 0.50–0.75 as good; and >0.75 as excellent. Missing data in the pain course item and the radiating pain item were treated as score = 0 due to high proportions of missing data in these items. At each score sum, the NeuPPS was assessed against the clinical verification of NP with the accuracy measures sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, and negative likelihood ratio. NeuPPS scores against clinically verified NP were assessed graphically with receiver operating characteristic (ROC) curves – plotting the true positive rate against the false positive rate – and by plotting the predicted probability of NP against NeuPPS scores. The area under the ROC curve was calculated with a corresponding 95% confidence interval (CI). In the primary analysis, the NeuPPS score was tested against NP with an intensity of NRS ≥1 at rest. Subsequently, it was tested against an NP of moderate to severe intensity (NRS ≥ 4) [27]. We also included an analysis of our initially tested NeuPPS scale [8], which included hypoesthesia in the sum score of the NeuPPS, with hypoesthesia giving 1 point (totally adding up to 6) to provide more data on the relevance of omitting this item [8]. ROC curves were also plotted for PainDETECT scores compared to clinically verified NP.
Statistical significance for item fit statistics and tests of DIF and local dependence used the Benjamini–Hochberg procedure [28] to account for multiple analyses. The significance level was set at alpha = 0.05. Analyses were performed using DIGRAM software (Department of Biostatistics, University of Copenhagen, Denmark) and SAS 9.4 for Windows (SAS Institute Inc., Cary, USA).
-
Ethical approval: The study complied with all relevant national regulations and institutional policies, is in accordance with the tenets of the Helsinki Declaration (as amended in 2013), and has been approved by the regional bioethics committee of the capital region in Denmark, H-D-2007-0098. Informed consent has been obtained from all individuals included in this study and data collection was approved by the Danish Data Protection Agency. It was registered with clinicaltrials.gov NCT01523132.
3 Results
The median age at the time of surgery of the 491 patients was 61 years (IQR: 51–67), distributed with age >50: 20%, 50–59: 27%, 60–69: 37%, <70: 16%. NeuPPS reporting at 6 and 12 months and pain reporting and pain intensity at the different NeuPPS scores are shown in Table 1. Increasing postsurgical pain prevalence and increasing pain intensity were observed with increasing NeuPPS scores.
NeuPPS scores at 6 (N = 491) and 12 (N = 475) months
| 6 months | 12 months | |||||
|---|---|---|---|---|---|---|
| NeuPPS score | Number (%) | Pain at rest*, N (row %) | Median (IQR) pain NRS score | Number (%) | Pain at rest†, N (row %) | Median (IQR) pain NRS score |
| 0 | 191 (38.9) | 34 (17.8) | 0 (0;0) | 200 (42.1) | 29 (14.5) | 0 (0;0) |
| 1 | 103 (21.0) | 48 (47.1) | 0 (0;0) | 105 (22.1) | 50 (47.6) | 0 (0;3) |
| 2 | 83 (16.9) | 52 (62.7) | 1 (0;3) | 80 (16.8) | 47 (58.8) | 1 (0;3) |
| 3 | 47 (9.6) | 41 (87.2) | 3 (2;4) | 47 (9.9) | 41 (89.1) | 3 (2;4) |
| 4 | 18 (3.7) | 17 (94.4) | 3 (2;4) | 17 (3.6) | 16 (94.1) | 3 (2;4) |
| 5 | 6 (1.2) | 6 (100.0) | 4.5 (3;7) | 6 (1.3) | 6 (100.0) | 4.5 (3;5) |
| Missing | 43 (8.8) | 18 (42.9) | 0 (0;3) | 20 (4.2) | 10 (50.0) | 0.5 (0;5) |
Abbreviations: IQR: interquartile range. NeuPPS: Neuropathic Pain for Post-Surgical Patients. Consists of five dichotomous items. Each item gives 1 point for yes and 0 point for no, giving a total score between 0 and 5. NRS: numerical rating scale. *Two patients with missing pain scores. †One patient with missing pain scores.
3.1 Accuracy of the NeuPPS compared to clinically verified NP and cutoff scores
Among the 290 patients who completed a QST examination, 281 (97%) patients had complete data on the NeuPPS. Accuracy measures for different cutoff scores for the NeuPPS compared to verified NP with an intensity of NRS ≥1 are shown in Table 2. The area under the curve (AUC) for the NeuPPS was 80.2% (95% CI: 75.2–85.3) (Figure 2). Figure 3 shows the observed and predicted probabilities of NP at different NeuPPS scores. The area under the curve for the models with NP intensity of NRS ≥4 and for the NeuPPS including the omitted “numbness” item was 71.1 and 82.1%, respectively.
Accuracy measures for NeuPPS scores according to clinically verified NP* among 281 patients
| NeuPPS cutoff score | Number (%) ≥ cutoff score | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR |
|---|---|---|---|---|---|---|---|
| ≥ 1 vs 0 | 168 (59.8) | 88.3 | 58.8 | 58.3 | 88.5 | 2.14 | 0.20 |
| ≥2 vs <2 | 101 (35.9) | 62.2 | 81.2 | 68.3 | 76.7 | 3.30 | 0.47 |
| ≥3 vs <3 | 46 (16.4) | 35.1 | 95.9 | 84.8 | 69.4 | 8.53 | 0.68 |
| ≥4 vs <4 | 15 (5.3) | 12.6 | 99.4 | 93.3 | 63.5 | 21.44 | 0.93 |
| 5 vs <5 | 4 (1.4) | 3.6 | 100 | 100 | 61.8 | — | 0.96 |
Abbreviations: LR: likelihood ratio. NeuPPS: Neuropathic Pain for Post-Surgical Patients. Consists of five dichotomous items. Each item gives 1 point for yes and 0 points for no, giving a total score between 0 and 5. NPV: negative predictive value; PPV: positive predictive value. *Defined as a numerical rating scale score ≥1 at rest. NEUPPS scores with increasing probability of neuropathic pain.
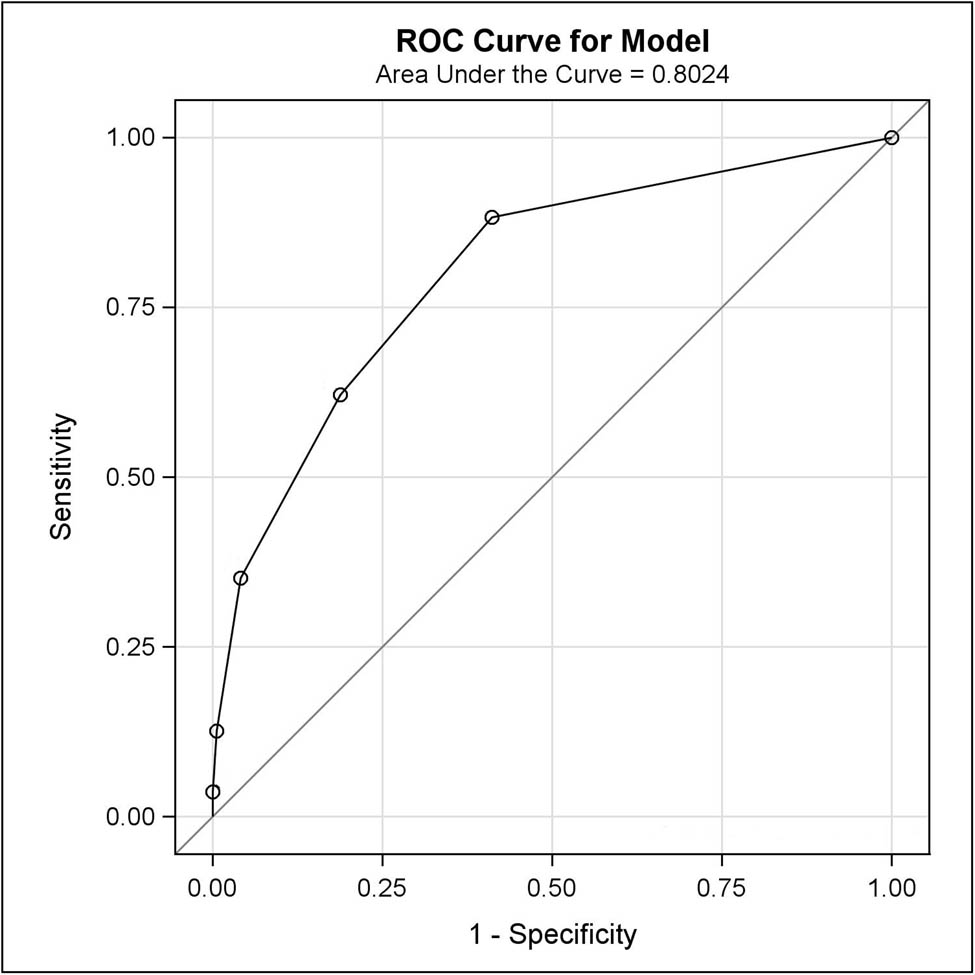
ROC curve for the NeuPPS.
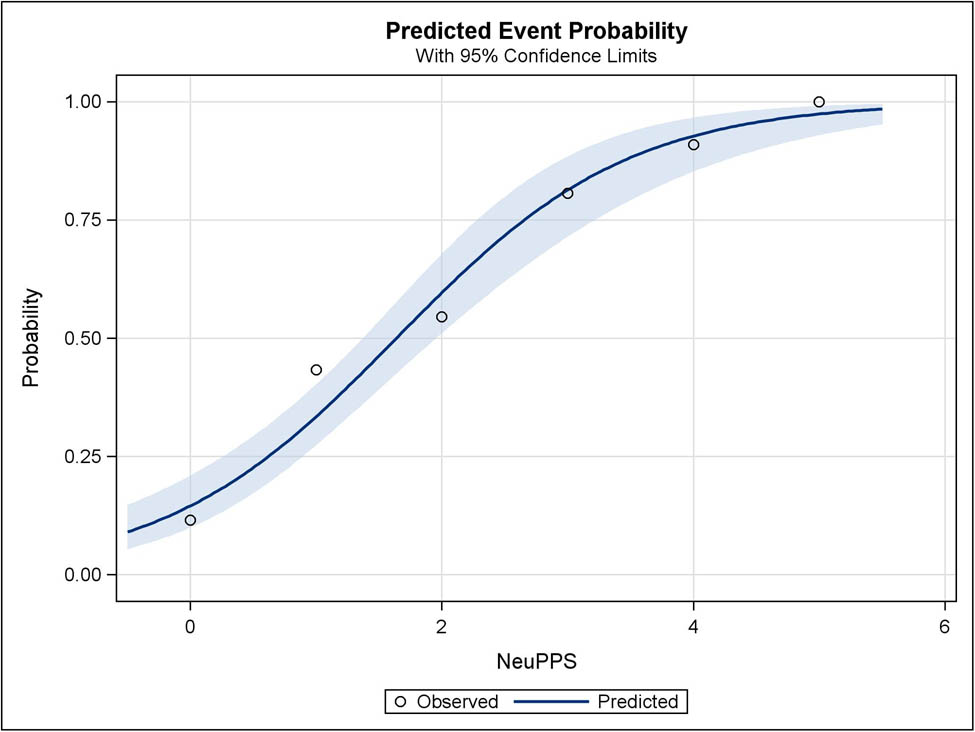
Predicted and observed probabilities of NP plotted against NeuPPS scores. Shaded blue is the 95% CI of predicted probabilities.
3.2 Rasch model
The final dataset at 6 months consisted of questionnaires from 448 patients (83% out of 537). Rasch analysis of the NeuPPS showed good overall model fit (Andersen CLR = 3.4 on 4 degrees of freedom, p = 0.4865) and no evidence of item misfit. There was evidence of DIF for item 1 with respect to breast procedure. In a comparison of patients with the same total score, patients who had received a mastectomy scored 0.12 (95% CI 0.05–0.19) higher on this item compared to breast-conserving surgery patients. The model did not show any signs of local dependence. At 12 months, the final dataset consisted of questionnaires from 455 patients (85% out of 537). The Rasch model showed a good overall fit (Andersen CLR = 5.8 on 4 degrees of freedom, p = 0.2172) and no evidence of item misfit. The model did not show any signs of DIF or local dependence.
Using a longitudinal item response model, we evaluated DIF between 6- and 12-month follow-ups. In this model, no evidence of DIF with respect to follow-up was disclosed.
3.3 PainDETECT
At 6-month follow-up, there were 357 patients with no missing data on either the NeuPPS items or the PainDETECT items. The Spearman rank correlation coefficient between NeuPPS and PainDETECT scores was p = 0.59. At 12 months, 369 patients had complete data on NeuPPS and PainDETECT, and the Spearman rank correlation coefficient between the NeuPPS score and the PainDETECT score was p = 0.63. Comparing the PainDETECT to clinically verified NP (N = 234), the sensitivity was 27%, the specificity was 94%, the PPV was 85%, and the NPV was 62% at a cutoff value of 13 vs 12 or favorable. At a cutoff of 19 vs 18 or less, the corresponding values were 8, 99, 89, and 57%, respectively. The area under the ROC curve was 0.81 (95% CI: 0.76–0.87). Graphically, the fit of observed-to-expected event probabilities was less good for the PainDETECT scores than for the NeuPPS scores (Figure 4).
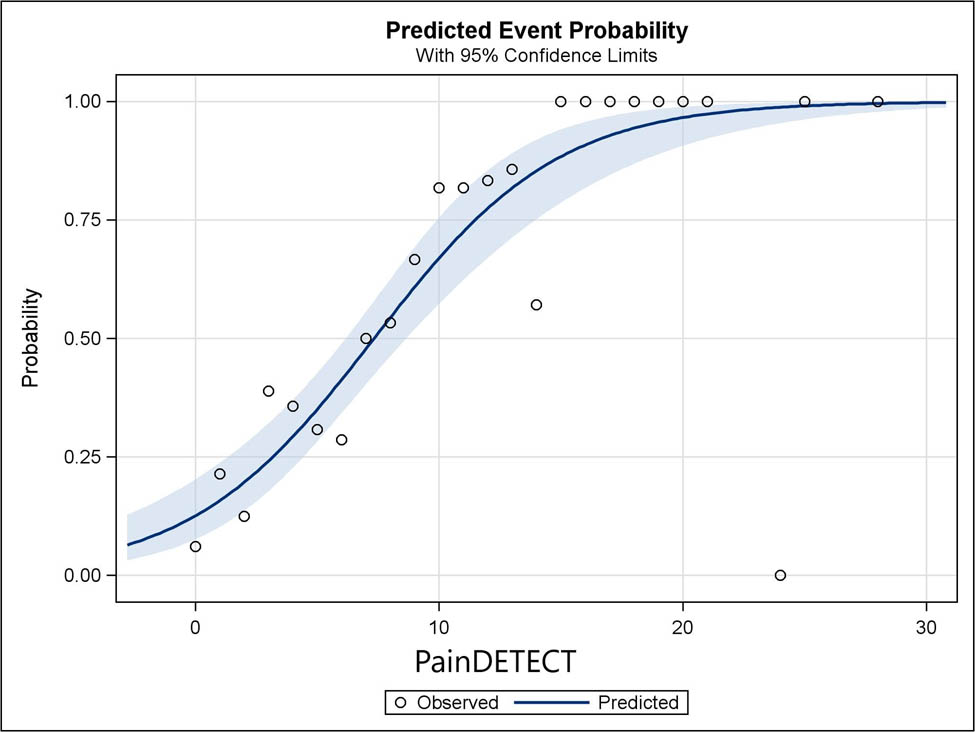
Predicted and observed probabilities of NP plotted against PainDETECT scores. Shaded blue is the 95% CI of predicted probabilities.
4 Discussion
The present study adds to the evidence of validity of the simple 5-item NeuPPS [8]. When comparing the NeuPPS to clinically diagnosed NP using QST methods, the NeuPPS showed good convergent validity. The psychometric properties of the NeuPPS demonstrated in a previous study [8] were confirmed at 6 and 12 months. We further showed in a longitudinal Rasch model that the psychometric properties of the NeuPPS were stable from 6 to 12 months. Finally, the data suggest that the conventionally used cutoff scores for the PainDETECT may be inappropriately high in a postsurgical breast cancer population.
Comparing the NeuPPS scale to clinically confirmed NP, the AUC was 0.8 (i.e., for a randomly selected case defined by clinically verified NP, the probability of scoring higher on the NeuPPS than a randomly selected non-case is 80%). Generally, the NeuPPS showed a high specificity and PPV, but the sensitivity quickly diminished with increasing NeuPPS cutoff scores. The NPV was more stable, only going from 77 to 62% between NeuPPS cutoff scores of 2 vs 1 and 5 vs 4. Using a cutoff score of NRS ≥4 for NP increased the NPV’s to 95–90% for the different NeuPPS cutoff scores at the expense of lower PPV’s. Adding the hypoesthesia item to the NeuPPS slightly increased the sensitivity and PPV at the expense of slightly lower specificity and NPV, supporting the argument for omitting this item.
The PainDETECT demonstrated a comparable AUC of 0.81, a sensitivity of 0.08, and a specificity of 0.99 with the proposed cutoff value of 18 (“likely NP”). Even with a cutoff of 13 (“ambiguous”), sensitivity and specificity were 0.27 and 0.94, respectively, thus suggesting weaknesses in cutoff levels in relation to the present population, with a too low prevalence estimate as also noted by Ilhan et al. [7]. In a study of breast cancer patients using PainDETECT, it was also noted a lower-than-expected prevalence of NP [29]. The observed and predicted values had a quite poor model fit, probably related to the psychometric properties of the scale, and could suggest a lack of monotonicity which would mean that it is less appropriate to use as a scale in its current form. This is supported by another Rasch analysis of the PainDETECT, where it is suggested to re-score the results [30].
Comparing the NeuPPS to other NP screening tools, the S-LANSS reported a sensitivity of 0.74 and a specificity of 0.76 with the proposed cutoff of 12 in a population with chronic pain [31]. The seven items of the self-administered Douleur Neuropathique and four questions (DN4) had a sensitivity of 0.78 and a specificity of 0.81 with a cutoff of 3 [32]. The validation study of PainDETECT in a low back pain population indicated a sensitivity of 0.85 and a specificity of 0.85 [11]. In contrast to our studies, the DN4 and PainDETECT validation studies had the patients examined twice [11,32] and had access to sophisticated equipment such as electrophysiology or imaging [11]. Despite this potential shortcoming, our results are comparable to these results, but with a simpler tool. Our population was relatively homogenous, including only women treated for breast cancer, and being unselected regarding pain. A problem with several NP screening tools when used in surgical populations is that they do not specify the surgical neuroanatomical distribution in relation to the pain. Several include a body map to mark the main pain, but this may relate to other pain conditions. In a setting of postsurgical breast cancer patients, patients may report other symptoms than surgically induced sensory dysfunction and pain (e.g., taxane induced peripheral neuropathy). This potentially leads to very heterogeneous reporting of symptoms in studies examining sequelae following surgery. Furthermore, pain at other locations than in the area of surgery may overshadow pain and sensory signs they experience in the surgical area, leading to loss of information.
We have defined NP as “probable” according to the classification [17]. QST is one of the accepted confirmatory tests [16], although not recommended as a standalone test [33]. In cases where the surgeon observes nerve damage, such a confirmatory test is not necessary [17].
The NeuPPS is a simple tool consisting of five questions with a binary response that may be summed together. In clinical use, we suggest cutoff 1 as a screening tool to detect patients with probable NP. For research purposes, it may be beneficial to include patients with a higher probability for NP. In such cases, a cutoff of 3 would be more appropriate.
Rasch analyses demonstrated a solid scale with good fit. A single DIF was observed, which however was not found in the previous two validation populations [8], suggesting that it could be a chance finding. The strong correlations between the clinical examination and QST and PainDETECT indicate an excellent convergent validity regarding NP in the population. The longitudinal Rasch analysis suggested stability between 6 and 12 months. The previous validation study did however indicate that there could be problems with the validity with large differences in follow-up probably due to the dynamic nature of neuropathy and NP [8].
The strengths of our study and scale are the very thorough validation process including content, construct, and convergence validity assessments, and together with our previous study [8], the large amount of patients we have included in our studies ranges from 6 months to 6 years of follow-up. Furthermore, the patients were from a well-defined surgical population, and the NeuPPS was compared to a structured clinical examination using QST. However, certain limitations apply to the present study. To determine the status of NP a single examination was performed and using a second clinician to examine the patients could have ensured a more secure diagnosis. Testing the NeuPPS only in breast cancer patients is also a potential limitation, as the generalizability of the results to other postsurgical populations is unknown. Finally, missing data could have introduced bias in the analyses. In the present study though, we observed only that 9% and 4% had missing data at 6 and 12 months, respectively, on the NeuPPS. Comparatively, for the PainDETECT, 22% and 20% had missing data at 6 and 12 months.
5 Conclusion
This study provides accuracy measures and adds to the data to support the construct validity of the simple and easy NeuPPS as a usable questionnaire screening tool to detect NP from an unselected population treated for breast cancer. Furthermore, it is not limited to patients reporting pain and therefore potentially giving valuable information also on postsurgical patients without pain.
6 Use of scale
The NeuPPS was validated in Danish and the translation from Danish to English was done using an iterative forward–backward translation sequence. We recommend a formal validation within the language it is intended to be used, to ensure cultural validity. We recommend adjustment of the wording of the questionnaire text concerning the neuroanatomical area affected by surgery (Figure 1). The scale is intended to be used free of charge upon agreement with the corresponding author.
-
Research ethics: Complied with national legislation. Information supplied in the text.
-
Informed consent: Complied with national legislation.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors declare no conflicts of interest.
-
Research funding: This study was funded by grants from the Danish Cancer Society (grant number is not applicable). The funder had no influence in the conception, design, data collection, analysis, interpretation, or writing of the article.
References
[1] Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367(9522):1618–25.10.1016/S0140-6736(06)68700-XSearch in Google Scholar PubMed
[2] Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725–46. 10.1016/j.jpain.2010.12.005.Search in Google Scholar PubMed
[3] Meretoja TJ, Andersen KG, Bruce J, Haasio L, Sipilä R, Scott NW, et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 2017;35(15):1660–7.10.1200/JCO.2016.70.3413Search in Google Scholar PubMed
[4] Mejdahl MK, Andersen KG, Gärtner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: Six year nationwide follow-up study. BMJ. 2013;346(7907):1–14.10.1136/bmj.f1865Search in Google Scholar PubMed
[5] Andersen KG, Christensen KB, Kehlet H, Bidstup PE. The effect of pain on physical functioning after breast cancer treatment development and validation of an assessment tool. Clin J Pain. 2015;31(9):794–802.10.1097/AJP.0000000000000156Search in Google Scholar PubMed
[6] Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA - J Am Med Assoc. 2009;302(18):1985–92.10.1001/jama.2009.1568Search in Google Scholar PubMed
[7] Ilhan E, Chee E, Hush J, Moloney N. The prevalence of neuropathic pain is high after treatment for breast cancer: A systematic review. Pain. 2017;158(11):2082–91.10.1097/j.pain.0000000000001004Search in Google Scholar PubMed
[8] Mejdahl MK, Christensen KB, Andersen KG. Development and validation of a screening tool for surgery-specific neuropathic pain: Neuropathic pain scale for postsurgical patients. Pain Physician. 2019;22(2):E81–90.10.36076/ppj/2019.22.E81Search in Google Scholar
[9] Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156(12):2413–22.10.1097/j.pain.0000000000000298Search in Google Scholar PubMed
[10] Andersen KG, Duriaud HM, Kehlet H, Aasvang EK. The Relationship Between Sensory Loss and Persistent Pain 1 Year After Breast Cancer Surgery. J Pain. 2017;18(9):1129–38. 10.1016/j.jpain.2017.05.002.Search in Google Scholar PubMed
[11] Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–20.10.1185/030079906X132488Search in Google Scholar PubMed
[12] Https://dbcg.dk/PDF/DBCG_2014_protokoller_oversigt. DBCG treatment protocol [Internet]. [cited 2021 Mar 1]. https://dbcg.dk/PDF/DBCG_2014_protokoller_oversigt.Search in Google Scholar
[13] Blichert-Toft M, Christiansen P, Mouridsen HT. Danish Breast Cancer Cooperative Group - DBCG: History, organization, and status of scientific achievements at 30-year anniversary. Acta Oncol (Madr). 2008;47(4):497–505.10.1080/02841860802068615Search in Google Scholar PubMed
[14] Andersen KG, Aasvang EK, Kroman N, Kehlet H. Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta Anaesthesiol Scand. 2014;58(10):1240–8.10.1111/aas.12393Search in Google Scholar PubMed
[15] Andersen KG, Kehlet H, Aasvang EK. Test-retest agreement and reliability of quantitative sensory testing 1 year after breast cancer surgery. Clin J Pain. 2015;31(5):393–403.10.1097/AJP.0000000000000136Search in Google Scholar PubMed
[16] Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. 10.1016/j.pain.2010.07.031.Search in Google Scholar PubMed
[17] Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain. 2016;157:1599–606.10.1097/j.pain.0000000000000492Search in Google Scholar PubMed PubMed Central
[18] Christensen KB, Kreiner S, Mesbah M. Rasch models in health. NJ, USA: John Wiley & Sons, Ltd; 2013.10.1002/9781118574454Search in Google Scholar
[19] Rasch G. Probabalistic models for some intelligence and attainment tests. Copenhagen, Denmark: Nielsen & Lydiche; 1960.Search in Google Scholar
[20] Fischer GH, Molenaar IW. Rasch models: Foundations, recent developments and application. Springer US; 1995.10.1007/978-1-4612-4230-7Search in Google Scholar
[21] Andersen EB. A goodness of fit test for the Rasch model. Psychometrika. 1973;38:123–40.10.1007/BF02291180Search in Google Scholar
[22] Kreiner S. A note on Item-Restscore association in rasch models. Appl Psychol Meas. 2011;35:557–61.10.1177/0146621611410227Search in Google Scholar
[23] Christensen KB. Conditional maximum likelihood estimation in polytomous rasch models using SAS. ISRN Comput Math. 2013;2013:617475.10.1155/2013/617475Search in Google Scholar
[24] Kelderman H. Loglinear Rasch model tests. Psychometrika. 1984;49:223–45.10.1007/BF02294174Search in Google Scholar
[25] Olsbjerg M, Christensen KB. Marginal and conditional approaches to longitudinal Rasch models. Pub Inst Stat Univ Paris. 2013;57:109–26.Search in Google Scholar
[26] Olsbjerg M, Christensen KB. %lrasch_mml: A SAS macro for marginal maximum likelihood estimation in longitudinal polytomous rasch models. J Stat Softw. 2015;67:codesnippet 2.10.18637/jss.v067.c02Search in Google Scholar
[27] Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. Br J Anaesth. 2011;107(4):619–26.10.1093/bja/aer195Search in Google Scholar PubMed
[28] Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300.10.1111/j.2517-6161.1995.tb02031.xSearch in Google Scholar
[29] Juhl AA, Christiansen P, Damsgaard TE. Persistent pain after breast cancer treatment: A questionnaire-based study on the prevalence, associated treatment variables, and pain type. J Breast Cancer. 2016;19(4):447–54.10.4048/jbc.2016.19.4.447Search in Google Scholar PubMed PubMed Central
[30] Packham TL, Cappelleri JC, Sadosky A, MacDermid JC, Brunner F. Measurement properties of painDETECT: Rasch analysis of responses from community-dwelling adults with neuropathic pain. BMC Neurol. 2017;17(1):1–9.10.1186/s12883-017-0825-2Search in Google Scholar PubMed PubMed Central
[31] Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: Validation for use in clinical and postal research. J Pain. 2005;6(3):149–58.10.1016/j.jpain.2004.11.007Search in Google Scholar PubMed
[32] Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36.10.1016/j.pain.2004.12.010Search in Google Scholar PubMed
[33] Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154(9):1807–19.10.1016/j.pain.2013.05.047Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)


