Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
-
Mohammed Abdelbagi
, Rusli Fidriyanto
Abstract
This study aimed to evaluate the effects of encapsulation and combining probiotics with different nitrate forms on methane emission and the in vitro fermentation process of ruminants. Sodium nitrate (NaNO3) and nitric acid (HNO3) were used as nitrate forms, while lactic acid bacteria Lactiplantibacillus plantarum TSD-10 was used as a probiotic source. Twelve different treatments with four replicates were allocated in the factorial block design (2 × 2 × 3). During each replicate, the test was conducted individually in a different week so that each block could be considered separately. Data analysis followed the analysis of variance (ANOVA) and then continued with the Duncan multiple range test. After encapsulation, significant increases (p < 0.05) in gas production, gas kinetics, total volatile fatty acids (TVFAs), and production of propionic acid were observed. In addition, encapsulation significantly decreased (p < 0.05) the pH, ammonia concentration (NH3), nutrient digestibility, and the ratio of acetic to propionic acid (p < 0.05). The addition of combined encapsulated probiotics and encapsulated nitrate significantly increased (p < 0.05) gas production, maximum gas production, TVFAs, and the molar portion of propionic acid, and significantly decreased (p < 0.05) enteric methane emission, acetic acid, ammonia concentration, pH, and nutrient digestibility. The addition of sodium nitrate significantly increased (p < 0.05) the concentration of TVFAs and acetic acid, while nitric acid significantly increased (p < 0.05) the gas production rate. However, there was no significant effect due to combining unencapsulated probiotics with unencapsulated nitrate forms on the rumen fermentation process. There was a significant interaction (p < 0.05) between encapsulation probiotics and nitrate on ammonia concentration. In conclusion, combining encapsulated probiotics with encapsulated nitrate is an alternative method for enhancing the fermentation process and mitigating enteric methane emission in ruminants.
1 Introduction
Methane (CH4) is normally produced as a result of microbial activity, especially archaeal methanogens, during the fermentation process in ruminants [1]. In addition, methane is the second greatest greenhouse gas (GHG) after carbon dioxide [1]. Methane gained its importance among the other greenhouse gases due to its potential contribution to climate change and global warming phenomena [2,3,4]. On the other hand, the livestock sector has an important contribution to methane emission, which reaches about 14.5% of the total emitted methane in the globe [5]. In addition, 12% of gross energy (GE) losses in ruminants are lost due to the enteric methane emission. Thus, the issue of enteric methane emission is in relation to feed utilization, productivity, and global warming [6]. Therefore, the dietary options that are used for mitigating enteric methane emissions in ruminants are effective in elevating environmental concerns, improving utilization, and improving animal productivity [7]. Among the dietary options for mitigating such enteric methane emissions, the use of feed additives has been considered a promising option [8].
Both nitrate and probiotics have been suggested as feed additives to inhibit methane emission in ruminant animals [9,10,11,12]. Nitrate has the potential to inhibit enteric methane in ruminants due to its ability to act as an electron acceptor [13,14]. Moreover, nitrate is toxic for both methanogens [15] and ruminants themselves due to its relation with methemoglobinemia that occurs as a result of consuming high nitrate diets [16,17,18]. Despite the effectiveness of nitrate as a methane inhibitor, nitrate is still widely unused in ruminant nutrition. On the other hand, probiotics were proposed to mitigate enteric methane emission in ruminants [19] through two different mechanisms: first, probiotics have the ability to stimulate the growth of lactic acid utilizing bacteria, resulting in high production of propionic acid and subsequently decrease hydrogen molecules for forming methane during the fermentation process in ruminant animals [20]. Second, probiotics contribute to providing some nutrients for bacterial growth. The nutrients include some metabolic intermediates and vitamins that are essentially used for bacterial growth, and therefore, this may negatively affect methanogen growth [21].
Many experiments have been investigated to determine the effects of combining nitrate with other inhibitors on reducing enteric methane emission, improving utilization, and improving animal productivity. For instance, nitrate and nitrate-reducing bacteria have been used to enhance nitrate reduction [22]. Also, combining nitrate with saponin was previously examined. The authors did not observe significant effects due to combining nitrate with saponin on rumen microorganisms [23]. Additionally, the effects of combining nitrate with garlic oils were examined by using different raw materials. The results showed a significant effect of combining methane with garlic oils on methanogens [24].
Encapsulation is a process that is used to prevent nutrients from undesirable conditions over time by improving their stability and bioavailability and controlling their release rate at specific times and places [25]. Encapsulated nitrate was reported to reduce methane production without negatively affecting the performance of animals [26]. To date, there has been no research on the effects of combining probiotics with nitrate forms. Therefore, we hypothesized that encapsulation and combining probiotics with nitrate forms would influence the rumen fermentation process and decrease enteric methane emissions in ruminants. Therefore, in this study, we aimed to evaluate the effects of encapsulation and combine probiotics with different nitrate forms on enteric methane emission and in vitro fermentation characteristics.
2 Materials and methods
All research procedures in the present study were performed at the Research Center for Applied Zoology, National Research and Innovation Agency (BRIN), Cibinong, Indonesia, and the Department of Nutrition and Feed Technology, IPB University, Bogor, Indonesia.
2.1 Materials
A commercial concentrate containing soya bean meal, rice bran, corn meal, corn gluten feed (CGF), distiller dried grains with solubles (DDGS), and others was purchased from the Indofeed Mini Feed mill, Bogor, West Java. In this study, elephant grass (Pennisetum purpureum) was used as a source of forage. Forage was collected from the surrounding area of the research farm of KST Soekarno-BRIN, Cibinong, West Java, Indonesia. Lactiplantibacillus plantarum (10 log CFU/ml (TSD-10)) was used as a probiotic source. Probiotics were prepared by culturing L. plantarum in a facultative fermentation medium at 30°C in deMan Rogosa Sharpe (MRS) broth medium (Merck, Darmstadt, Germany). Preparation was done in the Genomic and Environmental Laboratory of the National Research and Innovation Agency (BRIN), Cibinong. In this study, maltodextrin was used as a coating material for encapsulation. Maltodextrin was in a powder readily used form. Sodium nitrate (NaNO3), 99% purity, and nitric acid (HNO3), 70% purity, were used as sources of nitrate. Sodium nitrate was supplied by Merck(Darmstadt, Germany), while nitric acid was obtained from Loba Chemie Pvt. Ltd. (Mumbai, India).
2.2 Encapsulation process
Encapsulation was done by using a freeze dryer, according to Chen Man et al. [27]. Briefly, 10 mM of NaNO3 and HNO3 were dissolved in 10 ml of distilled water. Then, 10 ml of probiotics containing 10 (log CFU/ml) was mixed with 10 g of maltodextrin. The mixture was prepared from different nitrate forms (sodium nitrate, nitric acid), probiotics, and maltodextrin (1:1:1) to obtain 10 mM nitrate. A total of 10 g of maltodextrin, 10 ml of sodium nitrate and nitric acid, and 10 ml of probiotics L. plantarum TSD-10 were mixed. The mixture was immediately kept at 20°C in the freezer for 15 min to homogenize. Subsequently, samples were placed overnight in an 80°C deep freezer (CHRIST Alpha 1-4 LD plus) until they became completely dry. After that, samples were ground by using a mortar and pestle to be used in the next steps.
2.3 In vitro experimental procedure
Feed materials (concentrates and the forage) were ground to pass a 1 mm screen size. Then, feed samples (concentrate and forage materials) were analyzed before adding nitrate forms or probiotics using the method described by Ridwan et al. [28] (Table 1). A feed ratio of 60% concentrates and 40% forages was used. Further, diets were designed in a 2 × 3 × 3 factorial design with 12 different treatments. Treatments were prepared by adding 0.5 g of nitrate forms and 0.5 ml of L. plantarum TSD-10. Treatments included T1(encapsulated NaNO3 without probiotics), T2 (encapsulated NaNO3 with probiotics), T3 (encapsulated NaNO3 with encapsulated probiotics), T4 (non-encapsulated NaNO3 probiotics without probiotics), T5 (non-encapsulated NaNO3 probiotics with probiotics), T6 (non-encapsulated NaNO3 probiotics with encapsulated probiotics), T7 (encapsulated HNO3 without probiotics), T8 (encapsulated HNO3 with probiotics), T9 (encapsulated HNO3 with encapsulated probiotics), T10 (non-encapsulated HNO3 probiotics without probiotics), T11 (non-encapsulated HNO3 with probiotics), and T12 (non-encapsulated HNO3 probiotics with encapsulated probiotics). Treatments were quadruplicated according to the number of in vitro incubation runs. Each of the replicates was run individually in different weeks. Each week was considered a block by itself.
Chemical composition of fistulated cattle basal diet and in vitro substrate (% dry matter)
| Item | Basal diet | In vitro substrate | |
|---|---|---|---|
| Forage | Concentrate | ||
| Ash | 2.3 | 3.0 | 3.2 |
| CP | 8.25 | 16.0 | 16.6 |
| EE | 1.92 | 5.30 | 4.83 |
| CF | 35.8 | 15.8 | 21.1 |
| NDF | 61.1 | 44.1 | 44.1 |
| ADF | 40.7 | 33.0 | 28.1 |
CP, crude protein; EE, ether extract; CF, crude fiber; NDF, neutral detergent fiber; ADF, acid detergent fiber.
The buffer medium was prepared anaerobically following the method of McDougall [29]. Rumen fluids were collected from two rumen fistulated Ongole crossbred males with an average body weight of 550 ± 30 kg. Steers were handled and maintained in accordance with the protocols of animal welfare of the Animal Care and Use Committee of the Indonesian Institute of Sciences 2015. Animals were fed two times a day (morning and afternoon). The feed substrate consisted of 40% forages and 60% concentrates. Water was freely accessible by animals. The rumen fluid collection was done before the morning feeding, around 7:00 a.m. Rumen solutions were sieved through a four-layer cheesecloth. A total of 500 ml of rumen fluid from each animal was collected and kept separately in pre-warm bottles. After collection, solutions were brought immediately to the laboratory and kept in a water bath at 39°C. Each of the collected fluids was separately transferred to the conical flask, sealed with an aluminum foil. After that, the rumen pH was determined and recorded. The pH was measured by using a TRAI BP3001 pH meter, e.g., the average pH of the samples collected from steer No. 1 was 6.93, and that of the sample from steer No. 2 was 6.91. The rumen buffered solution was mixed at 1:2 of rumen fluid/buffered solution. Subsequently, a rumen buffer solution was placed in a conical flask, which was sealed with an aluminum foil. Each of the rumen buffer solutions was continuously purged with CO2 to maintain the pH value and the anaerobic conditions. The pH value of the mixture was also recorded (pH 7.2 and 7.1). Incubation was done in accordance with a modified protocol of Theodorou et al. [30]. Forty-eight vials (100 ml) were filled with 50 ml of rumen buffer fluid containing 500 mg of the experimental substrate. All bottles were sealed with butyl rubber stoppers and aluminum crimps before placing into a 39°C water bath. Then, all bottles were incubated for 72 h using a 39°C water bath. However, the bottles were frequently shaken every 1 h. Each of the treatments had two blank bottles. Incubation was run four times during four different weeks. Each week was considered as a replicate by itself.
After 72 h of incubation, the gas production of each bottle was vented and recorded at 2, 4, 6, 8, 10, 12, 24, 48, and 72 h. Methane concentration was measured at 8, 10, 12, 24, 48, and 72 h. Total gas production was measured using a 50 ml syringe, while methane concentration was measured using a methane analyzer (RIKEN KEIKI RX415). Gas production kinetics were estimated using the Ørskov equation: p = a + b (1 − e−c.t) [31]. After 72 h of incubation, the serum in each bottle was sieved carefully in the plastic corning and the pH of the residues was measured. However, calibration of the Cyberscan pH 310 Eutech equipment was done using a pH 7 buffer solution. Later, each corning was centrifuged at 6,000 for 10 min at −4°C to determine the nutrient digestibility (dry matter and organic matter digestibility). The nutrient digestibility was measured as described by Tilley and Terry [32]. Residues were added to 20 ml of 0.2% pepsin HCL solution. Then, all samples were incubated for another 24 h. After incubation, the samples were dried at 130°C for 8 h and then burnt at 600°C for 3 h to obtain the nutrient digestibility (DMD and the OMD). The in vitro nutrient digestibility of the dry matter (IVDMD) and organic matter (IVOMD) was determined by subtracting the amount of the initial substrate from the substrates after the drying and burning processes. Total and partial volatile fatty acids were determined by using 10 ml of the supernatant, which was filtered carefully and collected in a plastic corning. The concentration of total volatile fatty acids (TVFAs, mg/L) was determined by using a spectrophotometer (495λ), as described in the study of Biswabandhu and Radhakrishnan [33]. Further, the molar portions of partial volatile fatty acids were determined using a GC machine (GC-MS-QP2010 SE) using a MEGA-WAX MS column (025-02530). Another 5 ml of the supernatant was used for determining ammonia concentration. Ammonia concentrations were quantified using a spectrophotometer (630λ) in accordance with the study of Souza et al. [34].
2.4 Statistical analysis
Data were analyzed using the general linear model procedure with a 2 × 2 × 3 factorial arrangement. The first factor included two different physical forms (encapsulated and non-capsulated). The second factor included two different chemical forms (NaNO3 and HNO3). The third factor included three different probiotic treatments (without probiotics, with probiotics, and with encapsulated probiotics). The allocation of treatments to experimental units followed a completely randomized block factorial design. Different in vitro operations served as blocks due to population variations and rumen microbial activity with each sampling time (each week). Data were analyzed by analysis of variance (ANOVA) based on a completely randomized factorial block design. When the ANOVA results showed p < 0.05 for a particular parameter, a post-hoc test, namely Duncan’s multiple range test, was applied to the data. Data analysis was performed using SAS Statistics software version 9.1.4. The figures are presented using Microsoft Office Excel.
3 Results
The effects of encapsulated and combining probiotics with nitrate forms on gas production and methane production are shown in Table 2. Gas production kinetics on the effects of encapsulation and nitrate types are presented in Figures 1 and 2, respectively. The effects of encapsulated and combining probiotics with nitrate forms on rumen fermentation parameters are presented in Table 3. The effects of encapsulated and combined probiotics with nitrate forms on the partial volatile fatty acids are shown in Table 4. Treatments significantly influenced the fermentation process. After encapsulation, we observed a significant increase in the gas production, gas kinetics, TVFAs, and production of propionic acid. In addition, encapsulation significantly decreased the pH, ammonia concentration (NH3), nutrient digestibility, and the ratio of acetic to propionic acid (p < 0.05). The addition of combined encapsulated probiotics and encapsulated nitrate significantly increased the gas production, maximum gas production, TVFAs, and the molar portion of propionic acid and significantly decreased enteric methane emission, acetic acid, ammonia concentration, pH, and nutrient digestibility (p < 0.05). The addition of sodium nitrate significantly increased the concentration of TVFAs and acetic acid, while nitric acid significantly increased the gas production rate (p < 0.05). However, there was no significant effect due to combining unencapsulated probiotics with unencapsulated nitrate forms on the rumen fermentation process. There was a significant interaction among encapsulation probiotics and nitrate on ammonia concentration.
Effects of treatments on in vitro gas production and methane emission
| Item | Total gas (ml/g DM) | a + b (ml/g DM) | c (/h) | CH4 (% gas) | |
|---|---|---|---|---|---|
| Encapsulation | ENCAP | 216 ± 32.5b | 216 ± 29.4b | 0.112 ± 0.016b | 7.35 ± 4.25 |
| Non-ENCAP | 123 ± 75.6a | 128 ± 70.5a | 0.064 ± 0.043a | 7.05 ± 3.81 | |
| Nitrate type | N1 | 163 ± 77.1 | 162 ± 75.7a | 0.089 ± 0.035 | 7.66 ± 4.43 |
| N2 | 176 ± 72.9 | 181 ± 62.5b | 0.087 ± 0.045 | 6.75 ± 3.87 | |
| Probiotics | Without PRO | 140 ± 71.9a | 150 ± 62.4a | 0.068 ± 0.038a | 9.08 ± 4.16b |
| With PRO | 141 ± 73.8a | 140 ± 66.9a | 0.076 ± 0.035a | 8.35 ± 3.39b | |
| With ENCAP-PRO | 228 ± 63.7b | 232 ± 30.2b | 0.120 ± 0.025b | 4.18 ± 3.16a | |
| Treatment | T1 | 204 ± 14.5b | 194 ± 11.5c | 0.100 ± 0.018bc | 8.97 ± 5.05 |
| T2 | 206 ± 16.4b | 196 ± 14.1cd | 0.107 ± 0.005bc | 8.45 ± 4.52 | |
| T3 | 226 ± 51.7b | 239 ± 43.2de | 0.127 ± 0.032cd | 2.20 ± 1.24 | |
| T4 | 60.3 ± 12.9a | 63.0 ± 18.2a | 0.037 ± 0.005a | 8.03 ± 5.90 | |
| T5 | 63.5 ± 4.26a | 63.0 ± 4.31a | 0.050 ± 0.008ab | 7.35 ± 2.67 | |
| T6 | 222 ± 20.3b | 218 ± 13.2d | 0.102 ± 0.012bc | 5.51 ± 4.23 | |
| T7 | 210 ± 22.6b | 205 ± 13.7cd | 0.110 ± 0.001c | 10.6 ± 3.73 | |
| T8 | 215 ± 19.1b | 210 ± 9.54d | 0.112 ± 0.009c | 9.22 ± 4.17 | |
| T9 | 238 ± 21.3b | 249 ± 25.0de | 0.117 ± 0.015c | 4.74 ± 3.17 | |
| T10 | 84.8 ± 21.1a | 114 ± 22.6ab | 0.027 ± 0.012a | 8.78 ± 2.77 | |
| T11 | 79.5 ± 10.5a | 89.5 ± 6.96a | 0.035 ± 0.006a | 8.38 ± 3.17 | |
| T12 | 228 ± 22.5b | 221 ± 32.4cd | 0.135 ± 0.029cd | 4.26 ± 3.51 | |
| p-value | Encapsulation | <0.001 | <0.001 | <0.001 | 0.786 |
| Nitrate type | 0.117 | 0.003 | 0.634 | 0.421 | |
| Probiotics | <0.001 | <0.001 | <0.001 | 0.002 | |
| Treatment | <0.001 | 0.669 | 0.116 | 0.130 | |
| ENC*NITR | 0.691 | 0.212 | 0.924 | 0.518 | |
| ENC*PRO | <0.001 | <0.001 | <0.001 | 0.552 | |
| NITR*PRO | 0.949 | <0.275 | 0.305 | 0.982 | |
| ENC*NITR*PRO | 0.816 | 0.287 | 0.007 | 0.747 | |
| Block | <0.001 | <0.001 | <0.001 | <0.001 |
a + b, potential gas production; c, gas production rate; ENCAP, encapsulation; Non-ENCAP, non-encapsulation; N1, sodium nitrate; N2, nitric acid; PRO, probiotics; ENCAP-PRO, encapsulated probiotics; ENC*NTR, the interaction between encapsulated and the nitrate type; ENCA*PRO, the interaction between encapsulated and probiotics; NTR*PRO, the interaction between the nitrate type and probiotics; ENC*NITR*PRO, the interaction among the encapsulated, nitrate type, and probiotics; T1, encapsulated sodium nitrate without probiotics; T2, encapsulated sodium nitrate with probiotics; T3, encapsulated sodium nitrate with encapsulated probiotics; T4, non-encapsulated sodium nitrate without probiotics; T5, non-encapsulated sodium nitrate with probiotics; T6, non-encapsulated sodium with encapsulated probiotics; T7, encapsulated nitric acid without probiotics; T8, encapsulated nitric acid with probiotics; T9, encapsulated nitric acid with encapsulated nitric acid; T10, non-encapsulated nitric acid without probiotics; T11, non-encapsulated nitric acid with probiotics; T12, non-encapsulated nitric acid with encapsulated probiotics; SEM, standard error of means; probability was considered significant when p-value <0.05; Small letter superscripts are in ascending order.
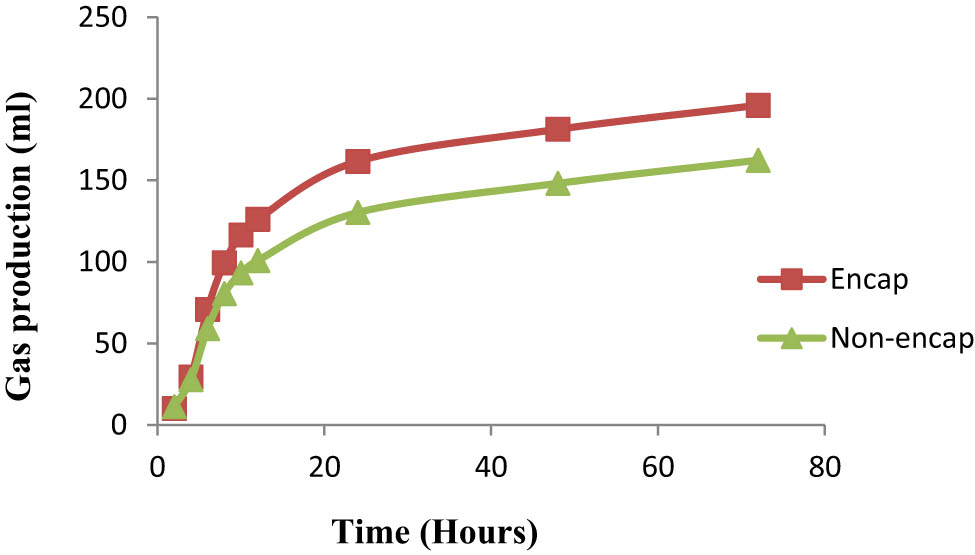
Effects of encapsulation on gas production kinetics.
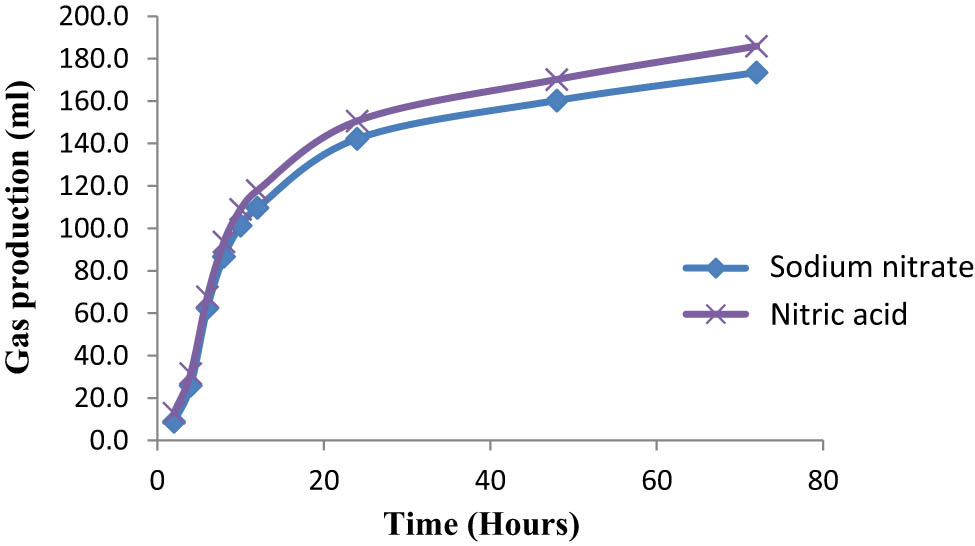
Effects of nitrate types on gas production kinetics.
Effects of treatments on the rumen fermentation characteristics
| Item | pH | NH3 (mg/ml) | IVDMD (%) | IVOMD (%) | TVFAs (mmol/g) | |
|---|---|---|---|---|---|---|
| Encapsulation | ENCAP | 5.75 ± 0.57a | 28.8 ± 15.6a | 47.7 ± 17.0b | 57.7 ± 15.2a | 111 ± 17.6b |
| Non-ENCAP | 6.53 ± 0.33b | 39.1 ± 11.0b | 58.8 ± 4.73a | 68.5 ± 5.16b | 64.6 ± 32.6a | |
| Nitrate forms | N1 | 6.19 ± 0.60 | 34.5 ± 14.4 | 53.3 ± 12.8 | 63.5 ± 12.3 | 91.9 ± 32.6b |
| N2 | 6.09 ± 0.61 | 33.4 ± 14.8 | 53.2 ± 14.6 | 62.7 ± 12.9 | 83.9 ± 37.5a | |
| Probiotics | Without encapsulation | 6.41 ± 0.34ab | 40.4 ± 8.74b | 59.2 ± 5.85b | 68.1 ± 4.49b | 77.7 ± 36.4a |
| With encapsulation | 6.38 ± 0.37ab | 36.6 ± 12.4b | 59.3 ± 17.1b | 67.9 ± 3.40b | 75.5 ± 37.0a | |
| With encapsulation | 5.63 ± 0.69c | 24.9 ± 16.7a | 41.4 ± 4.50a | 53.3 ± 17.4a | 110 ± 17.8b | |
| Treatment | T1 | 6.20 ± 0.07b | 40.9 ± 5.81cd | 61.1 ± 5.92b | 69.9 ± 4.46b | 98.9 ± 16.0b |
| T2 | 6.13 ± 0.07b | 30.2 ± 1.25bc | 57.6 ± 4.97b | 66.7 ± 3.77b | 110 ± 20.3b | |
| T3 | 5.21 ± 0.08a | 9.21 ± 3.63a | 27.8 ± 8.35a | 39.6 ± 6.58a | 97.0 ± 7.70b | |
| T4 | 6.76 ± 0.04c | 35.0 ± 11.1bc | 57.2 ± 4.99b | 65.8 ± 4.07b | 43.2 ± 11.5a | |
| T5 | 6.74 ± 0.03c | 41.9 ± 16.9bcde | 59.3 ± 3.65b | 67.8 ± 2.33b | 42.6 ± 12.1a | |
| T6 | 6.12 ± 0.08b | 42.9 ± 5.47cd | 57.0 ± 4.38b | 71.2 ± 10.5b | 112 ± 13.8bcd | |
| T7 | 5.98 ± 0.07b | 35.5 ± 4.76c | 58.6 ± 6.36b | 67.6 ± 4.83b | 121 ± 13.2cd | |
| T8 | 5.94 ± 0.09b | 47.7 ± 6.86de | 58.1 ± 4.84b | 66.9 ± 3.64b | 108 ± 10.5bc | |
| T9 | 5.05 ± 0.04c | 9.26 ± 1.74a | 23.3 ± 4.91a | 35.7 ± 4.09a | 131 ± 14.0d | |
| T10 | 6.70 ± 0.04c | 50.1 ± 2.42de | 59.9 ± 7.72b | 68.9 ± 5.03b | 47.2 ± 11.1a | |
| T11 | 6.71 ± 0.07c | 26.6 ± 6.12b | 62.1 ± 4.86b | 70.1 ± 3.89b | 41.4 ± 13.5a | |
| T12 | 6.14 ± 0.40b | 38.0 ± 5.27cd | 57.4 ± 2.16b | 66.9 ± 1.04b | 102 ± 14.5 | |
| p-value | Encapsulation | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Nitrate type | 0.215 | 0.584 | 0.938 | 0.572 | 0.049 | |
| Probiotics | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Treatment | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| ENC*NITR | 0.329 | 0.179 | 0.203 | 0.427 | 0.011 | |
| ENC*PRO | 0.187 | <0.001 | <0.001 | <0.001 | <0.001 | |
| NITR*PRO | 0.927 | 0.381 | 0.645 | 0.292 | 0.253 | |
| ENC*NITR*PRO | 0.999 | <0.001 | 0.926 | 0.707 | 0.074 | |
| Block | 0.948 | 0.838 | <0.001 | <0.001 | <0.001 |
pH; the rumen pH value, NH3; ammonia concentration, IVDMD; in vitro dry matter digestibility, IVOMD; in vitro organic matter digestibility, ENCAP; encapsulation, Non-ENCAP; non-encapsulation, N1; sodium nitrate, N2; nitric acid, PRO; probiotics ENCAP-PRO; encapsulated probiotics, INTR; the interaction among the factors, ENC*NTR; the interaction between encapsulated and the nitrate type, ENCA*PRO; the interaction between encapsulated and the probiotics, NTR*PRO; the interaction between the nitrate type and probiotics, ENC*NITR*PRO; the interaction among the encapsulation, nitrate type, and probiotics,T1; encapsulated sodium nitrate without probiotics, T2; encapsulated sodium nitrate with probiotics, T3; encapsulated sodium nitrate with the encapsulated probiotics, T4; non-encapsulated sodium nitrate without probiotics, T5; non-encapsulated sodium nitrate with probiotics, T6; non-encapsulated sodium with encapsulated probiotics, T7; encapsulated nitric acid without probiotics, T8; encapsulated nitric acid with probiotics, T9; encapsulated nitric acid with encapsulated nitric acid, T10; non-encapsulated nitric acid without probiotics, T11; non-encapsulated nitric acid with probiotics, T12; non-encapsulated nitric acid with encapsulated probiotics, SEM; standard error of means, probability was considered when p-value <0.05. Small letter superscripts a: z are in ascending order.
Effects of treatments on the partial volatile fatty acids
| Item | Acetate (%) | Propionate (%) | Isobutyrate (%) | Butyrate (%) | Isovalerate (%) | Valerate (%) | Acetate: Propionate (%) | |
|---|---|---|---|---|---|---|---|---|
| Encapsulation | Encap | 49.1 ± 4.02a | 40.9 ± 6.93b | 0.75 ± 0.32b | 5.97 ± 1.87b | 0.34 ± 0.34a | 1.46 ± 0.0.43b | 1.26 ± 0.34a |
| Non-encap | 60.3 ± 10.2b | 30.9 ± 10.8a | 0.43 ± 0.69a | 4.14 ± 2.0a | 0.60 ± 0.37b | 1.24 ± 0.51a | 2.28 ± 1.01b | |
| Nitrate type | N1 | 55.9 ± 9.77b | 36.7 ± 10.5 | 0.60 ± 0.54 | 5.30 ± 2.28 | 0.52 ± 0.37 | 1.45 ± 0.43 | 1.85 ± 0.96 |
| N2 | 53.5 ± 9.31a | 34.9 ± 10.3 | 0.57 ± 0.59 | 4.81 ± 1.99 | 0.51 ± 0.36 | 1.24 ± 0.52 | 1.67 ± 0.87 | |
| Probiotics | Without | 57.4 ± 9.23b | 33.6 ± 9.78a | 0.72 ± 0.62b | 6.12 ± 1.86b | 0.63 ± 0.34b | 1.20 ± 0.32a | 1.95 ± 0.94b |
| With | 58.3 ± 10.9b | 32.6 ± 11.4a | 0.68 ± 0.61b | 5.87 ± 1.97a | 0.58 ± 0.31b | 1.15 ± 0.44a | 2.12 ± 1.05b | |
| With-ENCAP | 48.5 ± 4.20a | 41.4 ± 7.67b | 0.38 ± 0.37a | 4.47 ± 2.25a | 0.32 ± 0.37a | 1.69 ± 0.49b | 1.23 ± 0.37a | |
| Treatment | T1 | 50.9 ± 3.65b | 38.0 ± 5.45b | 6.62 ± 0.26bcd | 6.62 ± 2.51cd | 0.67 ± 0.42bcde | 1.40 ± 0.24bc | 1.37 ± 0.27a |
| T2 | 50.2 ± 5.44b | 39.5 ± 10.4b | 6.35 ± 0.45bcd | 6.34 ± 2.48cd | 0.67 ± 0.45bcde | 1.34 ± 0.51abc | 1.37 ± 0.50a | |
| T3 | 51.0 ± 6.11b | 38.8 ± 10.2b | 6.49 ± 0.37bcd | 6.49 ± 1.80cd | 0.39 ± 0.38abc | 1.23 ± 0.56abc | 1.42 ± 0.58a | |
| T4 | 66.8 ± 1.32a | 25.5 ± 6.03a | 2.92 ± 0.85a | 2.92 ± 0.74a | 0.71 ± 0.46bcd | 0.90 ± 0.20ab | 2.78 ± 0.90b | |
| T5 | 70.0 ± 2.36a | 22.8 ± 4.32a | 2.68 ± 0.74a | 2.68 ± 0.32a | 0.51 ± 0.13bc | 0.88 ± 0.20ab | 3.16 ± 0.65b | |
| T6 | 46.8 ± 1.87b | 45.4 ± 4.58b | 3.81 ± 0.28ab | 3.81 ± 0.48ab | 0.16 ± 0.06a | 1.68 ± 0.31c | 1.06 ± 0.12a | |
| T7 | 46.8 ± 1.34b | 45.0 ± 4.33b | 4.36 ± 0.24ab | 4.36 ± 0.58bc | 0.25 ± 0.49ab | 1.47 ± 0.25bc | 1.05 ± 0.12a | |
| T8 | 46.6 ± 3.48b | 43.8 ± 6.01b | 5.09 ± 0.36bc | 5.89 ± 1.91c | 0.47 ± 0.23bc | 1.40 ± 0.61bc | 1.08 ± 0.22a | |
| T9 | 49.4 ± 1.84b | 40.1 ± 4.22b | 6.94 ± 0.24c | 6.94 ± 0.61d | 0.21 ± 0.08a | 1.90 ± 0.12c | 1.24 ± 0.16a | |
| T10 | 65.1 ± 2.37a | 26.0 ± 5.57a | 3.97 ± 0.89ab | 3.97 ± 0.36ab | 0.69 ± 0.07c | 1.02 ± 0.23ab | 2.61 ± 0.67b | |
| T11 | 66.4 ± 2.03a | 24.2 ± 5.17a | 4.23 ± 0.84ab | 4.23 ± 3.41bc | 0.87 ± 0.30cde | 0.98 ± 0.21ab | 2.85 ± 0.69b | |
| T12 | 46.7 ± 3.48b | 41.3 ± 10.9b | 7.25 ± 0.57de | 7.25 ± 2.13df | 0.63 ± 0.54abcde | 1.94 ± 0.58cd | 1.22 ± 0.44a | |
| p-value | ENCAP | <0.001 | <0.001 | 0.0574 | 0.0005 | 0.0757 | 0.0496 | <0.0001 |
| NITR | 0.021 | 0.384 | 0.7862 | 0.3104 | 0.8926 | 0.0621 | 0.229 | |
| PRO | <0.001 | 0.002 | 0.1870 | 0.0129 | 0.0218 | 0.0014 | 0.0001 | |
| Treatment | <0.001 | <0.001 | 0.5742 | 0.0012 | 0.0334 | 0.000 | <0.0001 | |
| ENC*NITR | 0.494 | 0.222 | 0.4325 | 0.0031 | 0.0041 | 0.6279 | 0.583 | |
| ENC*PRO | <0.001 | <0.001 | 0.6273 | 0.6282 | 0.8568 | 0.0163 | 0.0001 | |
| NITR*PRO | 0.507 | 0.531 | 0.8628 | 0.0985 | 0.2957 | 0.2674 | 0.6888 | |
| ENC*TR*PRO | 0.890 | 0.931 | 0.8708 | 0.9768 | 0.7481 | 0.6228 | 0.866 | |
| Block | 0.187 | 0.220 | <0.0001 | 0.4337 | 0.3773 | 0.0033 | <0.0001 |
ENCAP; encapsulation, Non-ENCAP; non-encapsulation, N1; sodium nitrate, N2; nitric acid, PRO; probiotics ENCAP-PRO; encapsulated probiotics, INTR; the interaction among the factors, ENC*NTR; the interaction between encapsulated and the nitrate type, ENCA*PRO; the interaction between encapsulated and the probiotics, NTR*PRO; the interaction between the nitrate type and probiotics, ENC*NITR*PRO; the interaction among the encapsulation, nitrate type, and probiotics,T1; encapsulated sodium nitrate without probiotics, T2; encapsulated sodium nitrate with probiotics, T3; encapsulated sodium nitrate with the encapsulated probiotics; T4; non-encapsulated sodium nitrate without probiotics, T5; non-encapsulated sodium nitrate with probiotics, T6; non-encapsulated sodium with encapsulated probiotics, T7; encapsulated nitric acid without probiotics, T8; encapsulated nitric acid with probiotics, T9; encapsulated nitric acid with encapsulated nitric acid; T10; non-encapsulated nitric acid without probiotics, T11; non-encapsulated nitric acid with probiotics, T12; non-encapsulated nitric acid with encapsulated probiotics, SEM; standard error of means, probability was considered when p-value < 0.05. Small letter superscripts a: z are in ascending order.
4 Discussion
We observed a significant influence of encapsulation on the fermentation process. Encapsulation increased significantly the TVFAs, gas production, maximum gas production, and gas production rate. Also, we observed a significant increase in gas production and TVFAs due to combining encapsulated probiotics with encapsulated nitrate forms. Among other treatments, the highest gas production and the highest TVFAs were scored as a result of combining encapsulated probiotics with encapsulated nitrate forms before or after encapsulation. The results were consistent with those of previous studies [9,35], which reported a significant increase in the total produced gas and TVFAs due to the addition of encapsulated probiotics and encapsulated nitrate. Also, there was a significant interaction between probiotics and encapsulation in gas production.
The increase in the gas production rate and amounts of TVFAs could probably be due to the effects of the encapsulation process. In this study, maltodextrin was used as a matrix for coating probiotics and nitrate during the encapsulation process. Maltodextrin is a readily fermented carbohydrate. Therefore, it could easily be attacked by rumen microorganisms, resulting in higher TVFAs and higher gas production [36]. However, several coating materials have been previously investigated [37,38]. For instance, maltodextrin was reported to increase the gas production rate and the amount of TVFAs produced, while a lower gas production rate was observed when sodium alginate was used as a matrix for encapsulation. Therefore, an increase in gas production and TVFAs is correlated with the types of coating materials. For example, carbohydrate materials are known to have a greater gas production rate as compared with other ingredients [39]. Generally, an increase in gas production does not indicate a deficiency of feed utilization; however, it indicates a higher fermentation rate of substrate degraded by rumen microorganisms [19]. According to Rahman et al. [40], changes in the gas production rates of different substrates are correlated with a significant shift in portions of TVFAs. Total gas naturally includes CO2, CH4, and small amounts of H2, N2, and O2 as a result of degrading nutrient substrates [41]. To avoid bias due to encapsulation, the amount of gas produced in this study as a result of degrading maltodextrin (about 20 ml) of the blank substrate was subtracted from the total gas production. On the other hand, there was no significant difference in gas production and TVFAs after adding unencapsulated probiotics to the diet before or after encapsulation. The results were consistent with those of previous studies [19,42]. This indicates the ability of probiotics to improve the fermentation process and maintain improved rumen conditions. Among nitrate forms, nitric acid has been shown to increase the gas production. Nitric acid is an acidic ion. It could reduce the pH values in rumen, resulting negatively in pathogen population and improving the fermentation rate by increasing both the gas production rate and amount of TFVAs.
After encapsulation, we observed a significant decrease in the pH value. Also, there was a significant decrease in the rumen pH value due to the addition of encapsulated probiotics and encapsulated nitrate in the diet. Among treatments, the lowest pH value was recorded due to combining encapsulated probiotics with encapsulated acid. There was a numerical reduction in the pH value due to combining encapsulated probiotics with encapsulated nitric acid as compared with combining encapsulated probiotics with encapsulated sodium nitrate. Among nitrate types, the pH value of nitric acid was numerically lower than sodium nitrate. Gawad and Fellner [35] found a significant decrease in the pH value due to encapsulation. In addition, Jiao et al. [42] observed no significant differences in the pH and TVFAs among encapsulated and non-encapsulated yeasts. Generally, the reduction of rumen pH is attributed to the rapid accumulation of TVFAs in the rumen [9]. The rapid commutation of organic acids is due to the significant increase of TFVAs in rumen as a result of improving the fermentation rate after encapsulation. This could correlate significantly with a significant reduction in the pH value in the rumen.
Also, the acid properties of nitric acid increase the reduction in pH. This indicates that both the encapsulation and acid properties of nitric acid would contribute to significantly reducing the rumen pH. Therefore, the lowest pH value among treatments was due to the addition of encapsulated probiotics and encapsulated nitric acid and can be attributed to the effects of both encapsulation and acid properties of nitric acid. After adding unencapsulated probiotics, the rumen pH value tends to be closer to the pH of treatments before encapsulation. We did not observe a significant difference between the addition of unencapsulated probiotics and combining unencapsulated with nitrate forms before and after encapsulation (T1 and T2, T4 and T5, T7 and T8, and T10 and T11). Thus, this indicates the ability of probiotics to sustain rumen conditions in the normal range. It is suggested that probiotics have the ability to sustain normal pH values [43]. According to Sari et al. [44], the normal pH value in the rumen is between 6.4 and 6.7.
We observed a significant decrease in nutrient digestibility and ammonia concentration after encapsulation. Moreover, the lowest digestion rate and the lowest ammonia concentration were observed due to the addition of combining encapsulated probiotics and encapsulated nitrate forms. Moreover, there was a significant interaction among encapsulation, probiotics, and nitrate forms on the ammonia concentration. On the other hand, by adding unencapsulated probiotics, we observed no significant effects on the digestion rate or ammonia concentration. Previously, Lund et al. [45] have reported a numerous reduction in nutrient digestion after encapsulation. Also, Lee et al. [46] found that ammonia concentration decreased linearly by adding encapsulated nitrate. Similarly, Makled et al. [38] reported lower concentrations of ammonia after encapsulation. The reduction in nutrient digestion follows the drastic reduction of pH value, which is caused by the rapid accumulation of TVFAs in the rumen because of encapsulation [47]. Faniyi et al. [48] reported that ammonia is produced mainly as a result of microbial activity on protein sources [49]. Therefore, ammonia production is usually affected positively or negatively by the digestion rate. The reduction in pH is usually linked to a reduction in nutrient digestibility (IVDMD and IVOMD), followed by a reduction in ammonia concentration. Sari et al. [44] stated that a normal range of pH indicates normal and appropriate conditions in the rumen. In addition, Vet [50] and Sari et al. [44] observed a significant reduction in digestibility and ammonia concentration due to a gastrointestinal shift at low pH. To improve the rate of digestion during encapsulation, a suitable matrix is suggested to be used based on the resistance of rumen microorganisms and degradability during the fermentation process. It is well known that rumen microorganisms are good and sophisticated in degrading a wide range of raw materials during the fermentation process. Therefore, resistant starch or any resistance material is recommended to be used as a suitable matrix for encapsulation in rumens. Probiotics are known to improve the fermentation process by increasing the digestion rates and ammonia concentration. By adding probiotics, we did not observe any significant differences in nutrient digestibility and ammonia concentration between the treatments before encapsulation and the treatment after adding unencapsulated probiotics to the diet. Similar results were obtained by Sheikh et al. [51]. This indicates the ability of probiotics to improve digestion and improve ammonia concentration in ruminants.
Despite the significant reduction in the molar portion of acetic acid and the significant decrease in the ratio of acetic to propionic acid, there was a significant increase in propionic acid after encapsulation. Also, we observed that by combining encapsulated probiotics with encapsulated nitrate, there was a significant decrease in enteric methane emission, thereby decreasing the concentration of acetic acid, decreasing the ratio of acetic to propionic acid, and increasing propionic acid. Similar results were observed in previous studies [9,35]. It is known that both nitrate and probiotics reduce enteric methane emissions [20,52,53]. Therefore, the reduction in methane concentration is attributed to the shallow release of probiotics and nitrate after encapsulation. This could positively affect the availability of both probiotics and nitrate to scavenge hydrogen, thereby reducing enteric methane concentration. Methane production is associated with acetic acid production due to the high production of hydrogen ion, which is used by methanogenic for the formation of methane during the process of methanogenesis [54]. The reduction in the concentration of the molar portion of acetic acid and an increase in the concentration of propionic inhibit methane formation due to a decrease of hydrogen molecules. The reduction in enteric methane emissions could also occur due to the reduction of the ratio of acetic to propionic acid. We observed in this study that there was a significant reduction in acetic acid and the ratio of acetic to propionic acid, and there was a significant increase in propionic acid after encapsulation. This indicates the effects of the encapsulation process on reducing enteric methane emissions. In addition, a significant decrease in methane concentration was observed by encapsulating nitrate in the long term [55].
5 Conclusion
Despite the limitation on digestibility, encapsulation is an effective method for enhancing the rumen fermentation process by increasing the total gas production and TVFAs. Nevertheless, encapsulation indicates effectiveness on enteric methane emission, thereby reducing the molar portion of acetic acid and the ratio of acetic to propionic acid and increasing the molar portion of propionic acid. Moreover, combining encapsulated probiotics with encapsulated nitrate forms is an effective method for improving the fermentation process in the rumen and reducing enteric methane emission by reducing the molar portion of acetic acid and the ratio of acetic to propionic acid and increasing the molar portion of propionic acid. Probiotics are effective in improving the fermentation process, thereby stabilizing and maintaining normal conditions in the rumen. Therefore, in vivo, long-term practices of combining probiotics with nitrate are recommended to improve the effectiveness of encapsulation on enteric methane emission.
Acknowledgment
The authors are grateful to Prof. Dr. Yantyati Widyastuti for providing the probiotic isolates used in this study.
-
Funding information: This research was supported by RIIM-LPDP-BRIN (Nos B-803/II.7.5/FR/6/2022 and B-1373/III.5/PR.03.08/6/2022) of the Research Center for Applied Zoology-BRIN. The authors are also grateful to the Directorate General of Higher Education, Research and Technology, Ministry of Education, Culture, Research and Technology, Republic of Indonesia, for the financial support through the “Hibah Penelitian Fundamental” scheme, the year 2024 grant number 102/E5/PG.02.00.PL/2024.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. RR, NN, and AJ designed the experiments, and MA and RF carried them out. MA and SN prepared the manuscript with contributions from all co-authors.
-
Conflict of interest: Authors state is no conflict of interest.
-
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
[1] Broucek J. Production of methane emissions from ruminant husbandry: A review. J Env Prot. 2014;5:1482–93. 10.4236/jep.2014.515141.Suche in Google Scholar
[2] Takahashi J, Mwenya B, Santoso B, Sar C, Umetsu K, Kishimoto T, et al. Mitigation of methane emission and energy recycling in animal agricultural systems. Asian-Aust J Anim Sci. 1997;18:1199–208.10.5713/ajas.2005.1199Suche in Google Scholar
[3] Knapp JR, Laur GL, Vadas PA, Weiss WP, Tricarico JM. Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J Dairy Sci. 2014;97:3231–61. 10.3168/jds.2013-7234.Suche in Google Scholar PubMed
[4] Gruca-Rokosz R. Quantitative fluxes of the greenhouse gases CH4 and CO2 from the surfaces of selected Polish reservoirs. Atmosphere. 2020;11:1–15. 10.3390/atmos11030286.Suche in Google Scholar
[5] Grossi G, Goglio P, Vitali A, Williams AG. Livestock and climate change: Impact of livestock on climate and mitigation strategies. Anim Front. 2019;9:69–76. 10.1093/af/vfy034.Suche in Google Scholar PubMed PubMed Central
[6] Subepang S, Suzuki T, Phonbumrung T, Sommart K. Enteric methane emissions, energy partitioning, and energetic efficiency of zebu beef cattle fed total mixed ration silage. Asian-Australas J Anim Sci. 2019;32:548–55. 10.5713/ajas.18.0433.Suche in Google Scholar PubMed PubMed Central
[7] Palangi V, Taghizadeh A, Abachi S, Lackner M. Strategies to mitigate enteric methane emissions in ruminants: A review. Sustainability. 2022;14:1–15. 10.3390/su142013229.Suche in Google Scholar
[8] Palangi V, Lackner M. Management of enteric methane emissions in ruminants using feed additives: A review. Animals. 2022;12:1–15. 10.3390/ani12243452.Suche in Google Scholar PubMed PubMed Central
[9] Abdelbagi M, Ridwan R, Nahrowi N, Jayanegara A. The potential of nitrate supplementation for modulating the fermentation pattern and mitigating methane emission in ruminants: A meta-analysis from in vitro experiments. IOP Conf Ser Earth Env Sci. 2021;902:012023. 10.1088/1755-1315/902/1/012023.Suche in Google Scholar
[10] Abdelbagi M, Ridwan R, Fidriyanto R, Rohmatussolihat R, Nahrowi N, Jayanegara A. Effects of probiotics and encapsulated probiotics on enteric methane emission and nutrient digestibility in vitro. IOP Conf Ser Earth Env Sci. 2021;788:012050. 10.1088/1755-1315/788/1/012050.Suche in Google Scholar
[11] Sharifi M, Taghizadeh A, Hosseinkhani A, Palangi V, Macit M, Salem AZM, et al. Influence of nitrate supplementation on in vitro methane emission, milk production, ruminal fermentation, and microbial methanotrophs in dairy cows fed at two forage levels. Ann Anim Sci. 2022;22:1015–26. 10.2478/aoas-2021-0087.Suche in Google Scholar
[12] Sharifi M, Taghizadeh A, Hosseinkhani A, Mohammadzadeh H, Palangi V, Macit M, et al. Nitrate supplementation at two forage levels in dairy cows feeding: milk production and composition, fatty acid profiles, blood metabolites, ruminal fermentation, and hydrogen sink. Ann Anim Sci. 2022;22:711–22. 10.2478/aoas-2021-0044.Suche in Google Scholar
[13] Lee C, Beauchemin KA. Une revue de l’ajout de nitrate dans l’alimentation des ruminants: Toxicité aux nitrates, émissions de méthane et performance de production. Can J Anim Sci. 2014;94:557–70. 10.4141/CJAS-2014-069.Suche in Google Scholar
[14] Abdelbagi M, Ridwan R, Fitri A, Nahrowi N, Jayanegarac A. Performance, methane emission, nutrient utilization, and the nitrate toxicity of ruminants with dietary nitrate addition: A meta-analysis from in vivo trials. Trop Anim Sci J. 2023;46:74–84. 10.5398/tasj.2023.46.1.74.Suche in Google Scholar
[15] Chen M-J, Kreuter JY-TK. Nanoparticles and microparticles for drug and vaccine delivery. J Anat. 1996;189:503–5. 10.1002/bit.Suche in Google Scholar
[16] Araujo RC, Soltan YA, Morsy AS. Encapsulated nitrate and cashew nut shell liquid on blood and rumen constituents, methane emission, and growth performance of lambs. Aust J Exp Agric. 2006;46:813–20. 10.2527/jas2013-7084.Suche in Google Scholar
[17] Guyader J, Doreau M, Morgavi DP, Gérard C, Loncke C, Martin C. Long-term effect of linseed plus nitrate fed to dairy cows on enteric methane emission and nitrate and nitrite residuals in milk. Animal. 2016;10:1173–81. 10.1017/S1751731115002852.Suche in Google Scholar PubMed
[18] De Raphélis-soissan V, Nolan JV, Godwin IR, Newbold JR, Perdok HB, Hegarty RS. Paraffin-wax-coated nitrate salt inhibits short-term methane production in sheep and reduces the risk of nitrite toxicity. Anim Feed Sci Technol. 2017;229:57–64. 10.1016/j.anifeedsci.2017.04.026.Suche in Google Scholar
[19] Antonius A, Wiryawan KG, Thalib A, Jayanegara A. Digestibility and methane emission of ration based on oil palm by products supplemented with probiotics and banana stem: An in vitro study. Pak J Nutr. 2015;14:37–43. 10.3923/pjn.2015.37.43.Suche in Google Scholar
[20] Doyle N, Mbandlwa P, Kelly WJ, Attwood G, Li Y, Ross RP, et al. Use of lactic acid bacteria to reduce methane production in ruminants: A critical review. Front Microbiol. 2019;10:1–13. 10.3389/fmicb.2019.02207.Suche in Google Scholar PubMed PubMed Central
[21] Mehdi I. Review paper on the mitigation strategies to reduce methane emissions from large ruminants: Specific intention to the dairy and beef cattle’s. J Bio Innov. 2018;7:335–59.Suche in Google Scholar
[22] Sakthivel PC, Kamra DN, Agarwal N, Chaudhary LC. Effect of sodium nitrate and nitrate reducing bacteria on in vitro methane production and fermentation with buffalo rumen liquor. Asian-Aust J Anim Sci. 2012;25:812–7.10.5713/ajas.2011.11383Suche in Google Scholar
[23] Patra AK, Yu Z. Effective reduction of enteric methane production by a combination of nitrate and saponin without adverse effect on feed degradability, fermentation, or bacterial and archaeal communities of the rumen. Bioresour Technol. 2013;148:352–60. 10.1016/j.biortech.2013.08.140.Suche in Google Scholar
[24] Patra AK, Yu Z. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J Appl Microbiol. 2015;119:127–38. 10.1111/jam.12819.Suche in Google Scholar
[25] Mujica-Alvares J, Barra PA. Encapsulation of vitamins A and E as spray-dried. Molecules. 2020;25:1357.10.3390/molecules25061357Suche in Google Scholar
[26] El-Zaiat HM, Araujo RC, Soltan YA, Morsy AS, Louvandini H, Pires AV, et al. Encapsulated nitrate and cashew nut shell liquid on blood and rumen constituents, methane emission, and growth performance of lambs. J Anim Sci. 2014;92:2214–24. 10.2527/jas.2013-7084.Suche in Google Scholar
[27] Che Man YB, Irwandi J, Abdullah WJW. Effect of different types of maltodextrin and drying methods on physico-chemical and sensory properties of encapsulated durian flavour. J Sci Food Agric. 1999;79:1075–80. 10.1002/(SICI)1097-0010(199906)79:8<1075:AID-JSFA329>3.0.CO;2-Q.Suche in Google Scholar
[28] Ridwan R, Rusmana I, Widyastuti Y, Wiryawan KG, Prasetya B, Sakamoto M, et al. Fermentation characteristics and microbial diversity of tropical grass-legumes silages. Asian-Australas J Anim Sci. 2015;28:511–8. 10.5713/ajas.14.0622.Suche in Google Scholar
[29] McDougall EI. Saliva 1. studies in ruminant saliva. Biochem J. 1944;43:99–109.10.1042/bj0430099Suche in Google Scholar
[30] Theodorou MK, Williams B, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol. 1994;48:185–97. 10.5138/506.Suche in Google Scholar
[31] Orskov ER, Mcdonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 1979;92:499–503. 10.1017/S0021859600063048.Suche in Google Scholar
[32] Tilley JMA, Terry RA. A two‐stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963;18:104–11. 10.1111/j.1365-2494.1963.tb00335.x.Suche in Google Scholar
[33] Biswabandhu C, Radhakrishnan L. New approach for determination of volatile fatty acid in anaerobic digester sample. Environ Eng Sci. 2018;35:333–51. 10.1089/ees.2017.0190.Suche in Google Scholar
[34] Souza NKP, Detmann E, Valadares Filho SC, Costa VAC, Pina DS, Gomes DI, et al. Accuracy of the estimates of ammonia concentration in rumen fluid using different analytical methods. Arq Bras Med Vet Zootec. 2013;65:1752–8. 10.1590/S0102-09352013000600024.Suche in Google Scholar
[35] Gawad R, Fellner V. Evaluation of glycerol encapsulated with alginate and alginate-chitosan polymers in gut environment and its resistance to rumen microbial degradation. Asian-Australas J Anim Sci. 2019;32:72–81. 10.5713/ajas.18.0110.Suche in Google Scholar PubMed PubMed Central
[36] Miranda-romero LA, Tirado-gonzález DN, Tirado-estrada G, Améndola-massiotti R, Sandoval-gonzález L, Ramírez-valverde R, et al. Quantifying non-fibrous carbohydrates, acid detergent fiber and cellulose of forage through an in vitro gas production technique. J Sci Food Agric. 2020;100:3099–110. 10.1002/jsfa.10342.Suche in Google Scholar PubMed
[37] Adejoro FA, Hassen A, Thantsha MS. Characterization of starch and gum arabic-maltodextrin microparticles encapsulating acacia tannin extract and evaluation of their potential use in ruminant nutrition. Asian-Australas J Anim Sci. 2019;32:977–87. 10.5713/ajas.18.0632.Suche in Google Scholar PubMed PubMed Central
[38] Makled A, Khorshed M, Gouda G, El-Garhi M, Ebeid H, Azzaz H, et al. In vitro evaluation of encapsulated probiotic bacteria supplementation to ruminant rations. Arab Univ J Agric Sci. 2019;27:375–82. 10.21608/ajs.2019.43550.Suche in Google Scholar
[39] Jayanegara A, Harahap RP, Rozi RF, Nahrowi N. Effects of lipid extraction on nutritive composition of winged bean (Psophocarpus tetragonolobus), rubber seed (Hevea brasiliensis), and tropical almond (Terminalia catappa). Vet World. 2018;11:446–51. 10.14202/vetworld.2018.446-451.Suche in Google Scholar PubMed PubMed Central
[40] Rahman MM, Salleh MA, Sultana N, Kim MJ, Ra CS. Estimation of total volatile fatty acid (VFA) from total organic carbons (TOCs) assessment through in vitro fermentation of livestock feeds. Afr J Microbiol Res. 2013;7:1378–84. 10.5897/ajmr12.1694.Suche in Google Scholar
[41] Harahap RP, Setiawan D, Nahrowi N, Suharti S, Obitsu T, Jayanegara A. Enteric methane emissions and rumen fermentation profile treated by dietary chitosan: A meta-analysis of in vitro experiments. Trop Anim Sci J. 2020;43:233–9. 10.5398/tasj.2020.43.3.233.Suche in Google Scholar
[42] Jiao PX, Wei LY, Walker ND, Liu FZ, Chen LY, Beauchemin KA, et al. Comparison of non-encapsulated and encapsulated active dried yeast on ruminal pH and fermentation, and site and extent of feed digestion in beef heifers fed high-grain diets. Anim Feed Sci Technol. 2017;228:13–22. 10.1016/j.anifeedsci.2017.04.001.Suche in Google Scholar
[43] Ellis JL, Bannink A, Hindrichsen IK, Kinley RD, Pellikaan WF, Milora N, et al. The effect of lactic acid bacteria included as a probiotic or silage inoculant on in vitro rumen digestibility, total gas and methane production. Anim Feed Sci Technol. 2016;211:61–74. 10.1016/j.anifeedsci.2015.10.016.Suche in Google Scholar
[44] Sari NF, Ridwan R, Rohmatussolihat R, Fidriyanto R, Astuti WD, Widyastuti Y. The effect of probiotics on high fiber diet in rumen fermentation characteristics. IOP Conf Ser Earth Env Sci. 2019;251:012057. 10.1088/1755-1315/251/1/012057.Suche in Google Scholar
[45] Lund P, Dahl R, Yang HJ, Hellwing ALF, Cao BB, Weisbjerg MR. The acute effect of addition of nitrate on in vitro and in vivo methane emission in dairy cows. Anim Prod Sci. 2014;54:1432–5. 10.1071/AN14339.Suche in Google Scholar
[46] Lee C, Araujo RC, Koenig KM, Beauchemin KA. Effects of encapsulated nitrate on enteric methane production and nitrogen and energy utilization in beef heifers. J Anim Sci. 2015;93:2391–404. 10.2527/jas.2014-8845.Suche in Google Scholar PubMed
[47] Dijkstra J, Ellis JL, Kebreab E, Strathe AB, López S, France J, et al. Ruminal pH regulation and nutritional consequences of low pH. Anim Feed Sci Technol. 2012;172:22–33. 10.1016/j.anifeedsci.2011.12.005.Suche in Google Scholar
[48] Faniyi TO, Adegbeye MJ, Elghandour MMMY, Pilego AB, Salem AZM, Olaniyi TA, et al. Role of diverse fermentative factors towards microbial community shift in ruminants. J Appl Microbiol. 2019;127:2–11. 10.1111/jam.14212.Suche in Google Scholar PubMed
[49] Andrade-Montemayor H, García Gasca T, Kawas J. Ruminal fermentation modification of protein and carbohydrate by means of roasted and estimation of microbial protein synthesis. Rev Bras Zootec. 2009;38:277–91. 10.1590/s1516-35982009001300028.Suche in Google Scholar
[50] Vet AM. Rumen microorganisms and fermentation. Arch Med Vet. 2014;361:349–61.10.4067/S0301-732X2014000300003Suche in Google Scholar
[51] Sheikh GG, Ganai AM, Ishfaq A, Afzal Y, Ahmad HA. In vitro effect of probiotic mix and fibrolytic enzyme mixture on digestibility of paddy straw. Adv Anim Vet Sci. 2017;5:260–6. 10.17582/journal.aavs/2017/5.6.260.266.Suche in Google Scholar
[52] Božic AK, Anderson RC, Carstens GE, Ricke SC, Callaway TR, Yokoyama MT, et al. Effects of the methane-inhibitors nitrate, nitroethane, lauric acid, Lauricidin® and the Hawaiian marine algae Chaetoceros on ruminal fermentation in vitro. Bioresour Technol. 2009;100:4017–25. 10.1016/j.biortech.2008.12.061.Suche in Google Scholar PubMed
[53] Elanthamil R, Bandeswaran C. Methane emission from ruminants and its mitigating measures using probiotic – A review. Int J Sci Env. 2017;6:319–25.Suche in Google Scholar
[54] Togtokhbayar N, Cerrillo MA, Rodríguez GB, Elghandour MMMY, Salem AZM, Urankhaich C, et al. Effect of exogenous xylanase on rumen in vitro gas production and degradability of wheat straw. Anim Sci J. 2015;86:765–71. 10.1111/asj.12364.Suche in Google Scholar PubMed
[55] Granja-Salcedo YT, Fernandes RMI, De Araujo RC, Kishi LT, Berchielli TT, De Resende FD, et al. Long-term encapsulated nitrate supplementation modulates rumen microbial diversity and rumen fermentation to reduce methane emission in grazing steers. Front Microbiol. 2019;10:1–12. 10.3389/fmicb.2019.00614.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal
Artikel in diesem Heft
- Regular Articles
- Supplementation of P-solubilizing purple nonsulfur bacteria, Rhodopseudomonas palustris improved soil fertility, P nutrient, growth, and yield of Cucumis melo L.
- Yield gap variation in rice cultivation in Indonesia
- Effects of co-inoculation of indole-3-acetic acid- and ammonia-producing bacteria on plant growth and nutrition, soil elements, and the relationships of soil microbiomes with soil physicochemical parameters
- Impact of mulching and planting time on spring-wheat (Triticum aestivum) growth: A combined field experiment and empirical modeling approach
- Morphological diversity, correlation studies, and multiple-traits selection for yield and yield components of local cowpea varieties
- Participatory on-farm evaluation of new orange-fleshed sweetpotato varieties in Southern Ethiopia
- Yield performance and stability analysis of three cultivars of Gayo Arabica coffee across six different environments
- Biology of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different types of plants feeds: Potency as a pest on various agricultural plants
- Antidiabetic activity of methanolic extract of Hibiscus sabdariffa Linn. fruit in alloxan-induced Swiss albino diabetic mice
- Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance
- Nicotinamide as a biostimulant improves soybean growth and yield
- Farmer’s willingness to accept the sustainable zoning-based organic farming development plan: A lesson from Sleman District, Indonesia
- Uncovering hidden determinants of millennial farmers’ intentions in running conservation agriculture: An application of the Norm Activation Model
- Mediating role of leadership and group capital between human capital component and sustainability of horticultural agribusiness institutions in Indonesia
- Biochar technology to increase cassava crop productivity: A study of sustainable agriculture on degraded land
- Effect of struvite on the growth of green beans on Mars and Moon regolith simulants
- UrbanAgriKG: A knowledge graph on urban agriculture and its embeddings
- Provision of loans and credit by cocoa buyers under non-price competition: Cocoa beans market in Ghana
- Effectiveness of micro-dosing of lime on selected chemical properties of soil in Banja District, North West, Ethiopia
- Effect of weather, nitrogen fertilizer, and biostimulators on the root size and yield components of Hordeum vulgare
- Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp.
- Growth, yield, and secondary metabolite responses of three shallot cultivars at different watering intervals
- Design of drainage channel for effective use of land on fully mechanized sugarcane plantations: A case study at Bone Sugarcane Plantation
- Technical feasibility and economic benefit of combined shallot seedlings techniques in Indonesia
- Control of Meloidogyne javanica in banana by endophytic bacteria
- Comparison of important quality components of red-flesh kiwifruit (Actinidia chinensis) in different locations
- Efficiency of rice farming in flood-prone areas of East Java, Indonesia
- Comparative analysis of alpine agritourism in Trentino, Tyrol, and South Tyrol: Regional variations and prospects
- Detection of Fusarium spp. infection in potato (Solanum tuberosum L.) during postharvest storage through visible–near-infrared and shortwave–near-infrared reflectance spectroscopy
- Forage yield, seed, and forage qualitative traits evaluation by determining the optimal forage harvesting stage in dual-purpose cultivation in safflower varieties (Carthamus tinctorius L.)
- The influence of tourism on the development of urban space: Comparison in Hanoi, Danang, and Ho Chi Minh City
- Optimum intra-row spacing and clove size for the economical production of garlic (Allium sativum L.) in Northwestern Highlands of Ethiopia
- The role of organic rice farm income on farmer household welfare: Evidence from Yogyakarta, Indonesia
- Exploring innovative food in a developing country: Edible insects as a sustainable option
- Genotype by environment interaction and performance stability of common bean (Phaseolus vulgaris L.) cultivars grown in Dawuro zone, Southwestern Ethiopia
- Factors influencing green, environmentally-friendly consumer behaviour
- Factors affecting coffee farmers’ access to financial institutions: The case of Bandung Regency, Indonesia
- Morphological and yield trait-based evaluation and selection of chili (Capsicum annuum L.) genotypes suitable for both summer and winter seasons
- Sustainability analysis and decision-making strategy for swamp buffalo (Bubalus bubalis carabauesis) conservation in Jambi Province, Indonesia
- Understanding factors affecting rice purchasing decisions in Indonesia: Does rice brand matter?
- An implementation of an extended theory of planned behavior to investigate consumer behavior on hygiene sanitation-certified livestock food products
- Information technology adoption in Indonesia’s small-scale dairy farms
- Draft genome of a biological control agent against Bipolaris sorokiniana, the causal phytopathogen of spot blotch in wheat (Triticum turgidum L. subsp. durum): Bacillus inaquosorum TSO22
- Assessment of the recurrent mutagenesis efficacy of sesame crosses followed by isolation and evaluation of promising genetic resources for use in future breeding programs
- Fostering cocoa industry resilience: A collaborative approach to managing farm gate price fluctuations in West Sulawesi, Indonesia
- Field investigation of component failures for selected farm machinery used in small rice farming operations
- Near-infrared technology in agriculture: Rapid, simultaneous, and non-destructive determination of inner quality parameters on intact coffee beans
- The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni)
- Weather index-based agricultural insurance for flower farmers: Willingness to pay, sales, and profitability perspectives
- Meta-analysis of dietary Bacillus spp. on serum biochemical and antioxidant status and egg quality of laying hens
- Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera
- Determination of C-factor for conventional cultivation and soil conservation technique used in hop gardens
- Empowering farmers: Unveiling the economic impacts of contract farming on red chilli farmers’ income in Magelang District, Indonesia
- Evaluating salt tolerance in fodder crops: A field experiment in the dry land
- Labor productivity of lowland rice (Oryza sativa L.) farmers in Central Java Province, Indonesia
- Cropping systems and production assessment in southern Myanmar: Informing strategic interventions
- The effect of biostimulants and red mud on the growth and yield of shallots in post-unlicensed gold mining soil
- Effects of dietary Adansonia digitata L. (baobab) seed meal on growth performance and carcass characteristics of broiler chickens: A systematic review and meta-analysis
- Analysis and structural characterization of the vid-pisco market
- Pseudomonas fluorescens SP007s enhances defense responses against the soybean bacterial pustule caused by Xanthomonas axonopodis pv. glycines
- A brief investigation on the prospective of co-composted biochar as a fertilizer for Zucchini plants cultivated in arid sandy soil
- Supply chain efficiency of red chilies in the production center of Sleman Indonesia based on performance measurement system
- Investment development path for developed economies: Is agriculture different?
- Power relations among actors in laying hen business in Indonesia: A MACTOR analysis
- High-throughput digital imaging and detection of morpho-physiological traits in tomato plants under drought
- Converting compression ignition engine to dual-fuel (diesel + CNG) engine and experimentally investigating its performance and emissions
- Structuration, risk management, and institutional dynamics in resolving palm oil conflicts
- Spacing strategies for enhancing drought resilience and yield in maize agriculture
- Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types
- Investigating Spodoptera spp. diversity, percentage of attack, and control strategies in the West Java, Indonesia, corn cultivation
- Yield stability of biofertilizer treatments to soybean in the rainy season based on the GGE biplot
- Evaluating agricultural yield and economic implications of varied irrigation depths on maize yield in semi-arid environments, at Birfarm, Upper Blue Nile, Ethiopia
- Chemometrics for mapping the spatial nitrate distribution on the leaf lamina of fenugreek grown under varying nitrogenous fertilizer doses
- Pomegranate peel ethanolic extract: A promising natural antioxidant, antimicrobial agent, and novel approach to mitigate rancidity in used edible oils
- Transformative learning and engagement with organic farming: Lessons learned from Indonesia
- Tourism in rural areas as a broader concept: Some insights from the Portuguese reality
- Assessment enhancing drought tolerance in henna (Lawsonia inermis L.) ecotypes through sodium nitroprusside foliar application
- Edible insects: A survey about perceptions regarding possible beneficial health effects and safety concerns among adult citizens from Portugal and Romania
- Phenological stages analysis in peach trees using electronic nose
- Harvest date and salicylic acid impact on peanut (Arachis hypogaea L.) properties under different humidity conditions
- Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential
- Use of different vegetation indices for the evaluation of the kinetics of the cherry tomato (Solanum lycopersicum var. cerasiforme) growth based on multispectral images by UAV
- First evidence of microplastic pollution in mangrove sediments and its ingestion by coral reef fish: Case study in Biawak Island, Indonesia
- Physical and textural properties and sensory acceptability of wheat bread partially incorporated with unripe non-commercial banana cultivars
- Cereibacter sphaeroides ST16 and ST26 were used to solubilize insoluble P forms to improve P uptake, growth, and yield of rice in acidic and extreme saline soil
- Avocado peel by-product in cattle diets and supplementation with oregano oil and effects on production, carcass, and meat quality
- Optimizing inorganic blended fertilizer application for the maximum grain yield and profitability of bread wheat and food barley in Dawuro Zone, Southwest Ethiopia
- The acceptance of social media as a channel of communication and livestock information for sheep farmers
- Adaptation of rice farmers to aging in Thailand
- Combined use of improved maize hybrids and nitrogen application increases grain yield of maize, under natural Striga hermonthica infestation
- From aquatic to terrestrial: An examination of plant diversity and ecological shifts
- Statistical modelling of a tractor tractive performance during ploughing operation on a tropical Alfisol
- Participation in artisanal diamond mining and food security: A case study of Kasai Oriental in DR Congo
- Assessment and multi-scenario simulation of ecosystem service values in Southwest China’s mountainous and hilly region
- Analysis of agricultural emissions and economic growth in Europe in search of ecological balance
- Bacillus thuringiensis strains with high insecticidal activity against insect larvae of the orders Coleoptera and Lepidoptera
- Technical efficiency of sugarcane farming in East Java, Indonesia: A bootstrap data envelopment analysis
- Comparison between mycobiota diversity and fungi and mycotoxin contamination of maize and wheat
- Evaluation of cultivation technology package and corn variety based on agronomy characters and leaf green indices
- Exploring the association between the consumption of beverages, fast foods, sweets, fats, and oils and the risk of gastric and pancreatic cancers: Findings from case–control study
- Phytochemical composition and insecticidal activity of Acokanthera oblongifolia (Hochst.) Benth & Hook.f. ex B.D.Jacks. extract on life span and biological aspects of Spodoptera littoralis (Biosd.)
- Land use management solutions in response to climate change: Case study in the central coastal areas of Vietnam
- Evaluation of coffee pulp as a feed ingredient for ruminants: A meta-analysis
- Interannual variations of normalized difference vegetation index and potential evapotranspiration and their relationship in the Baghdad area
- Harnessing synthetic microbial communities with nitrogen-fixing activity to promote rice growth
- Agronomic and economic benefits of rice–sweetpotato rotation in lowland rice cropping systems in Uganda
- Response of potato tuber as an effect of the N-fertilizer and paclobutrazol application in medium altitude
- Bridging the gap: The role of geographic proximity in enhancing seed sustainability in Bandung District
- Evaluation of Abrams curve in agricultural sector using the NARDL approach
- Challenges and opportunities for young farmers in the implementation of the Rural Development Program 2014–2020 of the Republic of Croatia
- Yield stability of ten common bean (Phaseolus vulgaris L.) genotypes at different sowing dates in Lubumbashi, South-East of DR Congo
- Effects of encapsulation and combining probiotics with different nitrate forms on methane emission and in vitro rumen fermentation characteristics
- Phytochemical analysis of Bienertia sinuspersici extract and its antioxidant and antimicrobial activities
- Evaluation of relative drought tolerance of grapevines by leaf fluorescence parameters
- Yield assessment of new streak-resistant topcross maize hybrids in Benin
- Improvement of cocoa powder properties through ultrasonic- and microwave-assisted alkalization
- Potential of ecoenzymes made from nutmeg (Myristica fragrans) leaf and pulp waste as bioinsecticides for Periplaneta americana
- Analysis of farm performance to realize the sustainability of organic cabbage vegetable farming in Getasan Semarang, Indonesia
- Revealing the influences of organic amendment-derived dissolved organic matter on growth and nutrient accumulation in lettuce seedlings (Lactuca sativa L.)
- Identification of viruses infecting sweetpotato (Ipomoea batatas Lam.) in Benin
- Assessing the soil physical and chemical properties of long-term pomelo orchard based on tree growth
- Investigating access and use of digital tools for agriculture among rural farmers: A case study of Nkomazi Municipality, South Africa
- Does sex influence the impact of dietary vitD3 and UVB light on performance parameters and welfare indicators of broilers?
- Design of intelligent sprayer control for an autonomous farming drone using a multiclass support vector machine
- Deciphering salt-responsive NB-ARC genes in rice transcriptomic data: A bioinformatics approach with gene expression validation
- Review Articles
- Impact of nematode infestation in livestock production and the role of natural feed additives – A review
- Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: A review
- Climate change and adaptive strategies on viticulture (Vitis spp.)
- The false tiger of almond, Monosteira unicostata (Hemiptera: Tingidae): Biology, ecology, and control methods
- A systematic review on potential analogy of phytobiomass and soil carbon evaluation methods: Ethiopia insights
- A review of storage temperature and relative humidity effects on shelf life and quality of mango (Mangifera indica L.) fruit and implications for nutrition insecurity in Ethiopia
- Green extraction of nutmeg (Myristica fragrans) phytochemicals: Prospective strategies and roadblocks
- Potential influence of nitrogen fertilizer rates on yield and yield components of carrot (Dacus carota L.) in Ethiopia: Systematic review
- Corn silk: A promising source of antimicrobial compounds for health and wellness
- State and contours of research on roselle (Hibiscus sabdariffa L.) in Africa
- The potential of phosphorus-solubilizing purple nonsulfur bacteria in agriculture: Present and future perspectives
- Minor millets: Processing techniques and their nutritional and health benefits
- Meta-analysis of reproductive performance of improved dairy cattle under Ethiopian environmental conditions
- Review on enhancing the efficiency of fertilizer utilization: Strategies for optimal nutrient management
- The nutritional, phytochemical composition, and utilisation of different parts of maize: A comparative analysis
- Motivations for farmers’ participation in agri-environmental scheme in the EU, literature review
- Evolution of climate-smart agriculture research: A science mapping exploration and network analysis
- Short Communications
- Music enrichment improves the behavior and leukocyte profile of dairy cattle
- Effect of pruning height and organic fertilization on the morphological and productive characteristics of Moringa oleifera Lam. in the Peruvian dry tropics
- Corrigendum
- Corrigendum to “Bioinformatics investigation of the effect of volatile and non-volatile compounds of rhizobacteria in inhibiting late embryogenesis abundant protein that induces drought tolerance”
- Corrigendum to “Composition and quality of winter annual agrestal and ruderal herbages of two different land-use types”
- Special issue: Smart Agriculture System for Sustainable Development: Methods and Practices
- Construction of a sustainable model to predict the moisture content of porang powder (Amorphophallus oncophyllus) based on pointed-scan visible near-infrared spectroscopy
- FruitVision: A deep learning based automatic fruit grading system
- Energy harvesting and ANFIS modeling of a PVDF/GO-ZNO piezoelectric nanogenerator on a UAV
- Effects of stress hormones on digestibility and performance in cattle: A review
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part II
- Assessment of omega-3 and omega-6 fatty acid profiles and ratio of omega-6/omega-3 of white eggs produced by laying hens fed diets enriched with omega-3 rich vegetable oil
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part II
- Special Issue on FCEM – International Web Conference on Food Choice & Eating Motivation: Message from the editor
- Fruit and vegetable consumption: Study involving Portuguese and French consumers
- Knowledge about consumption of milk: Study involving consumers from two European Countries – France and Portugal

