Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
-
Poonam Dwivedi
, Indu Jatrana
, Azmat Ali Khan
Abstract
This article reports a simple, cost-effective, and eco-friendly biosynthesis of ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves’ water extract. For the first time, we used a green synthetic route via C. viminalis leaves’ extract to prepare ZnO/Fe3O4 nanocomposites (NCs) using zinc acetate and ferric chloride as precursor materials. Fourier transform infrared (FTIR) spectroscopic results revealed polyphenolic compounds mainly phenolic acids present in the plant extract acted as both reducing and stabilizing agents to synthesize ZnO/Fe3O4 NCs. Outcomes of XRD and X-ray photoelectron spectroscopy confirmed the formation of ZnO–Fe3O4 heterojunction in ZnO/Fe3O4 NCs, with crystallite sizes of 45, 35, and 60 nm, respectively, according to Debye–Scherrer’s formula. EDX confirmed Zn, Fe, and O in the ZnO/Fe3O4 nanocomposite. Scanning electron microscopy and transmission electron microscopy (TEM) analyses revealed the existence of both ZnO and Fe3O4 in the NCs with some agglomeration. The thermal stability of NCs was evaluated using thermogravimetric analysis (TGA) and differential thermal analysis (DTA) in a nitrogen atmosphere. In addition, as-prepared ZnO/Fe3O4 NCs along with biosynthesized ZnO and Fe3O4 (prepared by C. viminalis extract) nanoparticles were examined for photodegradation of methylene blue under visible light irradiation for 150 min. The result reveals that the photodegradation efficiency of ZnO/Fe3O4 NCs (99.09%) was higher compared to that of monometallic ZnO (84.7%) and Fe3O4 (37.1%) nanoparticles.
1 Introduction
Industrialization and urbanization have increased water pollution to a great extent because of the direct disposal of organic and industrial waste into water bodies [1]. Among all, dying industries produce an enormous amount of wastewater containing unused dye along with other chemicals [2]. It reported that the majority of dyes are toxic and nonbiodegradable [3]. Hence, the dye effluent contaminates surface and underground water, which brings out adverse effects on flora and fauna [4,5]. Methylene blue (MB) is the most popular thiazine dye used in textile industries. The prolonged exposure to MB results in harmful effects, such as cyanosis, skin irritation, and gastrointestinal irritation, in living beings [6]. To solve this challenge, many physical and chemical techniques including flocculation–coagulation, surface adsorption, ion-exchange, chemical precipitation, and photocatalytic degradation have been used to remove the dye from waste water [7,8]. Because of the simple experimental procedure and decomposition of organic dye molecules into nontoxic simple products in the presence of semiconductors under proper light irradiation, the photodegradation process proves its superiority over other methods [9,10]. For semiconductor-assisted photocatalytic process, various materials, such as ZnO, CuO, TiO2, and more, have been widely used as photocatalysts previously [11,12,13]. Among different semiconductor materials, ZnO is a nontoxic, easily available, and cost-effective material with a bandgap of 3.2 and 60 MeV exciton binding energy [14]. That is why ZnO became the priority to many researchers working in the photocatalytic degradation of dyes using semiconductors. But ZnO semiconductor has a rapid tendency of recombination of photo-induced electron–hole pairs, which makes it difficult to gain the practical demand [15]. To overcome this problem, many metallic or nonmetallic materials, such as CdS, Ag2O, CuO, and g-C3N4, have doped with ZnO and showed improved efficiency toward photodegradation process [16,17,18,19].
However, the removal of nanocomposites (NCs) from the treated solution is very tedious and expensive, which is still a challenge. In this concern, magnetite nanoparticles (Fe3O4 NPs) play an effective role as they are easily separable from the solution by applying an external magnetic field [20]. Apart from this, doping of metallic NPs with magnetite NPs improves their functionality and recyclability and in turn cost effectiveness of the process [21,22]. Inspired by this, many researchers have synthesized and investigated the results of magnetite NCs [23]. After reviewing the literature, we observed that various approaches, such as sol–gel, hydrothermal synthesis, precipitation, and microemulsion, have been adopted for preparing magnetite composites [24,25,26,27]. But many of these methods have certain demerits, such as the use of expensive and hazardous chemicals and the formation of toxic byproducts, which makes it difficult to achieve the requirement of green synthesis. In recent decades, the use of bioproducts (including biomolecules, bacteria, fungi, or plant extracts) for the synthesis of NPs has attracted researchers as well [28]. Metals and metal oxide nanoparticles have also been used as homogeneous or heterogeneous nanocatalysts in various organic syntheses due to the large surface-to-volume ratio of nanoparticles compared to bulk materials [29,30]. This strategy provides a simple, cost-effective, and eco-friendly route for the fabrication of nanomaterials [31]. Although synthesis of ZnO/Fe3O4 NCs by different routes has been reported [32,33], a few reports on a green synthesis of ZnO/Fe3O4 NCs are available [34,35]. In our present study, we have synthesized ZnO/Fe3O4 NCs using Callistemon viminalis leaves’ extract as a green reducing and stabilizing agent. C. viminalis is a small tree that belongs to the family Myrtaceae with a characteristic brushlike flowers. It is a traditional medicine to treat hemorrhoids, gastroenteritis, diarrhea, and skin infection [36,37,38]. The phytochemical study reported that C. viminalis is rich in biomolecules, including viminadiones, quercetin, and betulinic acid, and can act as a green reducing and capping agent during the fabrication of nanomaterials.
After reviewing the literature, we understand that C. viminalis leaves’ extract-mediated biomimetic synthesis of ZnO/Fe3O4 has not been reported till date. In this study, we have attempted to understand the role of biomolecules present in leaves’ extract as a reducing and stabilizing agent for the fabrication of ZnO/Fe3O4 NCs. The novelty of this study is to show the efficacy of biosynthesized ZnO/Fe3O4 NCs as a photocatalyst in contrast to ZnO or Fe3O4 NPs for the degradation of MB solution under visible light irradiation.
2 Experimental methodology
2.1 Chemicals
All chemicals applied in this research study, including zinc acetate dihydrate (Thermo Fisher Scientific India Pvt. Ltd.), ferric chloride (Thermo Fisher Scientific India Pvt. Ltd.), sodium hydroxide (Merck Life Science India Pvt. Ltd.), and methylene blue (Merck Life Science India Pvt. Ltd.), were of analytical grade and used as received commercially without further purification. Deionised water was used throughout the experiment wherever required.
2.2 Preparation of C. viminalis leaves’ extract
Leaves of C. viminalis were collected from Jaipur National University campus, India. The collected leaves were thoroughly washed under tap water and finally washed using deionised water. After drying in shade, the leaves were powdered in an electrical grinder. About 25 g of the powdered leaves in 100 mL deionised water was refluxed in a Soxhlet apparatus (Sigma-Aldrich, India) for 2 h at 80°C on a magnetic stirrer. On cooling, the suspension was filtered through Whatmann’s filter paper, and the filtrate was collected as leaves’ extract and stored in a refrigerator at 2°C for further studies.
2.3 Biosynthesis of ZnO/Fe3O4 NCs
The ZnO/Fe3O4 NCs were prepared through an eco-friendly green route, and the synthesis procedure is briefly illustrated as follows: 30 mL of C. viminalis leaves’ extract was added slowly in a round-bottom flask containing 30 mL of zinc acetate solution (0.01 M) under stirring. After 10 min, 0.16 g of ferric chloride in 10 mL deionised water was introduced dropwise into the flask and heated to 60°C, followed by the addition of NaOH (0.1 M) to maintain pH 10. The color of the solution changes from black to blackish brown after 1 h stirring, which indicated the formation of ZnO/Fe3O4 NCs. Afterward, the solution was cooled to room temperature, centrifuged, collected in a China dish, washed several times with ethanol to remove unused extract and NaOH, dried in an oven at 80°C, and finally calcined at 300°C before storing for further studies. For comparison, monometallic nanoparticles (ZnO and Fe3O4) had also been synthesized using C. viminalis leaves’ extract. A schematic of aforementioned green synthesis is shown in Figure 1.
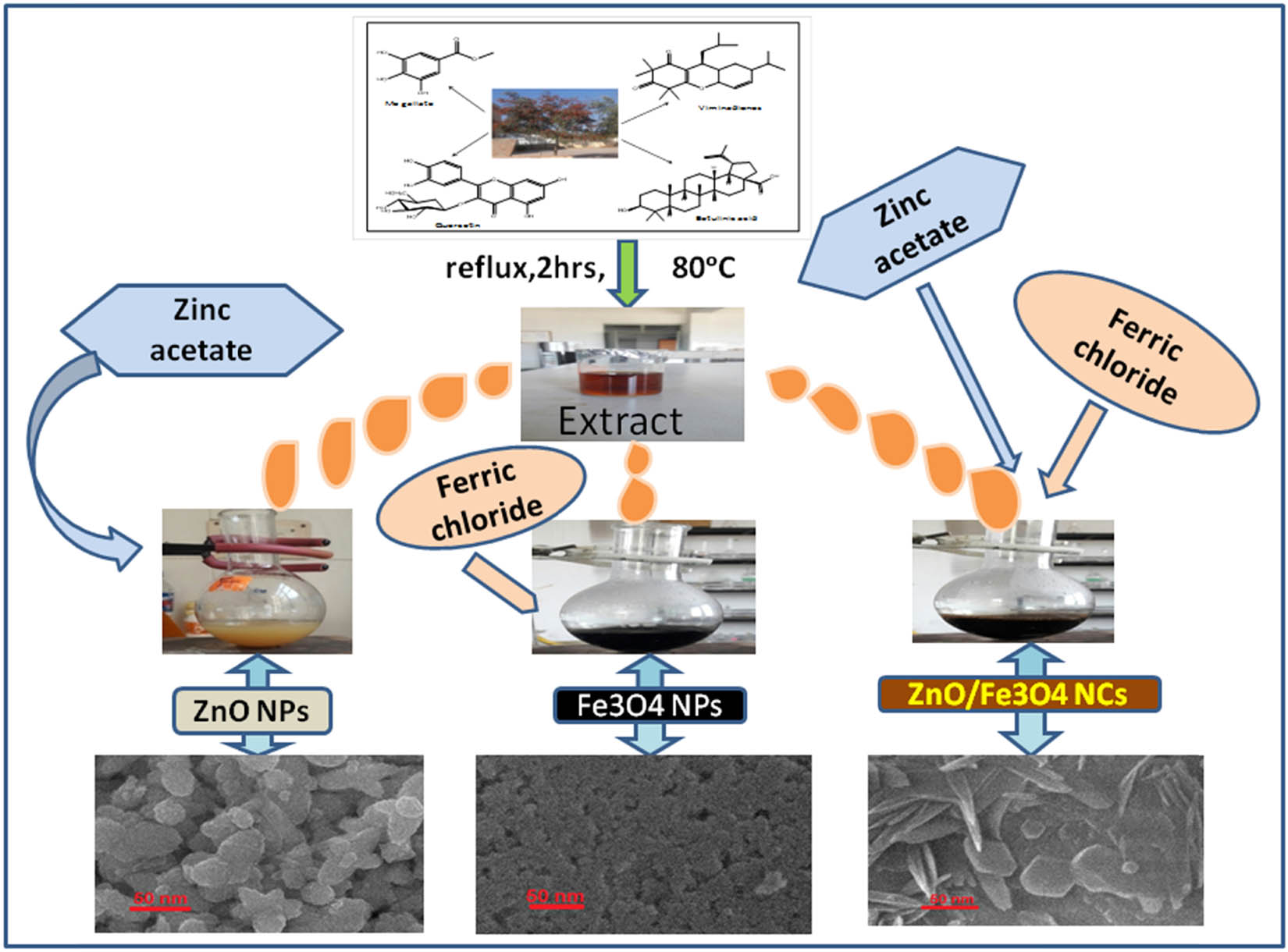
Schematic of green synthesis of ZnO NPs, Fe3O4 (NPs), and ZnO/Fe3O4 NCs.
2.4 Characterization
FTIR spectral analysis in the range of 4,000–400 cm−1 was carried out to investigate the role of C. viminalis leaf extract in the fabrication of ZnO, Fe3O4, and ZnO/Fe3O4 nanoproducts using a PerkinElmer spectrophotometer (MNIT, Jaipur). A powder X-ray diffraction technique was performed to determine the crystallinity and particle size of biosynthesized product by PAN analytical (XPART PRO) diffractometer in the scattering range (2θ) of 20–80° using Cu Kα radiation (λ = 1.5406 Å). The surface morphology of green synthesized samples was determined by scanning electron microscopy (SEM) using Nova Nano SEM 450 (MNIT, Jaipur) and transmission electron microscopy (TEM) at IIT Roorkee. The elemental composition was investigated using EDX analysis. Chemical states of elements present in ZnO/Fe3O4 NCs were analyzed by X-ray photoelectron spectroscopic technique (XPS, PHI 5000 Versa Probe III, IIT Roorkee). The thermal stability of ZnO/Fe3O4 sample was recorded in a nitrogen atmosphere at a heating rate of 5°C min−1 by TGA/DTA analyzer (EXSTAR TG/DTA 6300, IIT Roorkee).
2.5 Designing of the photocatalytic activity experiment
The photocatalytic efficiency of biosynthesized ZnO, Fe3O4, and ZnO/Fe3O4 nanomaterials for the degradation of MB dye was evaluated under visible light at pH 7. For the photodegradation study, three experimental sets were prepared. Each set comprised seven beakers (100 mL) with 25 mL solution of MB (32 mg L−1) in each. The dose of biosynthesized ZnO, Fe3O4, or ZnO/Fe3O4 nanoproducts taken was 0.004 g in each beaker. After certain intervals of time (15, 30, 45, 60, 75, 90, and 150 min), one beaker from each set was removed from irradiation, and dye solutions were centrifuged at 8,000 rpm followed by filtration to remove the photocatalyst. MB degradation was examined by measuring the absorbance of the dye solution at λ max = 665 nm using a UV-Vis spectrophotometer. The percentage of MB degradation was determined by the following equation:
In equation (1), η is the degradation percentage and A 0 and A t are the absorbances of MB dye solution at t = 0 and after time t, respectively.
3 Results and discussion
3.1 FTIR analysis
The involvement of biomolecules present in C. viminalis leaves’ extract, for the fabrication of nanomaterials, was screened by FTIR spectroscopic analysis. Figure 2 depicts the FTIR spectra of C. viminalis leaves’ extract as well as biosynthesized ZnO, Fe3O4, and ZnO/Fe3O4 nanomaterials. The FTIR spectrum of C. viminalis leaves’ extract (Figure 2a) showed some major absorption bands at 3,419, 2921.76, 1718.16, 1451.86, 1368.87, and 1180.95 cm−1 were assigned to O–H stretching of phenolic acids and phenols, C–H stretching in CH3 and CH2, C═O groups in phenolic acids and flavonoids, C═C stretching of the aromatic ring, and C–H deformation in CH3 and C–OH stretching in phenolic acids, respectively, as reported in various literature [39,40,41].
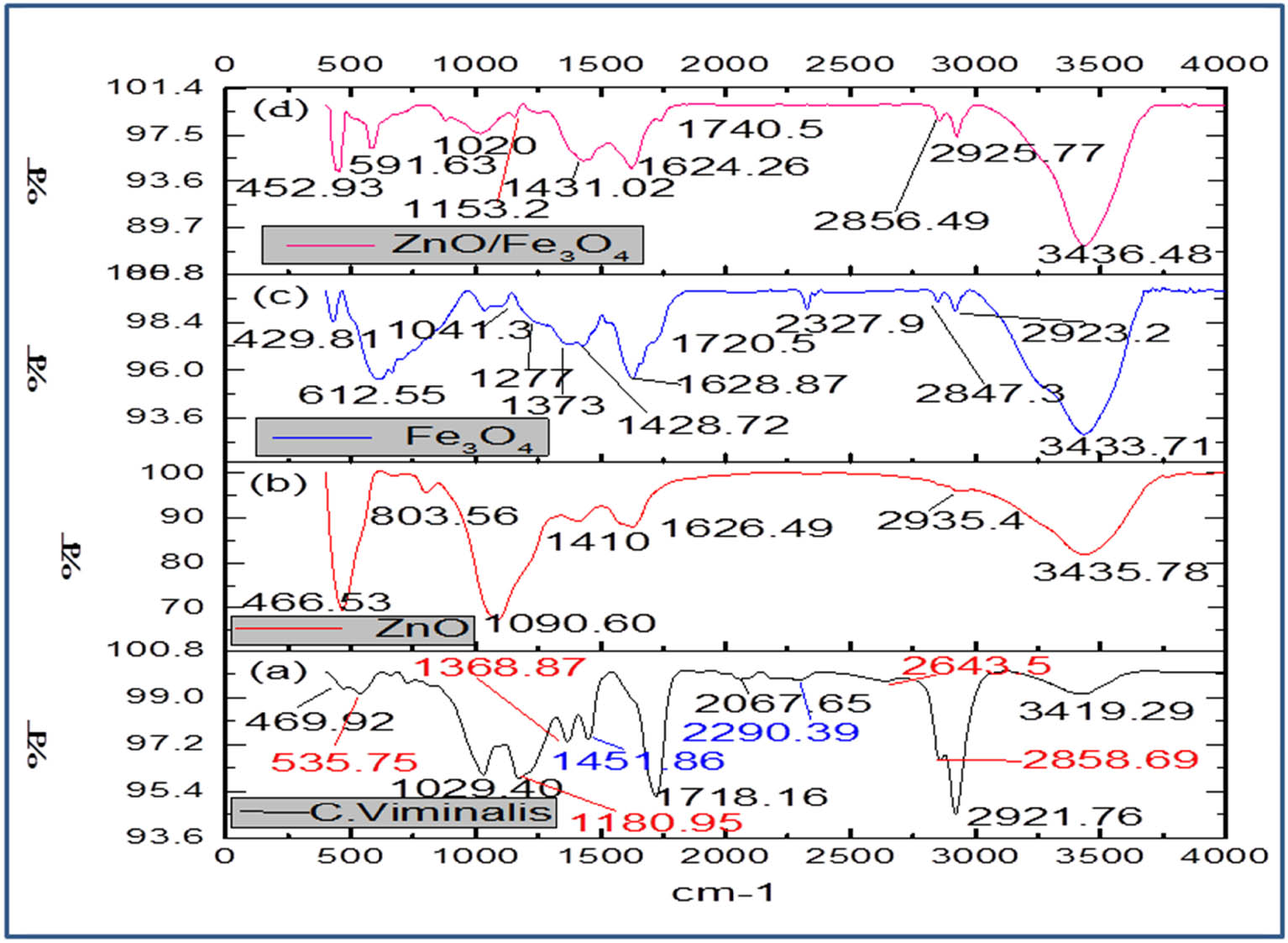
FTIR spectra of (a) C. viminalis leaf extract, (b) ZnO, (c) Fe3O4, and (d) ZnO/Fe3O4 NCs.
However, after the reduction of metal precursors into their respective metal nanoparticles, a remarkable difference in intensity, position, and shape of absorption peaks had been observed, which showed the participation of biomolecules (present in leaves’ extract) in the reduction and capping of nanomaterials. The additional peaks in the spectra of monometallic NPs at 466.53 cm−1 (Figure 2(b)) and 612 cm−1 (Figure 2(c)) were allocated to Zn–O and Fe–O stretching vibrations, respectively, confirming the formation of ZnO and Fe3O4 NPs [42]. Moreover, shifting in absorption peak values of Zn–O (452.93 cm−1) and Fe–O (591.63 cm−1) towards lower wave numbers in ZnO/Fe3O4 (Figure 2(d)) indicated the formation of bimetallic nanocomposite. Furthermore, shrinkage/shifting of peaks corresponding to C═O, C═C, and C–OH groups (1718.16, 1451.86, and 1180.95 cm−1) in the spectra of nanoproducts suggests that polyphenolic compounds mainly phenolic acids are responsible for the bioreduction of metal ions and capping of as-prepared nanoproducts.
3.1.1 Mechanism of biosynthesis
On the basis of FTIR results, a possible mechanism for the C. viminalis leaves’ extract-mediated synthesis of ZnO, Fe3O4, and ZnO/Fe3O4 has been proposed.
In short, betulinic acid present in leaves’ extract undergoes oxidation according to the free radical mechanism, that is, betulinic acid to dehydro betulinic acid (Scheme 1). Zn+2/Fe+3 ions (present in solution) form complex with dehydro betulinic acid via transfer of electrons from anionic dehydro betulinic acid to metal ions. On calcination, the resulting complex is converted into respective metal oxide nanoparticles because of the capping effect of biomolecules [43].
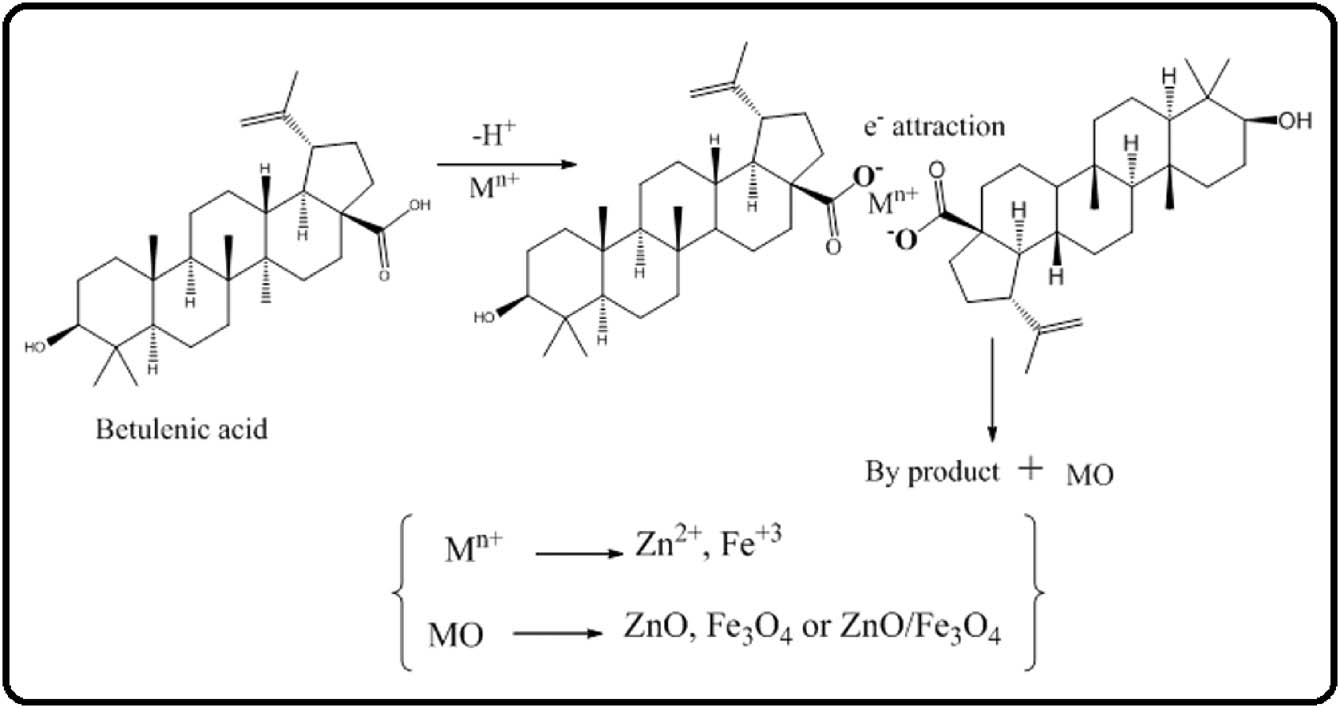
Proposed mechanism for the biosynthesis of ZnO, Fe3O4, and ZnO/Fe3O4 NCs.
3.2 XRD analysis
The phase and crystal structure of biosynthesized ZnO, Fe3O4, and ZnO/Fe3O4 nanomaterials were examined by powder X-ray diffraction analysis. Figure 3 depicts the X-ray patterns of ZnO (a), Fe3O4 (b), and ZnO/Fe3O4 (c). In Figure 3(a), XRD peaks at 2θ values = 32.03°, 34.73°, 36.66°, 48.03°, 57.05°, 68.32°, and 69.49° were indexed to the respective (100), (002), (101), (102), (110), (112), and (201) crystalline planes of hexagonal wurtzite phase of ZnO (JCPDS Card No. 36-1451), whereas in Figure 3(b), diffraction peaks at 2θ values = 30.16°, 35.29°, 43.12°, 56.83°, and 62.59° correspond to the miller indices (220), (311), (400), (511), and (440), respectively (JCPDS Card No. 19-0629), which confirm the face-centered cubic structure of Fe3O4 [44]. For ZnO/Fe3O4 NCs (Figure 3c), peaks at 32.56°, 34.87°, 36.07°, 57.28°, 68.92°, and 69.58° corresponding to (100), (002), (101), (110), (112), and (201) planes infer the presence of ZnO in hexagonal wurtzite phase, whereas peaks at 2θ = 30.10°, 35.65°, 43.15°, and 62.86° represent (220), (311), (400), and (440) planes of face-centered cubic structure of iron. The identification of a dual phase with shifting of the respective peak values in the XRD pattern of NCs indicates the formation of Zn–Fe heterojunction. Moreover, the crystallite size (D) of as-prepared nanoproducts is calculated using Debye–Scherrer’s formula (D = kλ/β cos θ) and at maximum intense peaks, the size of ZnO, Fe3O4, and ZnO/Fe3O4 is found to be 45, 35, and 60 nm, respectively.
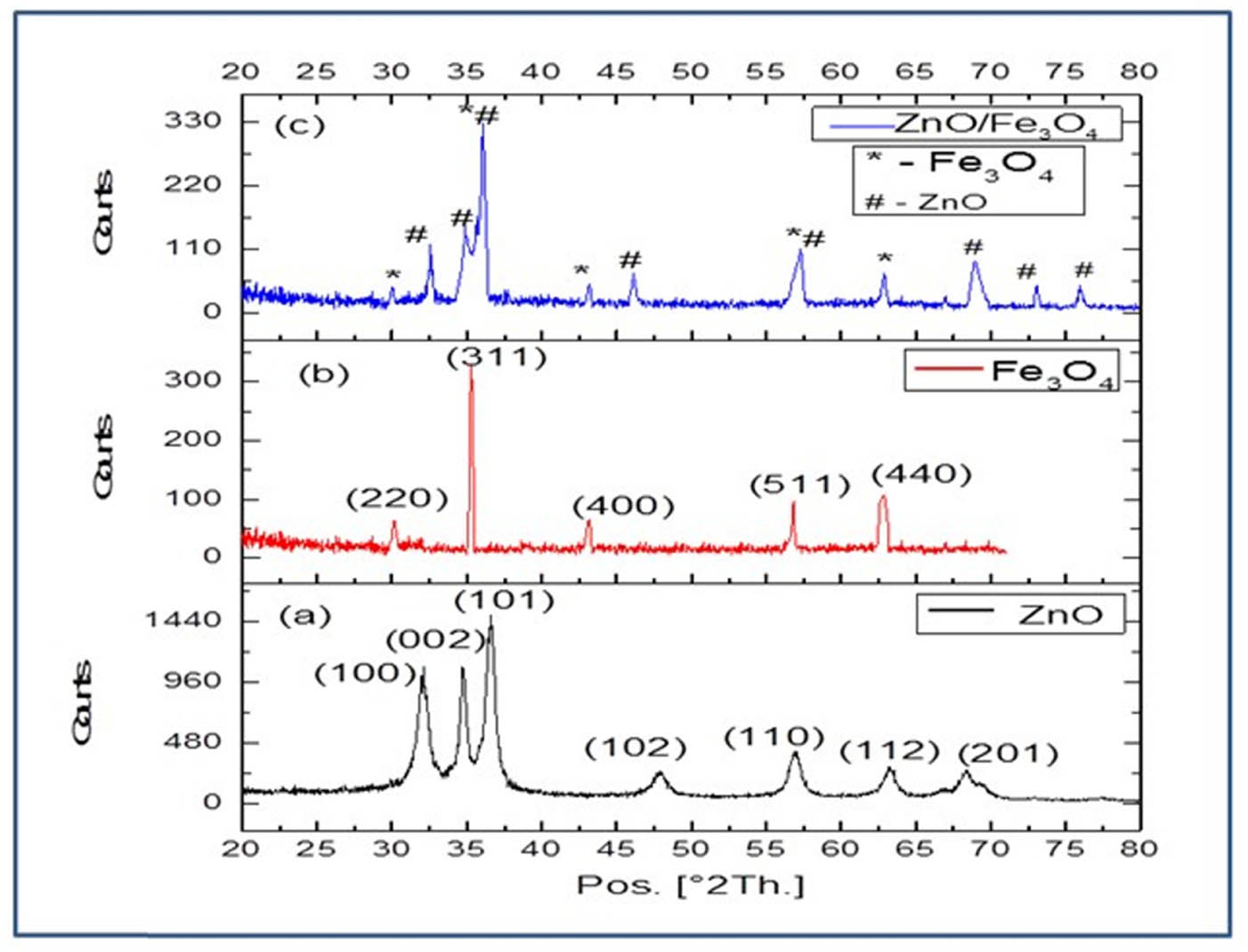
Powder XRD pattern of biosynthesized NPs: (a) ZnO, (b) Fe3O4, and (c) ZnO/Fe3O4 NCs.
3.3 SEM and TEM analysis
Morphology and nanoscale of the biosynthesized samples were analyzed using SEM and TEM. An overview of SEM images of Zn, Fe3O4, and ZnO/Fe3O4 samples is shown in Figure 4(a–c). As observed from images, the morphology of ZnO/Fe3O4 differs from ZnO or Fe3O4, which suggested the formation of ZnO/Fe3O4 NCs. Furthermore, in the SEM image of ZnO/Fe3O4, rod-shaped particles of ZnO and cubic-shaped structures of Fe3O4 are clearly visible, which interprets the interaction of Fe3O4 with ZnO. Morphological differences among as-prepared samples were also identified by TEM micrographs (Figure 4(d–f)). The spherical shape of ZnO NPs with a particle size of ∼45 nm can be seen in Figure 4(d), whereas Fe3O4 NPs have an irregular shape (Figure 4(e)) with a particle size of ∼35 nm. The coexistence of ZnO and Fe3O4 can be clearly identified in the TEM micrograph of NCs (Figure 4(f)), wherein Fe3O4 can identify as dark particles with some agglomeration because of its highly magnetic nature and ZnO as bright particles surrounding Fe3O4. Moreover, selected area electron diffraction (SAED) image of ZnO/Fe3O4 NCs (Figure 4(g)) also interprets the incorporation of Fe3O4 in the crystalline lattice of ZnO NPs, which resulted in a comparatively less crystalline and large-sized ZnO/Fe3O4 NCs. Thus, the results obtained from SEM and TEM analyses were in close agreement with XRD results.
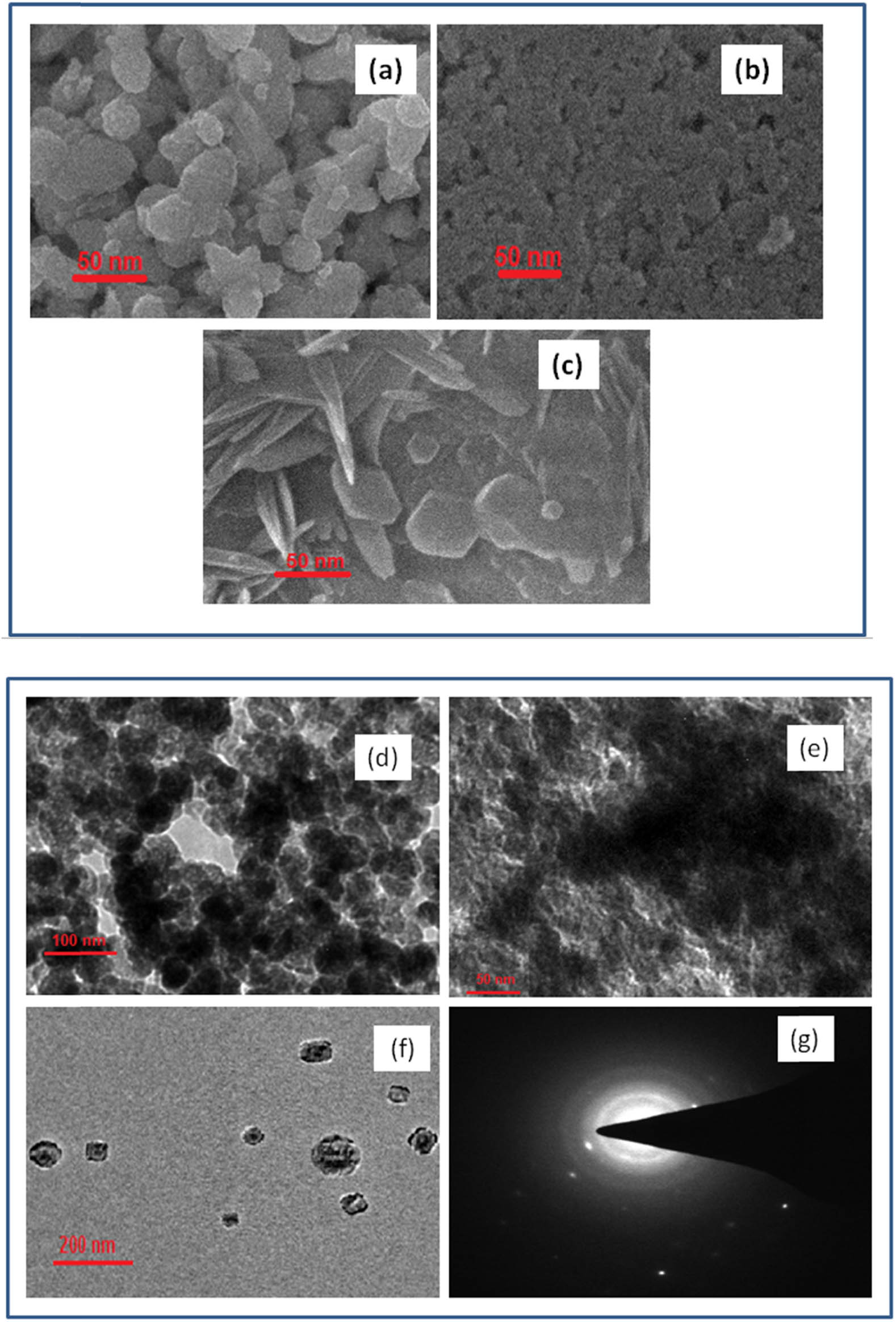
SEM images: (a): ZnO, (b) Fe3O4, and (c) ZnO/Fe3O4 NCs. TEM images: (d) ZnO, (e) Fe3O4, (f) ZnO/Fe3O4 NCs, and (g) SAED pattern of ZnO/Fe3O4 NCs.
3.4 EDX analysis
To determine the chemical composition of ZnO, Fe3O4, and ZnO/Fe3O4 nanoproducts, EDX analysis was carried out and results are displayed in Figure 5(a–c). It can be seen in Figure 5(a) that the EDX spectrum consists of strong peaks for Zn and O, whereas Figure 5(b) shows Fe and O elemental peaks. In case of ZnO/Fe3O4 NCs (Figure 5(c)), strong signals for Zn, Fe, and O elements were well recognized, which further confirmed the coexistence of ZnO and Fe3O4. The appearance of carbon in all three spectra may be due to biomolecular capping on the surface of nanoproducts [45]. Based on EDX outcomes, the weight percentage of elements in ZnO/Fe3O4 NCs was 32.56, 38.67, and 28.77% for Zn, Fe, and O, respectively.
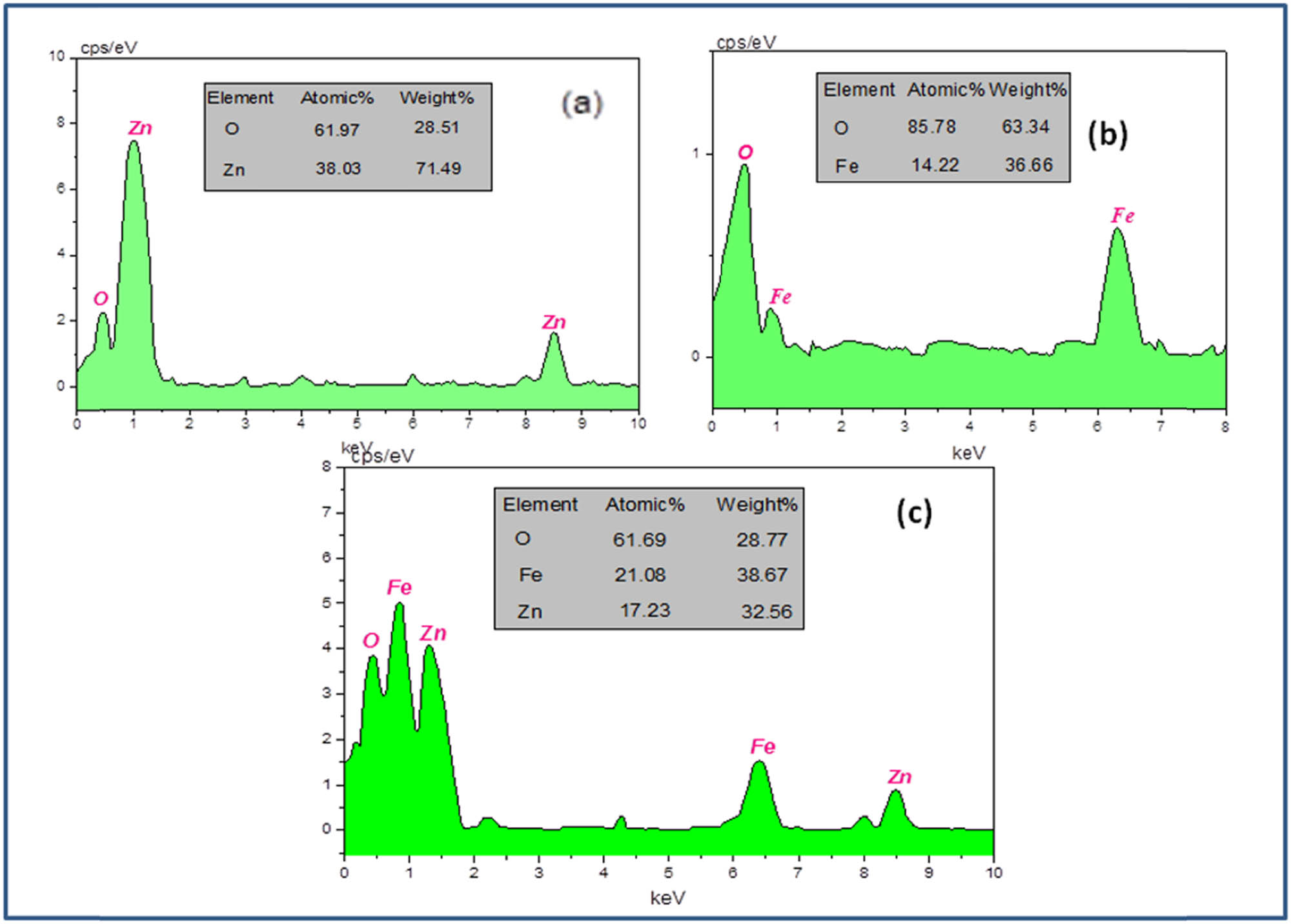
EDX spectrum of (a) ZnO, (b) Fe3O4, and (c) ZnO/Fe3O4 NCs.
3.5 XPS analysis
XPS analysis was carried out to demonstrate the chemical nature of the surface of biosynthesized ZnO/Fe3O4 NCs. Figure 6(a) depicts the full scan spectrum of ZnO/Fe3O4 and the appearance of major peaks at binding energies of 1,021 eV (Zn2p), 725 eV (Fe2p), 530 eV (O1s), and 284 eV (C1s) confirms the fabrication of ZnO/Fe3O4 NCs. High-resolution XPS spectra of Fe2p, Zn2p, O1s, and C1s are shown in Figure 6(b–e). In Figure 6(b), a doublet for Fe2p at 711.94 and 726.93 eV was assigned to respective binding energies of Fe2p3/2 and Fe2p1/2 of Fe3O4 [46,47]. In the Zn2p spectra (Figure 6(c)), the spin-orbit doublet Zn2p3/2 and Zn2p1/2 peaks were centered at binding energies of 1021.7 and 1044.65 eV, respectively [48]. It is evident from the literature that Zn2p3/2 and Zn2p1/2 peaks are separated by 23 eV in pure ZnO. From the XPS results, peaks of Zn2p in NCs were separated by 21 eV, which strongly manifests the synergistic effect between ZnO and Fe3O4 NPs, contributing to the enhancement in photocatalytic activity of NCs [49]. XPS spectrum of C1s (Figure 6(d)), consists of three peaks at 283 eV (C–C) [50], 284 eV (C═C) [46,51], and 285 eV (C═O) [52], attributed to the polyphenolic compounds of leaves’ extract acting as a stabilizing agent for ZnO/Fe3O4 NCs [53]. In the O1s spectrum shown in Figure 6(e), the peak position at 529.89 eV was assigned to oxygen in Fe–O of ZnO/Fe3O4, corresponding to ferro–ferric oxide. The deconvolution of O1s signals revealed the presence of Zn–O of ZnO at a binding energy of 530.89 eV. The peak at 531.89 eV may be due to the hydroxyl group of biomolecular capping and oxygen chemisorbed onto the surface of ZnO/Fe3O4 NCs [54]. Under visible light irradiation, these active oxygen species on the surface of NCs may contribute to MB degradation via oxygen ions, such as O−1 and O−2, which in turn improves the photocatalytic activity of ZnO/Fe3O4 NCs [55].
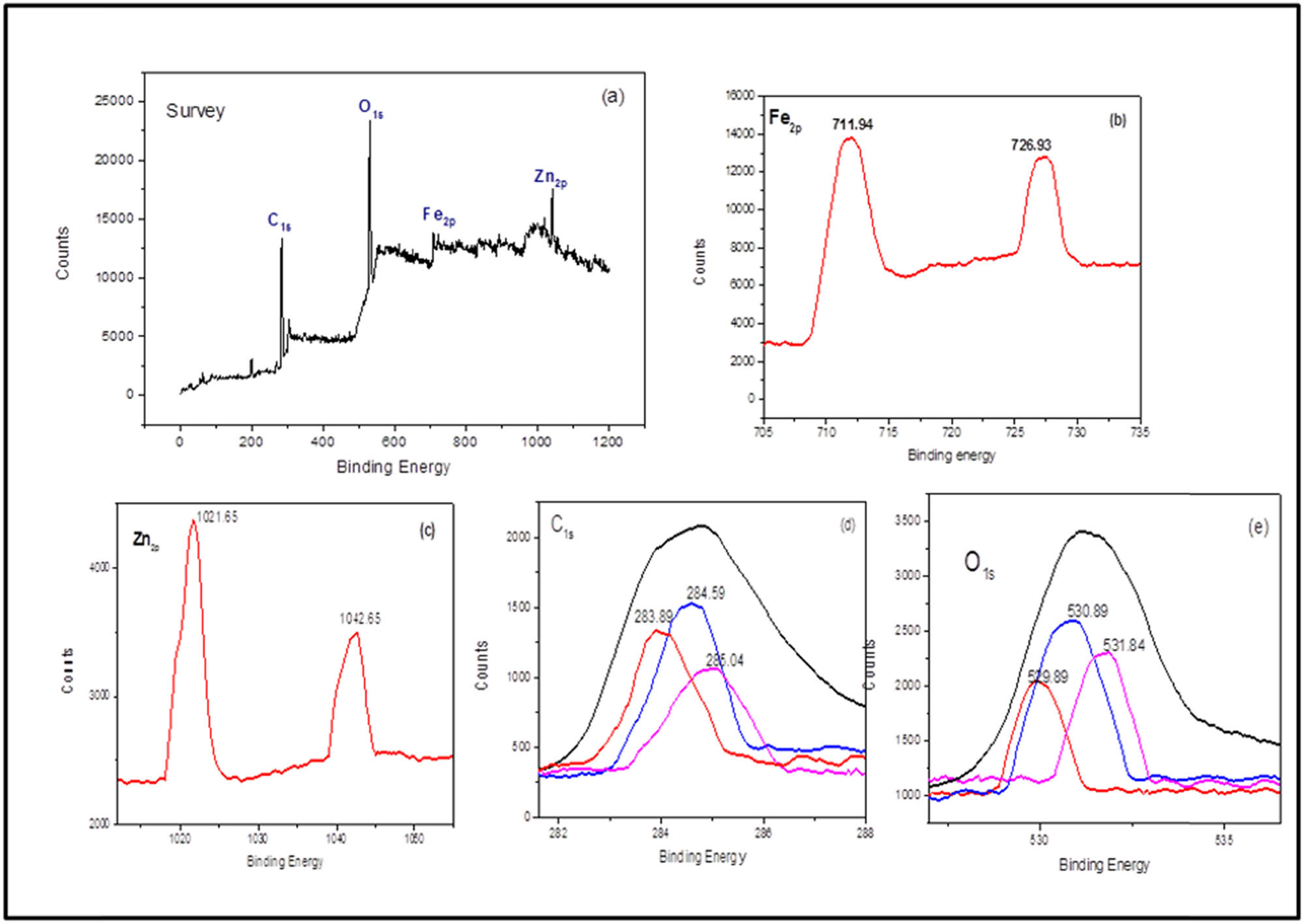
XPS spectra of ZnO/Fe3O4 NCs: (a) survey spectrum, (b) Fe2p, (c) Zn2p, (d) C1s, and (e) O1s.
3.6 Thermal analysis
Thermal characteristics of ZnO/Fe3O4 NCs were determined simultaneously in a single run by using TGA and DTA (Figure 7). TGA results showed that the thermal decomposition of ZnO/Fe3O4 NCs occurred in four steps. Initially, up to 100°C, the weight loss of 5.2% was due to the loss of adsorbed water on the surface of NCs. The weight loss observed in the second step (200–400°C) was 17.3%, which might be due to the dismissal of biomolecules capped on the surface of NCs. The loss in weight observed in between 400 and 600°C (third step) was assigned to the adsorbed oxygen species [56]. In the last step, a loss of 7.5% in weight was observed up to 800°C. DTA thermogram (Figure 7) displayed energy changes irrespective of change in weight. The peaks observed at 328 and 599°C were associated with the release of bioactive molecules and adsorbed oxygen, respectively. A peak at 927°C in DTA thermogram probably infers a crystalline transition of ZnO/Fe3O4 NCs.
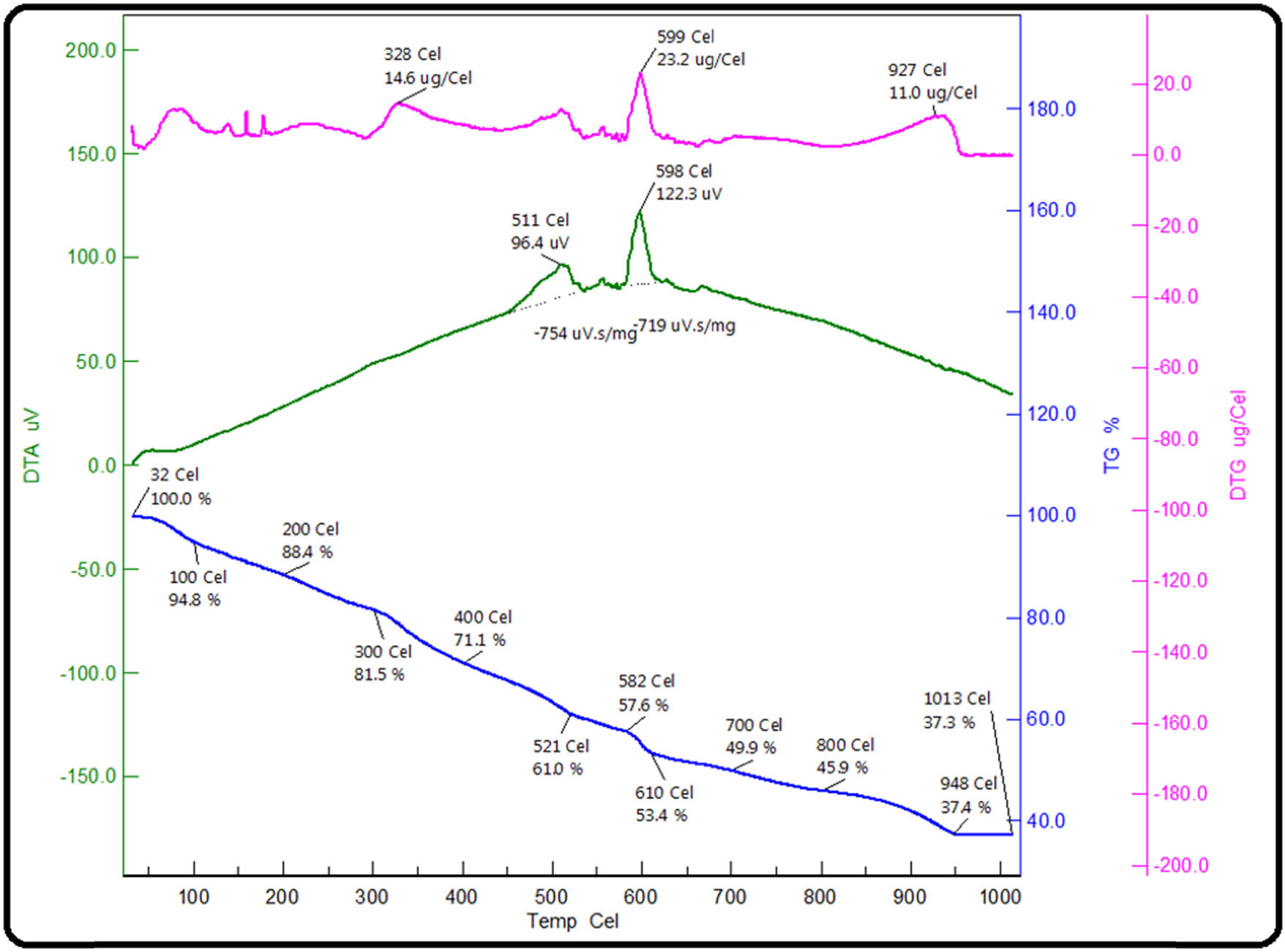
Thermograms of ZnO/Fe3O4 NCs.
3.7 Assessment of the photocatalytic activity
The photodegradation of MB in the presence of as-prepared nanoproducts was examined under visible light, and the extent of degradation was measured in terms of absorbance of MB solution using a UV-Vis spectrophotometer after certain intervals of time for 150 min. The results of degradation studies are showcased in (Figures 8 and (9, and Table 1, which revealed that the degradation of MB increases with an increasing irradiation time. From the absorbance spectra of MB (Figure 8 and Table 1), it can be seen clearly that initially, 15 min of exposure to sunlight, MB solution was degraded by 6.14, 2.27, and 28.03% and after 90 min, MB solution was degraded by 48.3, 29.94, and 63.25% in the presence of biosynthesized ZnO, Fe3O4, and ZnO/Fe3O4 samples, respectively.
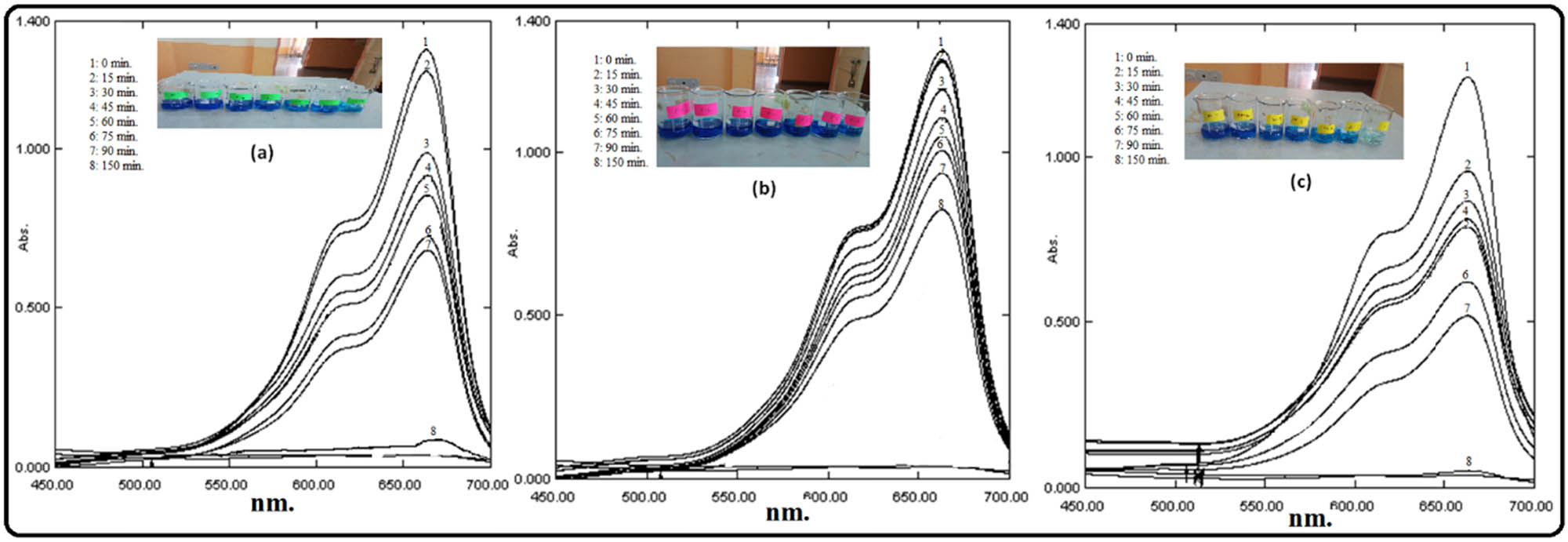
UV-Vis absorbance spectra of MB solution during the photocatalytic process with biosynthesized: (a) ZnO, (b) Fe3O4, and (c) ZnO/Fe3O4 NCs.
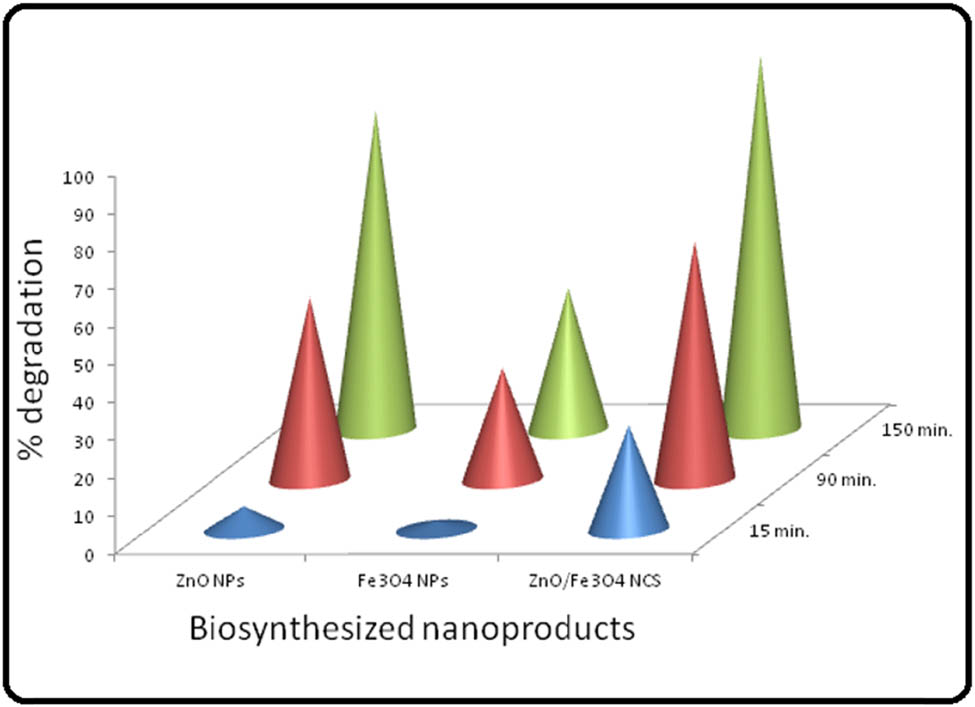
Graphical portrayal of percentage removal of MB at 15, 90, and 150 min in the presence of as-prepared ZnO, Fe3O4, and ZnO/Fe3O4.
Comparative analysis on the photoremediation of MB in the presence of biosynthesized ZnO, Fe3O4, and ZnO/Fe3O4 NCs for a period of 150 min
| Sr. no. | Time (min) | ZnO (E max) | Fe3O4 (E max) | ZnO/Fe3O4 (E max) |
|---|---|---|---|---|
| 1 | Initially | 1.32 | 1.32 | 1.32 |
| 2 | 15 | 1.239 | 1.29 | 0.956 |
| 3 | 30 | 0.987 | 1.19 | 0.854 |
| 4 | 45 | 0.914 | 1.12 | 0.806 |
| 5 | 60 | 0.865 | 1.023 | 0.772 |
| 6 | 75 | 0.722 | 0.992 | 0.578 |
| 7 | 90 | 0.682 | 0.93 | 0.485 |
| 8 | 150 | 0.202 | 0.829 | 0.012 |
As shown in Figure 8, MB dye was almost completely degraded (99.09%) by ZnO/Fe3O4 NCs in 150 min, whereas ZnO and Fe3O4 NPs degraded it by 84.7 and 37.1%, respectively. These outcomes of MB absorbance spectra revealed that the degradation efficiency of ZnO NPs was increased in the presence of Fe3O4 NPs. The enhancement in MB removal by ZnO/Fe3O4 NCs compared to ZnO NPs indicated the synergistic effect between ZnO and Fe3O4, assigning to the degradation of MB.
Moreover, the degradation efficiency of ZnO/Fe3O4 NCs was examined for three consecutive runs, and the results are shown in Figure 10.
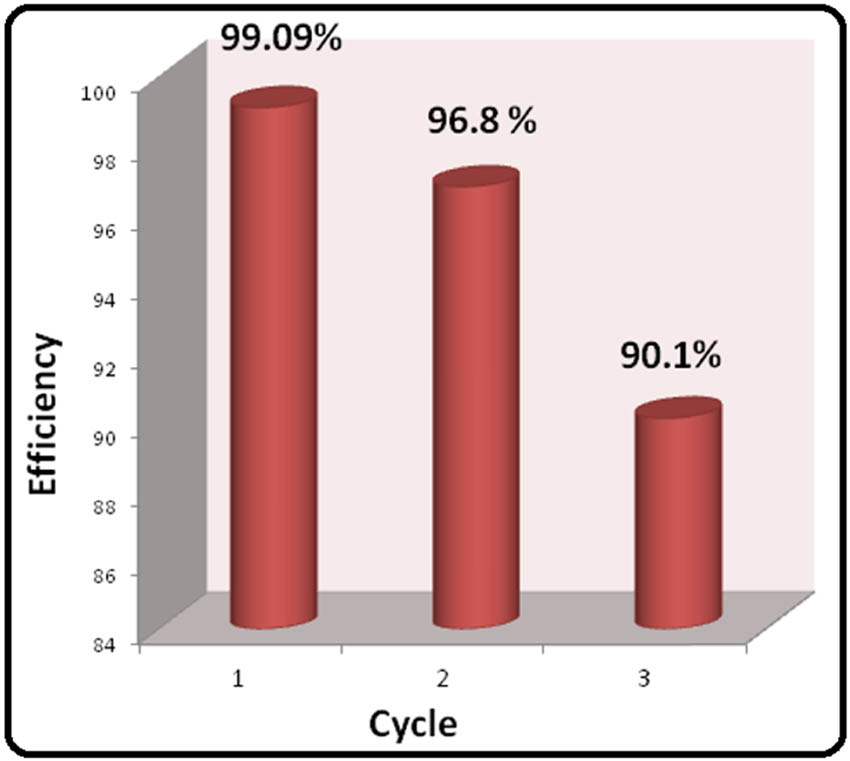
Reutilizing performance of ZnO/Fe3O4 up to three cycles.
From the results, it is clear that the composites were active up to three cycles. Although in the third cycle, the degradation efficiency was decreased (90.1%). This decrement may be due to the adsorbance of some MB on the surface of photocatalyst, which perhaps blocks some active sites of NCs and can also be due to some loss of NCs during the recovery process.
The possible mechanism for the photodegradation of MB dye over biosynthesized ZnO/Fe3O4 NCs under visible light is shown in Figure 11.
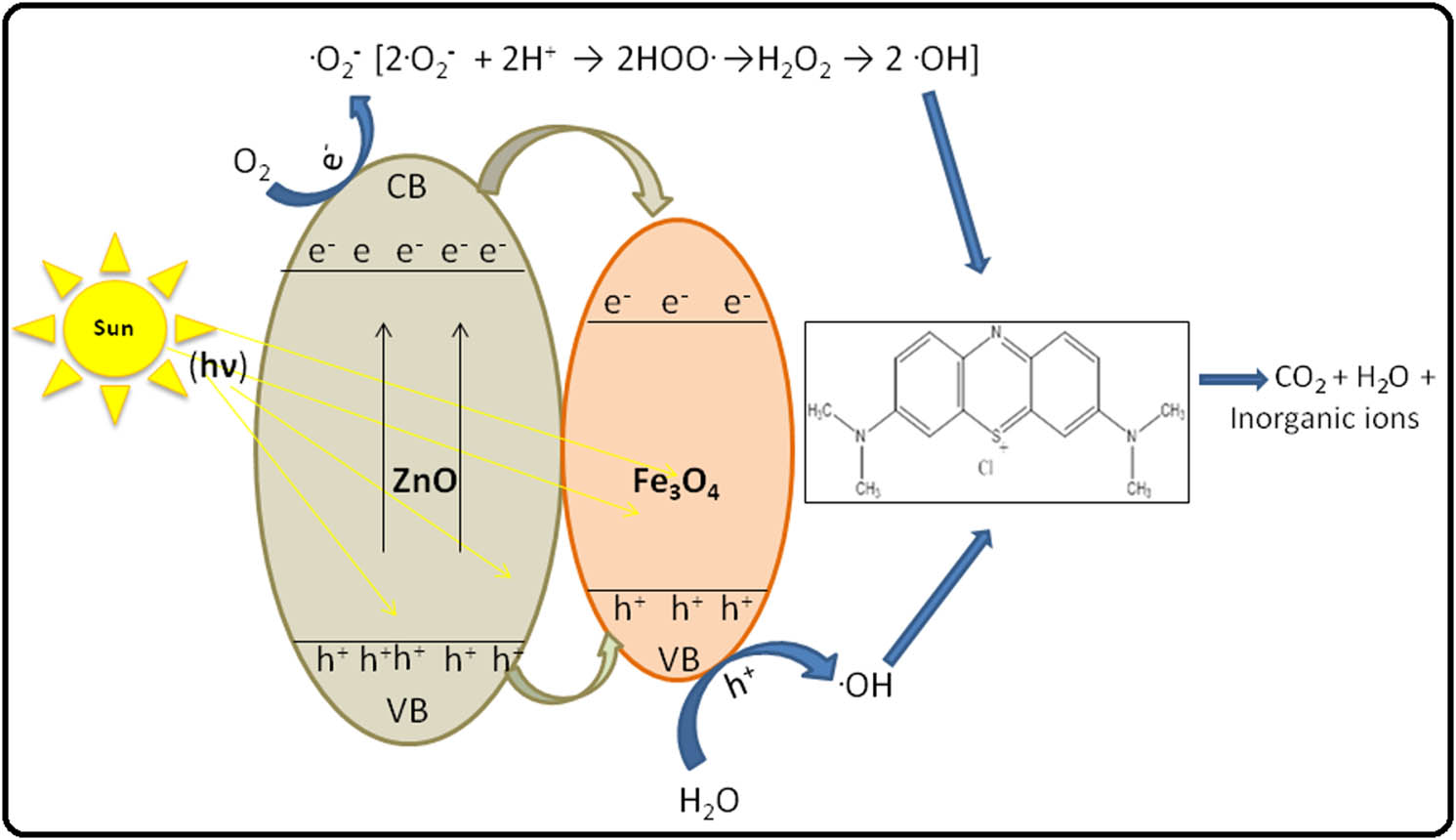
Possible mechanism of photocatalytic degradation of MB in the presence of C. viminalis leaves’ extract-mediated biosynthesized ZnO/Fe3O4 NCs.
The phenomenon of photodegradation of MB takes place when visible light is irradiated on the photocatalyst (ZnO, Fe3O4, or ZnO/Fe3O4), which leads to the generation of electron–hole pairs in conduction/valence bands (VBs) simultaneously on the surface of the photocatalyst (equation (2)). In the conduction band (CB), oxygen on the surface of the photocatalyst combines with the excited electron and forms ˙
As shown in Figure 8 and Table 1, the absorption intensity of dye gradually decreases with an increasing irradiation time and finally diminished in case of ZnO/Fe3O4. This is because of a breakdown of the heterocyclic conjugated structure of MB molecule into simple molecules, such as water, carbon dioxide, and inorganic ions. Apart from this, the photocatalytic efficiency of ZnO/Fe3O4 NCs was greater compared to ZnO NPs, and this can be summarized as follows: Fe3O4 NPs possess a narrow bandgap, and hence, e−/h+ pairs produced in it under irradiation recombines fastly, as a result charge carriers could not survive for a long time for the photocatalysis process [58]. In ZnO/Fe3O4 NCs, the energy level of CB and VB of ZnO differs from that of Fe3O4. During irradiation, some photogenerated electrons from ZnO are captured by Fe3O4 NPs at the composite’s interface, where they react with the surface oxygen to form superoxide radicals. However, some holes transfer to VB of Fe3O4 from ZnO and react with the surface water to produce hydroxyl radicals (˙OH). This phenomenon at the interface restricts an electron–hole recombination in ZnO. As a resultant, the generation of reactive species increases at the junction of ZnO–Fe3O4, which accelerates the degradation of dye molecules [59]. Hence, the efficient charge transfer separation at heterojunction of two different semiconductor materials attributes to an enhanced photo-catalytic activity of ZnO/Fe3O4 NCs to degrade MB solution. However, a comparison data for photodegradation of MB dye solution by ZnO/Fe3O4 NCs synthesized by different routes are displayed in Table 2. The results show considerable proficiency for the current study of MB degradation by C. viminalis-synthesized ZnO/Fe3O4 NCs.
A comparable study of MB dye removal by ZnO/Fe3O4 NCs (from the literature) with present study
| S. no. | ZnO/Fe3O4 (ppm) | MB (ppm) | Synthesis method | Degradation time (min) | Degradation efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 50 | 10 | Hydrothermal | 180 | 89.2 | [29] |
| 2 | 500 | 20 | One-pot synthesis | 90 | 98.9 | [37] |
| 3 | 2,500 | 20 | Microwave reactor | 60 | 63.02 | [60] |
| 4 | 160 | 32 | Biosynthesis | 150 | 99.09 | Present study |
4 Conclusion
In this study, ZnO/Fe3O4 NCs as well as ZnO and Fe3O4 NPs were successfully synthesized via eco-friendly route using C. viminalis leaves’ extract without using any toxic additives. FTIR study indicated that the leaves’ extract played a key role in the reduction and stabilization of ZnO/Fe3O4 NCs, ZnO NPs, and Fe3O4 NPs, through the interaction of O–H, C═O, and C═C groups of phytochemicals present in C. viminalis leaves’ extract. Nanosize and the presence of pure phase in the crystal structure of biosynthesized products without major impurities, were confirmed by XRD analysis. XRD results were supported by XPS outcomes and revealed the formation of Zn–Fe hetero-junction in ZnO/Fe3O4 NCs. Evidence of SEM, TEM, and EDX analyses also confirms the existence of Zn and Fe in composite nanoparticles. In addition, the photodegradation of MB under visible light irradiation was carried out using synthesized ZnO, Fe3O4, and ZnO/Fe3O4 as a photocatalyst. The results obtained in this study demonstrate that compared to monometallic (ZnO and Fe3O4) NPs, bimetallic NCs of Zn and Fe (ZnO/Fe3O4) accelerated the degradation of MB. The enhanced photocatalytic activity was attributed to the synergistic effect between ZnO and Fe3O4 nanoparticles in ZnO/Fe3O4 NCs. Moreover, ZnO/Fe3O4 NCs can be reused for three successive runs. Therefore, these findings suggest that ZnO/Fe3O4 NCs synthesized via C. viminalis leaves’ extract have a potential as an efficient photocatalyst for water purification.
Acknowledgements
The authors would like to thank the Chancellor, Jaipur National University, Jaipur of India for providing laboratory facilities for this study.
-
Funding information: This study was funded by the Researchers Supporting Project Number (RSP-2021/339) King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Ajmal M, Ahmad A, Nomani AA. Physico-chemical studies on industrial pollutants. J Sci Res. 1980;2:51–3.Suche in Google Scholar
[2] Daneshvar N, Ayazloo M, Khataee A, Pourhassan M. Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp. Bioresour Technol. 2007;98(6):1176–82.10.1016/j.biortech.2006.05.025Suche in Google Scholar
[3] He C, Yu Y, Hu X, Larbot A. Influence of silver doping on the photocatalytic activity of titania films. Appl Surf Sci. 2002;200(1–4):239–47.10.1016/S0169-4332(02)00927-3Suche in Google Scholar
[4] Nilsson I, Möller A, Mattiasson B, Rubindamayugi MS, Welander U. Decolorization of synthetic and real textile wastewater by the use of white-rot fungi. Enzyme Microb Technol. 2006;38(1–2):94–100.10.1016/j.enzmictec.2005.04.020Suche in Google Scholar
[5] Mathur N, Bhatnagar P. Mutagenicity assessment of textile dyes from Sanganer (Rajasthan). J Env Biol. 2007;28(1):123–6.Suche in Google Scholar
[6] Hamdaoui O, Chiha M. Removal of methylene blue from aqueous solutions by wheat bran. Acta Chim Slov. 2007;54:2.Suche in Google Scholar
[7] López C, Valade A-G, Combourieu B, Mielgo I, Bouchon B, Lema JM. Mechanism of enzymatic degradation of the azo dye Orange II determined by ex situ 1H nuclear magnetic resonance and electrospray ionization-ion trap mass spectrometry. Anal Biochem. 2004;335(1):135–49.10.1016/j.ab.2004.08.037Suche in Google Scholar PubMed
[8] Nagaveni K, Hegde M, Ravishankar N, Subbanna G, Madras G. Synthesis and structure of nanocrystalline TiO2 with lower band gap showing high photocatalytic activity. Langmuir. 2004;20(7):2900–7.10.1021/la035777vSuche in Google Scholar PubMed
[9] Aarthi T, Madras G. Photocatalytic degradation of rhodamine dyes with nano-TiO2. Ind Eng Chem Res. 2007;46(1):7–14.10.1021/ie060948nSuche in Google Scholar
[10] Kumar SG, Rao KKJ Tungsten-based nanomaterials (WO3 & Bi2WO6): modifications related to charge carrier transfer mechanisms and photocatalytic applications. Appl Surf Sci. 2015;355:939–58.10.1016/j.apsusc.2015.07.003Suche in Google Scholar
[11] Chu D, Masuda Y, Ohji T, Kato K. Formation and photocatalytic application of ZnO nanotubes using aqueous solution. Langmuir. 2010;26(4):2811–5.10.1021/la902866aSuche in Google Scholar PubMed
[12] Singh P, Borthakur A, Mishra P, Tiwary D. Nano-materials as photocatalysts for degradation of environmental pollutants: challenges and possibilities. Amsterdam: Elsevier; 2019.Suche in Google Scholar
[13] Anjum M, Miandad R, Waqas M, Gehany F, Barakat MA. Remediation of wastewater using various nano-materials. Arab J Chem. 2019;12(8):4897–919.10.1016/j.arabjc.2016.10.004Suche in Google Scholar
[14] Lee KM, Lai CW, Ngai KS, Juan JC. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016;88:428–48.10.1016/j.watres.2015.09.045Suche in Google Scholar PubMed
[15] Wang Y, Wang Q, Zhan X, Wang F, Safdar M, He J. Visible light driven type II heterostructures and their enhanced photocatalysis properties. Nanoscale. 2013;5(18):8326–39.10.1039/c3nr01577gSuche in Google Scholar PubMed
[16] Kundu P, Deshpande PA, Madras G, Ravishankar N. Nanoscale ZnO/CdS heterostructures with engineered interfaces for high photocatalytic activity under solar radiation. J Mater Chem. 2011;21(12):4209–16.10.1039/c0jm03116jSuche in Google Scholar
[17] Kadam A, Dhabbe R, Gophane A, Sathe T, Garadkar K. Template free synthesis of ZnO/Ag2O nanocomposites as a highly efficient visible active photocatalyst for detoxification of methyl orange. J Photochem Photobiol B. 2016;154:24–33.10.1016/j.jphotobiol.2015.11.007Suche in Google Scholar PubMed
[18] Pal S, Maiti S, Maiti UN, Chattopadhyay KC. Low temperature solution processed ZnO/CuO heterojunction photocatalyst for visible light induced photo-degradation of organic pollutants. CrystEng Comm. 2015;17(6):1464–76.10.1039/C4CE02159BSuche in Google Scholar
[19] Liu W, Wang M, Xu C, Chen S. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem Eng Sci. 2012;209:386–93.10.1016/j.cej.2012.08.033Suche in Google Scholar
[20] Thangavel S, Thangavel S, Raghavan N, Krishnamoorthy K, Venugopal G. Visible-light driven photocatalytic degradation of methylene-violet by rGO/Fe3O4/ZnO ternary nanohybrid structures. J Alloy Compd. 2016;665:107–12.10.1016/j.jallcom.2015.12.192Suche in Google Scholar
[21] Liu S-Q. Magnetic semiconductor nano-photocatalysts for the degradation of organic pollutants. Env Chem Lett. 2012;10(3):209–16.10.1007/s10311-011-0348-9Suche in Google Scholar
[22] Mamba G, Mishra A. Advances in magnetically separable photocatalysts: smart, recyclable materials for water pollution mitigation. Catalysts. 2016;6(6):79.10.3390/catal6060079Suche in Google Scholar
[23] Boruah PK, Sharma B, Karbhal I, Shelke MV, Das M. Ammonia-modified graphene sheets decorated with magnetic Fe3O4 nanoparticles for the photocatalytic and photo-Fenton degradation of phenolic compounds under sunlight irradiation. J Hazard Mater. 2017;325:90–100.10.1016/j.jhazmat.2016.11.023Suche in Google Scholar PubMed
[24] Mahmoudzadeh G, Khorrami S, Madani S, Frounchi M. Influence of different fuel additives at different molar ratios on the crystallite phase formation process, structural characteristics and morphology of dispersed zinc ferrite powders by sol-gel auto combustion. J Ceram Process Res. 2012;13(4):368–72.Suche in Google Scholar
[25] Lee H, Jung JC, Kim H, Chung Y-M, Kim TJ, Lee SJ, et al. Preparation of ZnFe2O4 catalysts by a co-precipitation method using aqueous buffer solution and their catalytic activity for oxidative dehydrogenation of n-butene to 1,3-butadiene. Catal Lett. 2008;122(3):281–6.10.1007/s10562-007-9371-7Suche in Google Scholar
[26] Yadav RS, Havlica J, Masilko J, Tkacz J, Kuřitka I, Vilcakova J. Anneal-tuned structural, dielectric and electrical properties of ZnFe2O4 nanoparticles synthesized by starch-assisted sol–gel auto-combustion method. J Mater Sci: Mater Electron. 2016;27(6):5992–6002.10.1007/s10854-016-4522-5Suche in Google Scholar
[27] Zhu H, Gu X, Zuo D, Wang Z, Wang N, Yao K. Microemulsion-based synthesis of porous zinc ferrite nanorods and its application in a room-temperature ethanol sensor. Nanotechnology. 2008;19(40):405503.10.1088/0957-4484/19/40/405503Suche in Google Scholar PubMed
[28] Nagajyothi PC, Lee SE, An M, Duk LK. Green synthesis of silver and gold nanoparticles using Lonicera Japonica flower extract. Bull Korean Chem Soc. 2012;33(8):2609–12.10.5012/bkcs.2012.33.8.2609Suche in Google Scholar
[29] Nagajyothi PC, An TNM, Sreekanth TVM, Lee J-L, Lee DJ, Lee KD. Green route biosynthesis: Characterization and catalytic activity of ZnO nanoparticles. Mater Lett. 2013;108(1):160–3.10.1016/j.matlet.2013.06.095Suche in Google Scholar
[30] Ben AS, Sbihi HM, Azam M, Al-Resayes SI, Ayadi MT, Ayari F. Local iron ore identification: comparison to synthesized Fe3O4 nanoparticles obtained by ultrasonic assisted reverse co-precipitation method for Auramine O dye adsorption. Desalination Water Treat. 2021;220:446–58.10.5004/dwt.2021.27080Suche in Google Scholar
[31] Nasrollahzadeh M, Atarod M, Sajadi SM. Green synthesis of the Cu/Fe3O4 nanoparticles using Morinda morindoides leaf aqueous extract: a highly efficient magnetically separable catalyst for the reduction of organic dyes in aqueous medium at room temperature. Appl Surf Sci. 2016;364:636–44.10.1016/j.apsusc.2015.12.209Suche in Google Scholar
[32] Goyal P, Chakraborty S, Misra SKJEN. Monitoring, Management. Multifunctional Fe3O4-ZnO nanocomposites for environmental remediation applications. Env Nanotechnol Monit Manag. 2018;10:28–35.10.1016/j.enmm.2018.03.003Suche in Google Scholar
[33] Nadafan M, Sabbaghan M, Sofalgar P, Anvari JZ. Comparative study of the third-order nonlinear optical properties of ZnO/Fe3O4 nanocomposites synthesized with or without Ionic Liquid. Opt Laser Technol. 2020;131:106435.10.1016/j.optlastec.2020.106435Suche in Google Scholar
[34] Matinise N, Kaviyarasu K, Mongwaketsi N, Khamlich S, Kotsedi L, Mayedwa N, et al. Green synthesis of novel zinc iron oxide (ZnFe2O4) nanocomposite via Moringa Oleifera natural extract for electrochemical applications. Appl Surf Sci. 2018;446:66–73.10.1016/j.apsusc.2018.02.187Suche in Google Scholar
[35] Ghasemian Dazmiri M, Alinezhad H, Hossaini Z, Bekhradnia AR. Green synthesis of Fe3O4/ZnO magnetic core–shell nanoparticles by Petasites hybridus rhizome water extract and their application for the synthesis of pyran derivatives: Investigation of antioxidant and antimicrobial activity. Appl Organomet Chem. 2020;34(9):e5731.10.1002/aoc.5731Suche in Google Scholar
[36] Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–82.10.1128/CMR.12.4.564Suche in Google Scholar PubMed PubMed Central
[37] Wheeler GJBS. Maintenance of a narrow host range by Oxyops vitiosa; a biological control agent of Melaleuca quinquenervia. Ecology. 2005;33(4):365–83.10.1016/j.bse.2004.10.010Suche in Google Scholar
[38] El-Barasi NM, Miloud MM, El-ajaily MM, Mohapatra RK, Sarangi AK, Das D, et al. Synthesis, structural investigations and antimicrobial studies of hydrazone based ternary complexes with Cr(III), Fe(III) and La(III) ions. J Saudi Chem Soc. 2020;24(6):492–503.10.1016/j.jscs.2020.04.005Suche in Google Scholar
[39] Taghreed HAL-N, Ranjan KM, Azam M, Leka KABK, Pranab KM, Abeer AI, et al. Mixed-ligand complexes of ampicillin derived Schiff base ligand and Nicotinamide: Synthesis, physico-chemical studies, DFT calculation, antibacterial study and molecular docking analysis. J Mol Struct. 2020;229:29832.Suche in Google Scholar
[40] Islam MR, Ahamed R, Rahman MO, Akbar MA, Al-Amin M, Alam KD, et al. In vitro antimicrobial activities of four medicinally important plants in Bangladesh. Eur J Sci Res. 2010;39(Suppl 2):199–206.Suche in Google Scholar
[41] Cîntă-Pînzaru S, Dehelean CA, Soica C, Culea M, Borcan F. Evaluation and differentiation of the Betulaceae birch bark species and their bioactive triterpene content using analytical FT-vibrational spectroscopy and GC-MS. Chem Cent J. 2012;6(1):1–12.10.1186/1752-153X-6-67Suche in Google Scholar PubMed PubMed Central
[42] Singh H, Kumar A, Thakur A, Kumar P, Nguyen V-H, Vo D-VN, et al. One-pot synthesis of magnetite-ZnO nanocomposite and its photocatalytic activity. Top Catal. 2020;63(11):1097–108.10.1007/s11244-020-01278-zSuche in Google Scholar
[43] Bibi I, Nazar N, Iqbal M, Kamal S, Nawaz H, Nouren S, et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: characterization and photo-catalytic activity. Adv Powder Technol. 2017;28(9):2035–43.10.1016/j.apt.2017.05.008Suche in Google Scholar
[44] Awwad AM, Salem NM. A green and facile approach for synthesis of magnetite nanoparticles. J Nanosci Nanotechnol. 2012;2(6):208–13.10.5923/j.nn.20120206.09Suche in Google Scholar
[45] Karthikeyan B, Loganathan B. Rapid green synthetic protocol for novel trimetallic nanoparticles. J Nanomater. 2013;2013:2013–8.10.1155/2013/168916Suche in Google Scholar
[46] Yang T, Shen C, Li Z, Zhang H, Xiao C, Chen S, et al. Highly ordered self-assembly with large area of Fe3O4 nanoparticles and the magnetic properties. J Phys Chem B. 2005;109(49):23233–6.10.1021/jp054291fSuche in Google Scholar PubMed
[47] Yamashita T, Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci. 2008;254(8):2441–9.10.1016/j.apsusc.2007.09.063Suche in Google Scholar
[48] Haldorai Y, Voit W, Shim J-J. Nano ZnO@ reduced graphene oxide composite for high performance supercapacitor: Green synthesis in supercritical fluid. Electrochim Acta. 2014;120:65–72.10.1016/j.electacta.2013.12.063Suche in Google Scholar
[49] Wang L, Gu H, He J, Zhao T, Zhang X, Xiao C, et al. Scale synthesized cubic NaNbO3 nanoparticles with recoverable adsorption and photodegradation for prompt removal of methylene blue. J Alloy Compd. 2017;695:599–606.10.1016/j.jallcom.2016.10.327Suche in Google Scholar
[50] Cirone J, Ahmed SR, Wood PC, Chen A. Green synthesis and electrochemical study of cobalt/graphene quantum dots for efficient water splitting. J Phys Chem C. 2019;123(14):9183–91.10.1021/acs.jpcc.9b00951Suche in Google Scholar
[51] Zhan S, Zhu D, Ma S, Yu W, Jia Y, Li Y, et al. Highly efficient removal of pathogenic bacteria with magnetic graphene composite. ACS Appl Mater Interfaces. 2015;7(7):4290–8.10.1021/am508682sSuche in Google Scholar PubMed
[52] Sahu SK, Chakrabarty A, Bhattacharya D, Ghosh SK, Pramanik P. Single step surface modification of highly stable magnetic nanoparticles for purification of His-tag proteins. J Nanopart Res. 2011;13(6):2475–84.10.1007/s11051-010-0140-ySuche in Google Scholar
[53] Akhlaghi N, Najafpour-Darzi G, Younesi H. Facile and green synthesis of cobalt oxide nanoparticles using ethanolic extract of Trigonella foenumgraceum (Fenugreek) leaves. Adv Powder Technol. 2020;31(8):3562–9.10.1016/j.apt.2020.07.004Suche in Google Scholar
[54] Wahab R, Kim Y-S, Shin H-S. Synthesis, characterization and effect of pH variation on zinc oxide nanostructures. Mater Trans. 2009;50(8):2092–7.10.2320/matertrans.M2009099Suche in Google Scholar
[55] Li J, Ng DH, Song P, Song Y, Kong C, Liu S. Synthesis of hierarchically porous Cu–Ni/C composite catalysts from tissue paper and their catalytic activity for the degradation of triphenylmethane dye in the microwave induced catalytic oxidation (MICO) process. Mater Res Bull. 2015;64:236–44.10.1016/j.materresbull.2014.12.046Suche in Google Scholar
[56] Basavegowda N, Mishra K, Lee YR. Trimetallic FeAgPt alloy as a nanocatalyst for the reduction of 4-nitroaniline and decolorization of rhodamine B: A comparative study. J Alloy Compd. 2017;701:456–64.10.1016/j.jallcom.2017.01.122Suche in Google Scholar
[57] Li N, Zhang J, Tian Y, Zhao J, Zhang J, Zuo W. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ ZnO core-shell photocatalyst with enhanced activity: ciprofloxacin degradation and mechanism insight. Chem Eng Sci. 2017;308:377–85.10.1016/j.cej.2016.09.093Suche in Google Scholar
[58] Shekofteh-Gohari M, Habibi-Yangjeh A. Fabrication of novel magnetically separable visible-light-driven photocatalysts through photosensitization of Fe3O4/ZnO with CuWO4. J Ind Eng Chem. 2016;44:174–84.10.1016/j.jiec.2016.08.028Suche in Google Scholar
[59] Xia J, Wang A, Liu X, Su Z. Preparation and characterization of bifunctional, Fe3O4/ZnO nanocomposites and their use as photocatalysts. Appl Surf Sci. 2011;257(23):9724–32.10.1016/j.apsusc.2011.05.114Suche in Google Scholar
[60] Długosz O, Szostak K, Krupiński M, Banach M. Synthesis of Fe3O4/ZnO nanoparticles and their application for the photodegradation of anionic and cationic dyes. Int J Env Sci Technol. 2021;18(3):561–74.10.1007/s13762-020-02852-4Suche in Google Scholar
© 2021 Poonam Dwivedi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

