Abstract
Nanomedicine is ongoing current research in the applications of nanotechnology for cancer therapy. Simply from a technology perspective, this field of research has an enormous broadening and success to date. Recently, nanomedicine has also made inroads in the treatment of cancer. Stimuli-responsive nanoparticles are an emerging field of research because its targeting capacity is of great interest in the treatment of cancer. The responsive nanoparticles are efficient in encountering different internal biological stimuli (acidic, pH, redox, and enzyme) and external stimuli (temperature, ultrasounds, magnetic field, and light), which are used as smart nanocarriers for delivery of the chemotherapeutic and imaging agents for cancer therapy. In-depth, the responsive nanocarrier that responds to the biological cues is of pronounced interest due to its capability to provide a controlled release profile at the tumor-specific site. The outlook of this review focuses on the stimuli-responsive nanocarrier drug delivery systems in sequence to address the biological challenges that need to be evaluated to overcome conventional cancer therapy.
Abbreviations
- DTT
-
dithiothreitol
- GSH
-
glutathione
- GO
-
graphene oxide
- DOX
-
doxorubicin
- CS
-
chitosan
- FA
-
folic acid
- P45
-
peptide
- 5-FU-5
-
fluorouracil
- CUR
-
curcumin
- PEI
-
polyethylamine
- PEG
-
polyethylene glycol
- PBLA
-
poly(β-benzyl l-aspartate)
- TPP
-
triphenylphosphine
- PNIPAM
-
poly(N-iso propyl acrylamide)
- LCST
-
lower critical solution temperature
- Fe3O4
-
Iron(ii,iii) oxide
- US
-
ultrasound
- TNP
-
titanium nanoparticles
- PDT
-
photodynamic therapy
- SiRNA
-
small interfering RNA
1 Introduction
Cancer is the second biggest populate disease to go after cardiovascular disease with the leading cause of death [1,2,3,4]. Based on the world health organization, around 26 million people will be affected by cancer and the mortality rates will increase by up to 17 million worldwide by 2030 [5]. Surgery, radiation therapy, and chemotherapy are commonly used in clinics [6]. However, it is crucial to minimize the long-lasting side effects due to the heterogeneous characteristics of cancer cells [7,8,9,10,11]. The recognition of nanomedicine, which is most impressive for cure cancer, has developed a new platform for targeted therapeutics. The mechanism of nanoparticles for targeting the tumor region is known as an enhanced permeation and retention (EPR) effect. Schematic sketch of EPR is shown in Figure 1.
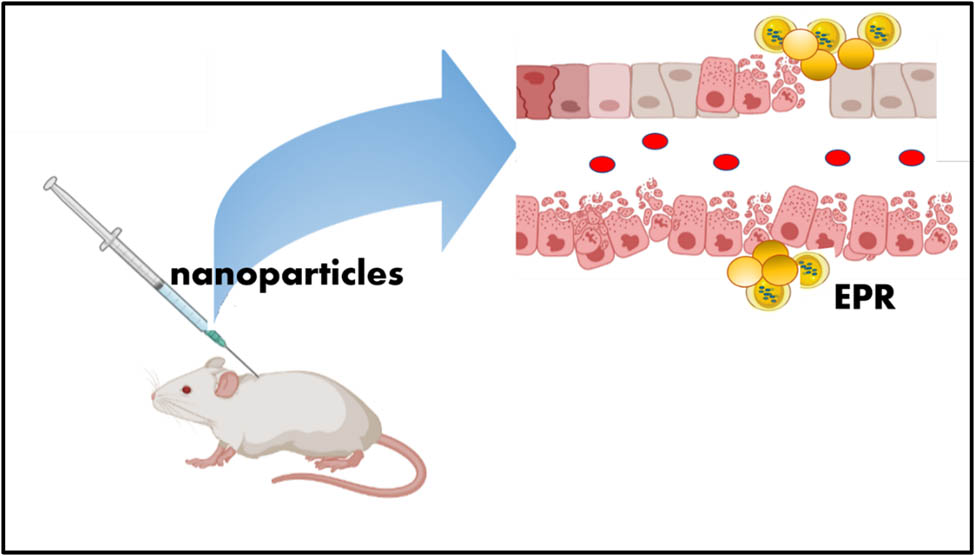
Schematic sketch of EPR.
Specific targeting of cancer cells is the challenge of current clinical treatment. Chemotherapy has been used as a stand-alone strategy for cancer treatment, as adjuvant therapy after surgery, and as neoadjuvant therapy before surgery [12]. Many different types of chemotherapeutic agents have been used to treat cancer, both individually and in combination. However, because these drugs are unable to distinguish between normal and cancer cells, they cause a slew of unfavorable side effects [13,14,15,16,17]. Targeted therapy involves the use of agents that interact with specific molecular targets that are involved in the progression and survival of cancer [18,19,20]. Nanoparticles otherwise ultrafine particles range from 1 to 100 nm and these particles can reveal physical and chemical properties. Nanoparticle delivery systems are broadly evaluated preclinically with other nanoparticle-constructed formulations and technologies that have been used so far in the clinic setup [21,22,23,24]. There are several methods such as oral, local, topical, and systemic (e.g., intravenous) that are established by Food and Drug Administration (FDA)-approval for the delivery of nanoparticles/microparticles, upon the targeted site and preferred applications [25,26,27,28,29,30,31]. Therapeutic and diagnostic nanoparticles are classified into two different types: (a) inorganic nanoparticles (e.g., gold, silica, and iron oxide) and (b) organic nanoparticles (e.g., polymeric, liposomes, and micelles). Inorganic nanoparticles are already used in preclinical studies, for various applications [32,33,34,35]. The use of nanoparticles can compensate for several shortcomings of conventional cancer therapies. A variety of nanoparticles, including polymeric, inorganic, and lipid-based carrier systems, have been reported for drug delivery to cancer cells [36,37,38,39,40,41,42].
Surface modification of nanoparticles with poly(ethylene glycol) (PEG) chains can result in longer circulation time in the body by slowing the opsonization process [43]. The addition of cancer cell-specific ligands to the surface of nanoparticles confers target-specificity [44]. Folic acid (FA) and transferrin are commonly used as cancer-targeting ligands [45]. However, identifying cancer-specific surface markers for targeting is a difficult task that has spawned a separate field of study. Furthermore, many solid tumors develop a stromal layer around cancer that restricts nanoparticle contact with the cancer tissue, limiting their therapeutic potential [46]. The use of external or internal stimuli to trigger the release of the drug from the carrier is one strategy that can improve the therapeutic potential of nanoparticles carrying the therapeutic agent as shown in Figure 2. There are numerous reports in the literature on the development of modified nanoparticles for stimuli-responsive drug release [47].
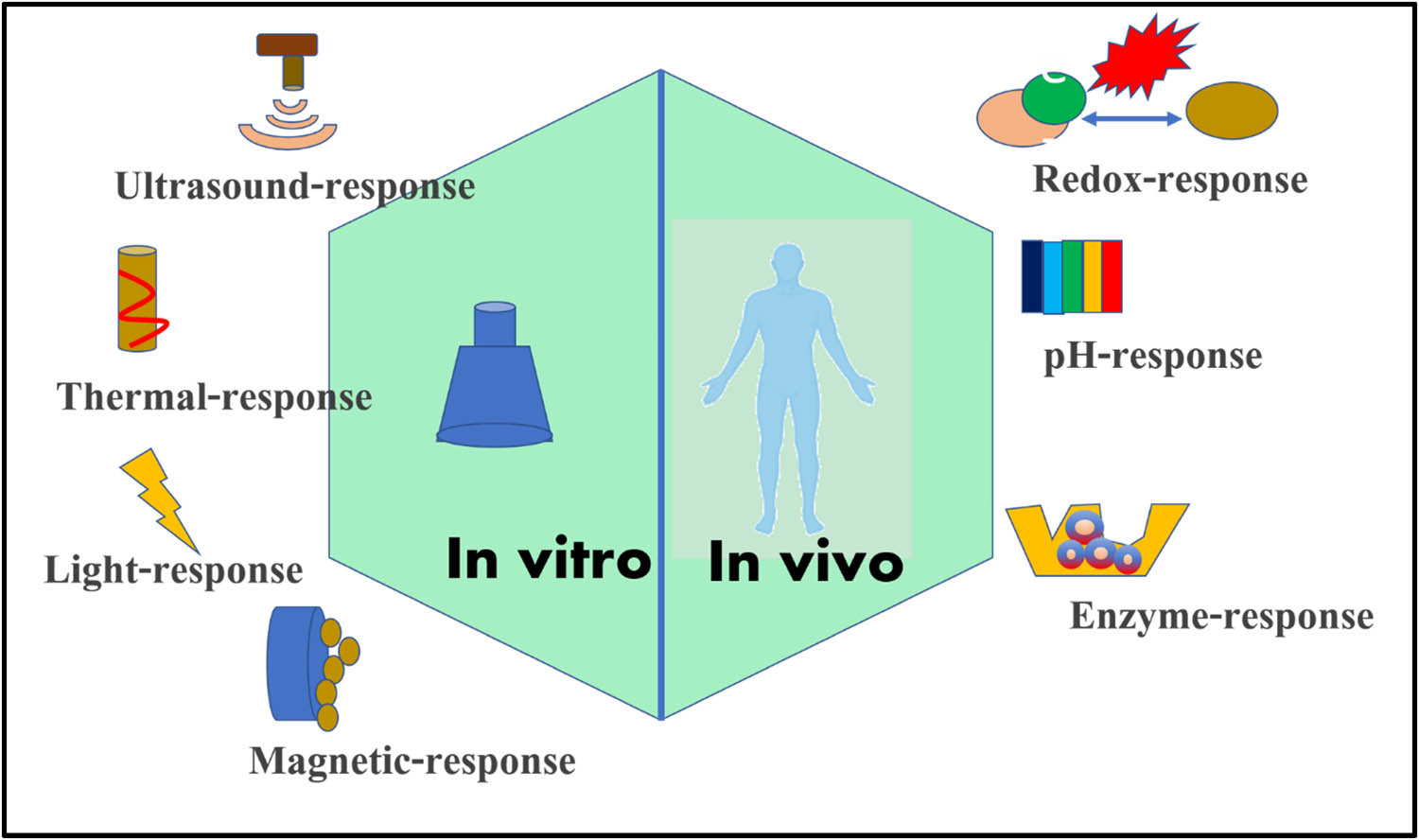
Schematic sketches of different types of stimuli for cancer therapeutics.
However, it is now recognized that the type of carrier, size of the carrier, type of linkers used for capping agent conjugation, nature of the capping agent, cell uptake, intracellular localization, and cell type all influence the carrier’s chemoresponsiveness as well as a therapeutic effect [48]. As a result, the field for investigating various types of carriers for cancer treatment remains wide open. Up to date, few clinical trials that use nanoparticles-based drug delivery systems to treat various cancer types have been revealed. This review debates the development of nanoparticles for stimuli-responsive drug delivery research and clinical developments around the globe.
2 An approach to smart delivery strategies
2.1 Internal stimuli for drug delivery systems
Here, we elaborate on the advances in internal stimuli for drug delivery systems. Different internal stimuli have been used to trigger the release of drug molecules towards cancer therapy.
2.1.1 pH responsiveness
pH stimuli trigger the drug release in response to the changes in the pH inside the body. In general, pH in normal tissues such as the brain and subcutaneous tissues is in the span of 7.2–7.5. But the pH in tumor cells is generally more acidic (pH 6.0–7.0). The variation between the normal cells and tumor cells attributes to the development of pH-responsive drug delivery systems, which trigger the release of the cargo into the cancer cells. Otto Warburg et al., a German scientist first describe the abnormal action of the cancer cells. The glycolysis process in the cancer cells completes, even in the presence of oxygen is known as aerobic glycolysis. An unusual relying on glycolysis as the only source of ATP conception is seen in many types of cancer cells is called the “Warburg effect” [49]. There are various gatekeeping molecules, which can be tagged to the surface of the nanoparticles through the pH labile groups such as ester bond, hydrazine bond, and acetal bond, which can be lysed in acidic pH [50]. The transformation from a neutral milieu to an acidic milieu could cause remarkable changes in the physical properties of the material activity. The mechanisms behind the pH-triggered targeted drug delivery at the tumor are as follows;
Protonation caused by the internalization
Extracellular drug release owing to dissociation
Detachment of PEG, which promotes the endocytosis of nanocarriers followed by destabilization of endosomal membrane and hence the release of the drug [51].
Wang et al. [52] constructed a novel pH-responsive block copolymer, poly(ethylene glycol)–poly(ε-caprolactone)–poly(l-histidine) (PEG–PCL–PHis), synthesized, and characterized for anti-cancer drug delivery with excellent advantages such as biocompatible, biodegradable, and strong drug-loading efficiency inner core. This self-assemble micelle has a constant circulation system in blood and tissue-specific targeting on cancer cells with pH sensitivity. Moreover, the cell viability of MCF-7 cells treated doxorubicin-loaded self-assemble micelle proved the biocompatibility of the drug-loaded system and the release mechanism pathway of DOX-loaded micelles. Anirudhan et al. [53] developed a graphene oxide (GO) and modified it to amine-functionalized GO (AGO), which acts as a cationic polyelectrolyte. Further, chitosan (CS) was conjugated with FA through N,N-dicyclohexylcarbodiimide coupling to form FA-CS. Subsequently, itaconic acid and acrylic acid monomers are grafted to the hydroxyl group of CS using ethylene glycol dimethacrylate as cross-linker and potassium peroxydisulfate as an initiator to generate –COOH functional groups and forming chemically modified chitosan (CMCS). In addition, doxorubicin (DOX) was loaded into the FA–CMCS/AGO through π–π stacking interactions. This carrier forms the complex with DOX through π–π stacking and hydrogen bonding interactions, which enhances the increased encapsulation and loading efficiency of DOX. The encapsulated DOX into the polymeric matrix specifically transit the cargo into HeLa cells through FA receptors. This system demonstrated the pH-responsive DOX release behavior at acidic conditions. Liu et al. [54] developed a targeted pH-responsive conjugated with polysaccharide and constructed with boronate linkage of N-(2-aminoethyl)-gluconamide- and grafted hyaluronic acid (HA) loaded with anticancer drug bortezomib, which could mediate the targeted drug release behaviors at acidic pH and exhibits decreased cytotoxicity and an increased inhibition rate toward cancer cells. Men et al. [55] were motivated by overexpression of CD44 receptor in tumor microenvironment on the outside of the tumor cells. Through this inspiration, they have designed and developed a pH-sensitive liposome–polymer nanoparticle (NP) consisting of lipid, HA, and poly(β-amino ester) (PBAE) by layer-by-layer (LbL) technique for targeted delivery and controlled release of DOX to augment the cancer treatment efficacy. The release profile of the DOX from multilayered nanoparticles was depending on pH. The percentage of release at low pH was notably stimulated in contrast to that at the base or normal pH, determining the promise for controlled drug release as shown in Figure 3. Further, A549 cells treated with DOX-loaded nanoparticles resulted in very low toxicity as compared to free DOX. In vivo efficacy of the DOX-loaded NPs showed an excellent anticancer efficacy with minimal side-effects in contrast to free DOX and PBS causing an active targeting of HA and pH-triggered drug-release profile (Table 1).
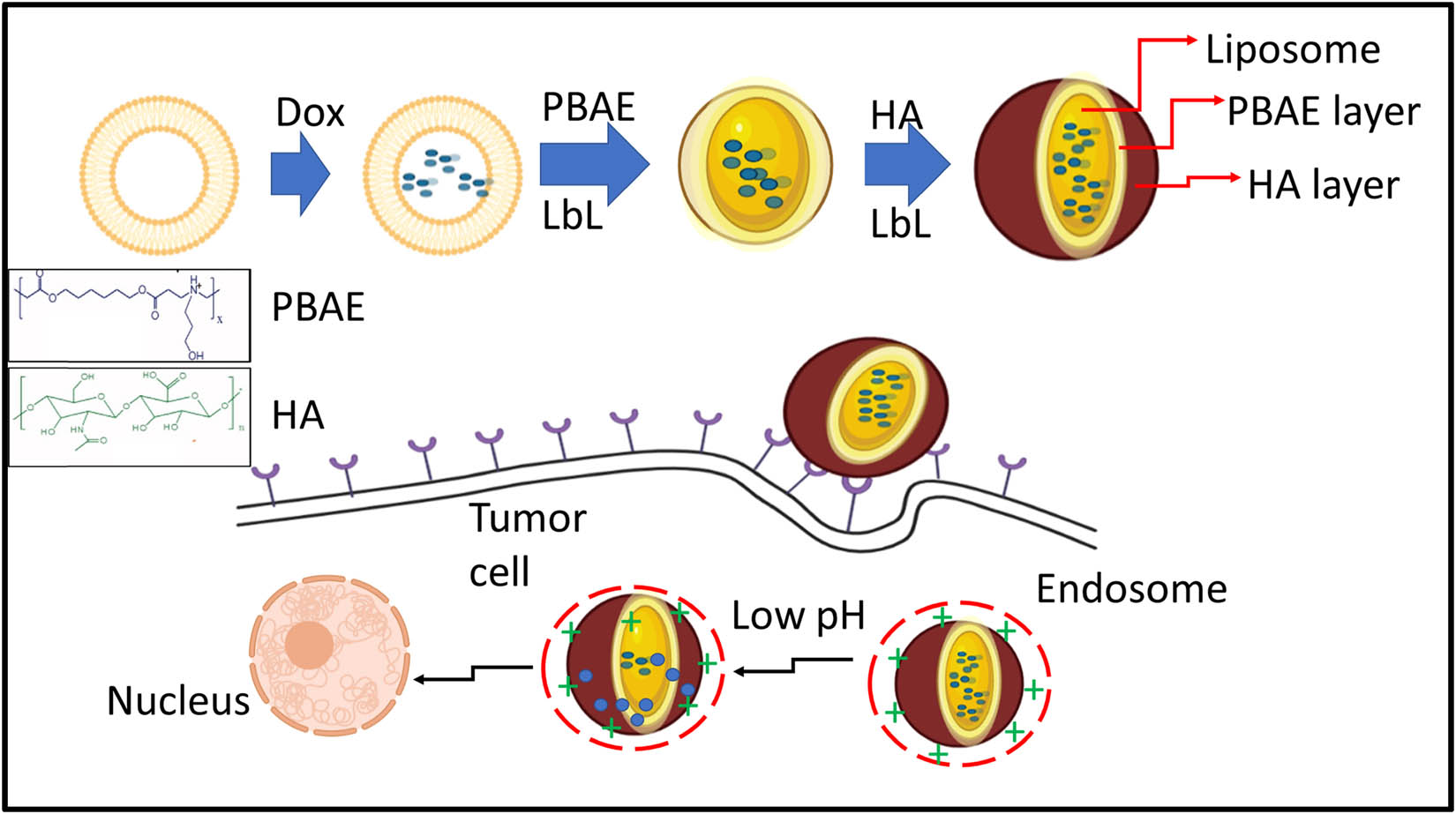
Schematic illustration of the development of LbL DOX-loaded NPs for targeted drug-delivery systems.
pH responsive DDS for mono and combination cancer therapy
| Nanoparticles used | Composition | Payload for delivery | Outcome | References |
|---|---|---|---|---|
| Amphiphilic polymer | PEG-BHyd-dC12 via an acid-labile hydrazone bond | Paclitaxel (PTX) | • Exhibited pH-dependent drug release profile | [56] |
| • Enhanced endosomal escape | ||||
| • Intracellular delivery, and enhanced anti-tumor activity | ||||
| Hybrid nanoparticles | pH-responsive hybrid ATRAM-BSA-PLGA | DOX-triphenylphosphine | • Nanoparticles exhibited highly efficient pH-dependent cellular uptake | [57] |
| • Energy-independent and -dependent internalization mechanisms | ||||
| • Significant cytotoxicity | ||||
| Hollow mesoporous silica nanoparticle | Mesoporous silica nanoparticles tagged with folic acid, coupled with polyethyleneimine (PEI–FA) | DOX and siRNA toward anti-apoptotic genes | • pH-responsive intracellular drug and siRNA release | [58] |
| • Lowers off-target effects, | ||||
| • Down regulation of anti-apoptotic protein Bcl-2 and triggered apoptosis | ||||
| Black phosphorous nanoparticles | DOX-loaded BP@PDA-PEOz-BTZ platform | DOX and bortezomib | • Exhibits high encapsulation efficiency | [59] |
| • Enhanced cellular uptake and cytotoxicity | ||||
| • Demonstrated photo-responsive, and fast drug release triggered by low pH | ||||
| pH sensitive polymerosomes | Angiopep-2-tagged pH-responsive polymersomes (Au-DOX@PO-ANG) | DOX | • pH sensitivity and counter the tumor microenvironment | [60] |
| • These targeted polymerosomes has ability to cross blood brain barrier |
2.1.2 Redox responsiveness
Redox responsive mechanism is the second most commonly used method to pH responsiveness for constructing stimuli drug delivery systems. The substances such as vitamin E, vitamin C, and glutathione are the reducing agents that are broadly present in our humans. Out of the reducing agents present in our body, glutathione (GSH) is most involved in the metabolic process [61]. The snap switch between the oxidized and reduced forms of glutathione secures the cells from pro-oxidant stress. This consists of a tripeptide sequence of glutamic acid-cysteine-glycine. The distinct feature of the peptide is the peptide bond configured between the gamma carboxylic acid of glutamic acid and the alpha-amino group present in cysteine. The stability in circulation as GSH levels are low in the extracellular environment is about 0.002 mM. Good response to high intracellular levels of GSH is about 1–10 mM indicating that the GSH-triggered release of redox responsive nanocarriers can take place inside the tumor cells and not in the extracellular environment. An intracellular concentration of GSH in tumor tissues is at least four times higher than that in normal tissues. The ratio has been varied in different cancer cells such as head and neck, ovarian, breast, pancreatic, and lung cancer cells [62]. The unique aspect of the redox responsive nanocarrier systems is the S–S bond that is chemically cross-linked as a gating or capping molecule on the surface of the nanoparticle is cleaved upon the addition of GSH, causing the rapid drug release to the tumor cells [63]. Sauraj et al. [64] developed a redox responsive nanocarrier system using Xyl-SS-Cur for dual delivery of curcumin and 5-FU in human colorectal cancer therapy. Researchers have synthesized Xyl-SS-Cur through covalent conjugation of curcumin to xylan via the disulfide bridge. The redox-sensitive Xyl-SS-Cur facilitates the self-assembly into the nanocarrier through the encapsulation of lipophilic 5-fluorouracil-stearic acid into the hydrophobic core containing Xyl-SS-Cur NPs/5-FUSA NPs via the dialysis membrane method. The Xyl-SS-Cur/5-FUSA NPs demonstrated a significant redox-responsiveness system because of the disulfide bridge causing the release of dual drug curcumin and 5-FUSA predominantly in the presence of 10 mM glutathione (GSH). The blood compatibility results determined hemolytic activity even at higher temperature Xyl-SS-Cur/5-FUSA NPs. The cytotoxicity results showed a significant reduction in the cell viability levels and an inhibition activity in cancer cells (HT-29, HCT-15). Hence, these dual drug delivery redox responsive nanocarrier systems would be potential and ideal candidates for multiple drug delivery research soon. Jia et al. [65] presented the two-stage rocket mimetic redox responsive system for a better tumor intracellular and tumor microenvironment. They designed a redox responsive nanocarrier, organo silica-micellar hybrid nanoparticles doped disulfide, and surface functionalized with PEG and amido-bonded polyethyleneimine (PEI), called as DOSN-PEI-SS-PEG, the disulfide tagged PEG of DOSN-PEI-SS-PEG, guards the positive charges of PEI, make sure the elongated circulation time, and inhibits the non-specific adsorption of protein. At stage 1, the long chain of PEG is broken with an extracellular concentration of GSH (2–10 μM). At stage 2, the positively charged PEI enhances the cell internalization through electrostatic interactions. This PEI allows the nanocarrier to get away from the endosomes and enters the cytoplasm through the proton sponge mechanism. In addition, the second stage of endocytosis by tumor cells is stimulated with an elevated intracellular concentration of GSH redox-responsive system by lysis of the disulfide bonds of the silsesquioxane matrix, causing the drug release. This two-stage rocket-mimic nanocarrier would be a novel approach to ameliorate the selective or target specific, in vivo efficacy, enhanced tumor accumulation, and safety chemotherapy.
Chang et al. [66] presented a novel redox-sensitive system, with polyethylene glycol-poly(β-benzyl-l-aspartate) (PEG-PBLA)-SS-paclitaxel (PPSP) prodrug conjugated with disulfide bridges, constructed, and synthesized successfully. This system was developed to improve the faster release of drug molecules to the cancer cells HepG2 and MCF-7 from the hydrophobic layer (prodrug) through lysis of the disulfide bridges upon exposure to GSH. This redox-sensitive (PEG-PBLA)-SS-paclitaxel (PPSP) system enhances the anti-tumor efficacy and biosafety. Chen et al. [67] reported a novel system, transferrin is a glycoprotein and an upregulated receptor present on the surface of the cancer cells. This protein is conjugated on the surface of the mesoporous silica nanoparticles via redox lysed disulfide bonds, which function both as a targeting and capping agent concurrently. This system (DOX@MSNs-S-S-Tf) showed a very slow release in the presence of GSH, at the high concentration of GSH, it triggers the drug release, limiting the premature leakage, and enhances the anti-tumor efficacy. The unique aspect of this system is three functions in one moiety such as targeting, capping, and intracellular uptake of cells. In summary, this protein conjugated MSNs for GSH-triggered drug delivery system would be an ideal and potential candidature for our new insights of upcoming research. Zid et al., [68] developed DOX-loaded hexagonal mesoporous silica nanoparticles to confirm the redox responsive system, cysteamine-based ligand was grafted on the surface of the SBA 15. Further, to prove the premature leakage of thioglycolic acid-functionalized ZnS nanoparticles (ZnS–COOH NPs) as pore capping agents. These cysteamine-grafted SBA 15 was bonded by peptide to thioglycolic acid groups of ZnS–COOH NPs. In vitro results of this system demonstrated that the cleavage of the disulfide bond through the addition of thiol reducing agents caused the drug to release and kill the cancer cells resulting in significant cytotoxicity effects. Schematic representation for the disulfide-linked oxidized cysteine-phenylalanine nanoparticles drug carrier is shown in Figure 4 (Table 2).
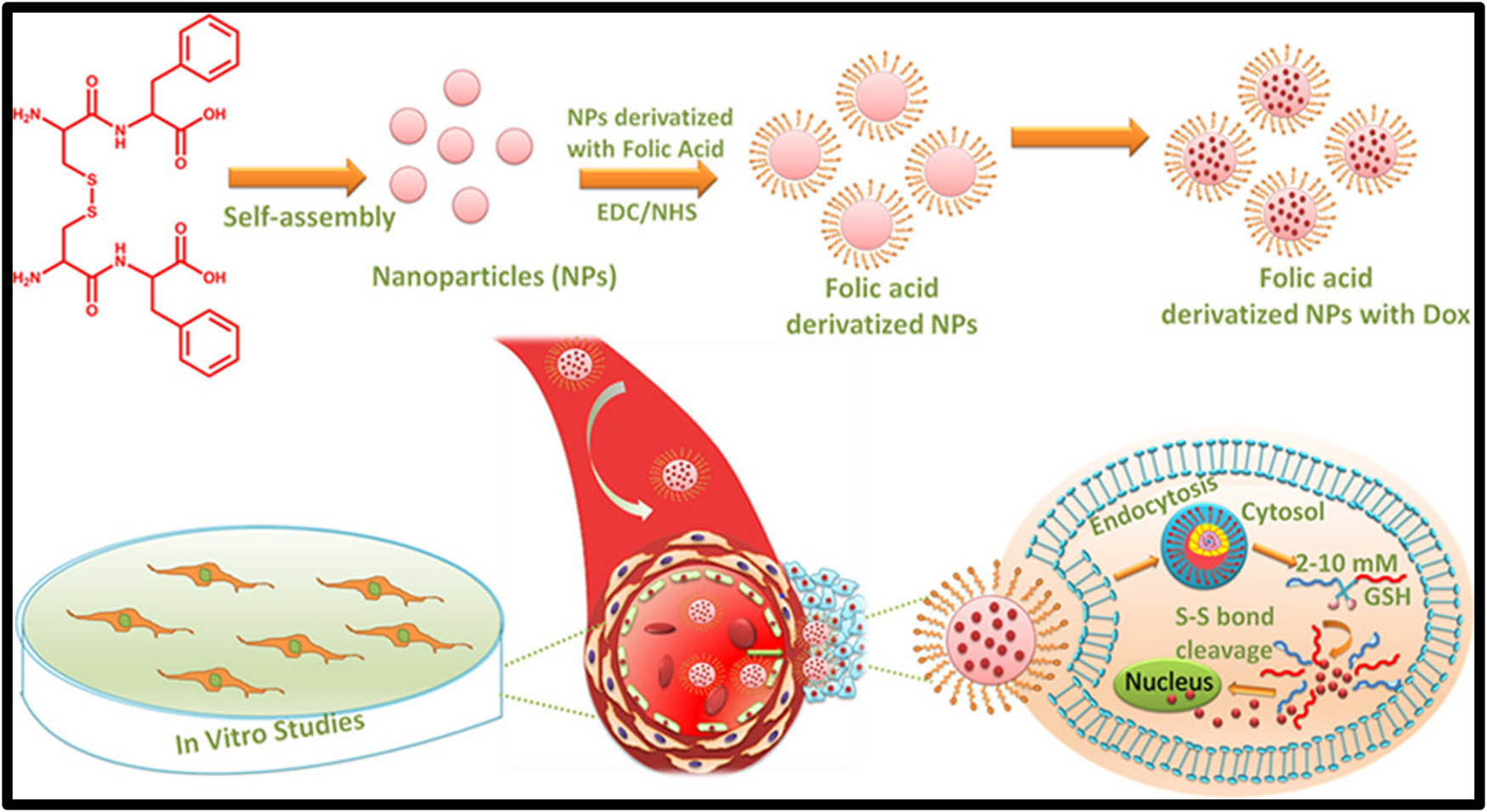
Schematic representation for the disulfide-linked oxidized cysteine-phenylalanine nanoparticles drug carrier.
Redox responsive DDS for mono and combination cancer therapy
| Nanoparticles used | Composition | Payload for delivery | Outcome | References |
|---|---|---|---|---|
| Disulfide-linked oxidized cysteine-phenylalanine nanoparticles | Folic acid-CFO-Dox-nanoparticles | DOX | • Increased cell uptake of FA tagged nanoparticles | [69] |
| • Significant reduction in cell viability compared to plain drug | ||||
| PAEs/RNA complex nanoparticles (PAEN) | Bioreducible poly(b-amino esters) (PAEs), poly[bis(2-hydroxylethyl)-disulfide-diacrylatetetraethylenepentamine] (PAP) | iMdr-1-shRNA, iSurvivin-shRNA | • Demonstrated low IC50 of DOX in multidrug resistance cells, down-regulated P-gland reduced tumor size of survivin, in vivo | [70] |
| Anti-carbonic anhydrase IX antibody on the surface of mesoporous silica nanoparticles via disulfide linkages | Doxorubicin@MSNs-CAIX | DOX | • Doxorubicin@MSNs-CAIX would facilitate cell internalization with response to glutathione trigger | [71] |
| • It suppresses the tumor growth efficiently | ||||
| Mesoporous silica nanoparticle | Amino-gated alkyl chains with disulfide bonds modified on the surface of nanoparticle by the reaction of S-(2 aminoethylthio)-2 thiopyridine hydrochloride (SATH) and thiol-modified nanoparticles (MSNPSH) | Negatively charged ssDNA, DOX | • Increased cellular internalization and significant apoptosis induction | [72] |
| Biodegradable polymeric NPs | Solid poly(disulfide amide) (PDSA)/cationic lipid core and a lipid–PEG shell | siRNA | • GSH-triggered intracellular siRNA release | [73] |
| • Potent gene silencing |
2.1.3 Enzyme responsiveness
Enzyme responsive drug delivery system is well developed and functions in many biochemical effects inside the body. There are many enzymatic reactions such as esterase’s produce ester hydrolytic reactions; glycosidases induce the hydrolysis of glycosidic bonds; peptidases invoke the breakdown of proteins into amino acids, and so on. The attractive feature of the enzyme is to provide the biodegradation of biomacromolecules and other important enzymes that are up-regulated in cancer cells would be advantageous for emerging of an enzyme-responsive drug delivery system [74]. Naz et al. [75] presented an enzyme-responsive drug delivery system by constructing mesoporous silica nanoparticles for multiple targeted anti-cancer activities. This mesoporous silica nanoparticle acquires both CD44 and triphenylphosphine (TPP) for mitochondria targeting effects and is further encapsulated with DOX then by capping with tumor-targeting molecules HA through electrostatic interactions. The HA capped on the surface of the mesoporous silica nanoparticles impart a powerful sealing ability in normal cells and improved selective uptake by cancer cells through CD44 receptor-mediated endocytosis pathway. These systems would be great for exhibiting enzyme responsive systems via the degradation property of HA in cancer cells as shown in Figure 5.
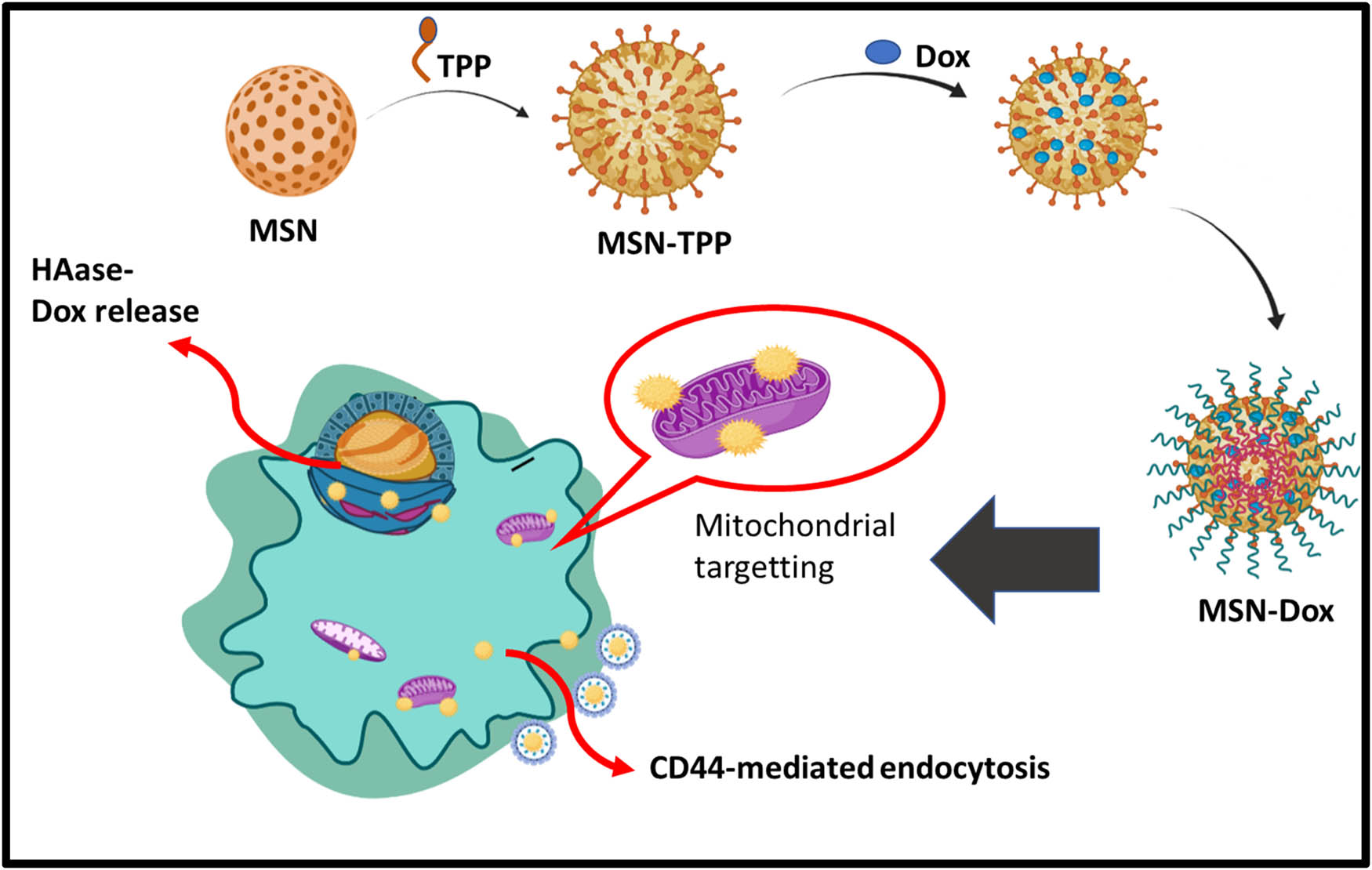
Schematic representation of enzyme responsive system.
β-gal enzyme upon exposure to elevated concentration and go along with the emission produced by the fluorescence turned on, the DOX is released to the tumor cells. The in vitro and in vivo results showed a significant therapeutic potent on GalDOX specifically in ASGP overexpressed HT-29 cells and significant retardation of tumor growth was observed in the xenograft mice model [76]. Zhou et al. [77] developed an enzyme-responsive drug delivery system involving mesoporous silica nanoparticles covalently conjugated with extracellular matrix biomacromolecules such as collagen I and HA enhances biocompatibility and limits premature leakage. Drug release was attained by biodegradation provoked by hyaluronidase (HAase) and matrix metalloproteinases 2 (MMP-2), which are over-expressed in cancer cells. The in vitro studies enhance the biocompatibility and targeting of the cells. The in vivo results demonstrated that this system would retard the tumor growth and reduces the side effects to the normal cells (Table 3).
Enzyme responsive DDS for mono and combination cancer therapy
| Nanoparticles used | Composition | Payload for delivery | Outcome | References |
|---|---|---|---|---|
| Theragnosticnanohybrids | GO capped with poly(ethylenimine)-co-poly(ethylene glycol) (PEI–PEG) via MMP2 cleavable PLGLAG peptide linkage | DOX, DNA | • Significant drug toxicity against tumor cells | [78] |
| • Coherent transfection in contrast to that with PEI25k | ||||
| Dendrimers (PAMAM) | γ-Glutamyl transpeptidase (GGT)-triggered transcytosis of the dendrimer–camptothecin conjugate | Camptothecin | • Increased anti-tumor activity in mouse models | [79] |
| Gold nanocluster | Fabricated gold nanocluster@bovine serum albumin (AuNC@CBSA-ICG), indocyanine green | Indocyanine green | • Hyaluronidase-dependent drug release | [80] |
| • Suppress tumor growth on mice breast cancer model | ||||
| Dendrimers | Dendritic thiolated hyperbranched polyglycerol | Maleimide-bearing DOX prodrug, maleimide-bearing methotrexate prodrug | • Exhibit high cytotoxicity against human cancer cells | [81] |
2.2 External stimuli for drug delivery systems
The external stimuli are man-designed stimuli, which are induced or signals from outside the body to stimulate the drug delivery systems. Recent advancements on the external stimuli developed by researchers have achieved a controlled release manner. There are quite a few parameters behind the interior body known as “external stimuli” such as magnetic fields, ultrasound, and light. In contrast with the “internal-stimuli” acts within tumor microenvironment like pH value, temperature, and redox as described earlier, the external stimuli-responsive drug delivery systems would introduce contrast agents to image, that of nanoparticles located in the target tissues, cells, or organelles, further triggers the nanocarriers outside the body through particular stimuli at a specific time. Hence, the controlled release is an increased spatiotemporal and has a great potential for clinical applications.
2.2.1 Magnetic field responsiveness
Magnetic systems are broadly utilized in targeting as well as imaging. As magnetic-responsive nanotherapeutics is a noninvasive signal, an externally applied magnetic field can damage the moving particles and increase the accumulation of anticancer agents in tumors by a magnetic field that would be employed for in vivo applications, this could have greater advantages for targeted cancer therapy as compared with intrinsic stimulus-responsive nanotherapeutics [82]. Furthermore, core/shell magnetic nanoparticles showed a collection of unique magnetic properties. The large surface-to-volume ratio of magnetic nanoparticles support a large number of active sites for conjugating a biomolecule, thus provides precise design and engineering to gain their smart functions by exploiting a localized external magnetic field, prolonged circulation in the bloodstream, targeting the damaged tissues, and therapeutic delivery [83]. Kong et al. [84] demonstrated a novel lipid–polymer hybrid nanoparticles encapsulated with magnetic beads and anti-cancer drug camptothecin for breast cancer therapy. The drug release was stimulated by external RF magnetic field actuation. In the presence of RF magnetic field actuation induces the heat by Fe3O4 in the inner side of the polymeric cores, which relaxes the polymer matrices and triggers the drug release, causing the elevated significant cytotoxicity in vitro results toward breast cancer cells.
Guisasola et al. [85] constructed a new system of magnetic field responsive systems that can control the release of drug molecules and proteins upon an altering on and off the magnetic field. Mesoporous silica nanoparticles were encapsulated with iron oxide and surface functionalized with temperature below lower critical solution temperature (LCST) based polymer called PNIPAM. The release was stimulated by altering the on and off switch of the magnetic field. As the temperature of the polymer increased above LCST upon the alternative magnetic field on and off switch, hence the drug is released on the conformational change of the surface-coated polymer. Chen et al., [86] presented a magnetic field responsive based drug delivery using (MSN@Fe3O4) capped with amine group with 2,3-dimercaptosuccinic acid-functionalized Fe3O4 nanoparticles via chemical amidation. Upon the induction of the magnetic field, nanocaps were detached by lysing the chemical bonds, resulting in faster drug release. These release profiles were controlled by the duration of strength and time. Hence, these MSN@Fe3O4 nanocarriers also play a role in T2 magnetic resonance contrast agents that could be useful for molecular imaging. Schematic representation for the rocket-mimetic mechanism of DOSN-PEI-SS-PEG drug carrier is shown in Figure 6 (Table 4).
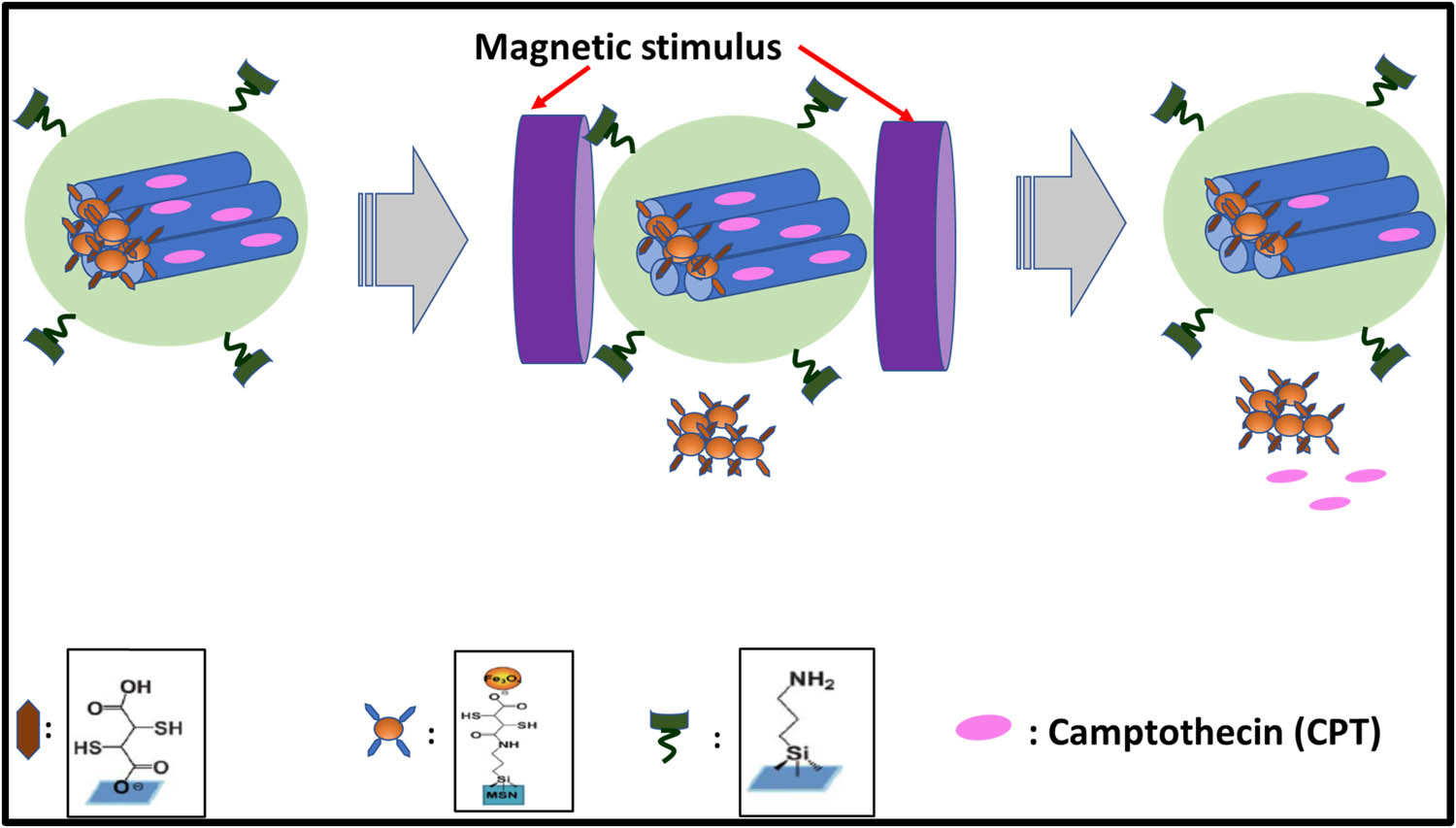
Schematic illustration of magnetic field responsive system.
Magnetic field responsive DDS for mono and combination cancer therapy
| Nanoparticles Used | Composition | Payload for delivery | Outcome | References |
|---|---|---|---|---|
| Iron oxide-double emulsions nano capsules | Polyvinyl alcohol shell inside iron oxide nanoparticles (core shell), IVO24, a peptide targeting cancer cells, capped to double emulsion capsules | Paclitaxel, DOX | • Controllable drug release | [87] |
| • Intensified dual magneto-chemotherapy and magneto-hyperthermia in vitro and in vivo | ||||
| Magnetic nanoparticles | Amine groups attached to the carboxylic functional groups coating magnetic nanoparticles (fluid MAG-CMX) | DOX | • Tumor retardation rate of CMX–DOX NPs under a magnetic field was significantly higher than the control group | [88] |
| Polymersomes | Superparamagnetic iron oxide nanoparticles (USPIO; γ-Fe2O3) | DOX | • Upon magnetic field triggers an increased intracellular drug release | [89] |
| Lipid-coated magnetic nanoparticles | Magnetic lipid microcapsules (MLMs) containing lipid-coated magnetic nanoparticles | 5-(6)-Carboxyfluorescein | • Effectively reduces the aggregation | [90] |
| • Improved stability and biocompatability |
2.2.2 Ultrasound responsiveness
Ultrasound is one of the most commonly used exogenous stimuli methods in cancer therapy. The unique advantages of ultrasound responsiveness are safety and deeper penetration into tissue, which is a non-invasive method. In the drug delivery systems, ultrasound has the pressure waves at frequencies of 20 kHz or greater is a most important factor for local stimulus concerning site-specific delivery and spatial release control of drugs tempt an increasing awareness on the cancer therapy [91]. Paris et al., [92] developed an ultrasound responsive system based on mesoporous silica nanoparticles capped p(MEO2MA)-co-THPMA on the surface connecting US-cleavable hydrophobic tetrahydropyranyl moieties, confer an LCST below physiological temperature. At 4°C, the polymer in the state of coil-like confirmation, allows the drug to be encapsulated inside the pores of the silica. As the temperature elevates to 37°C, the polymer transforms to the collapsed state and the drug molecule continue to be inside the pores. On exposure to the ultrasound irradiation, hydrophobic tetrahydropyranyl moieties are cleaved and the polymer turns to the hydrophilic nature and thereby increases in LCST temperature above 37°C. This exhibits the conformational change of the polymer, allowed to open the gates of the pores to release the encapsulated drug as shown in Figure 7.
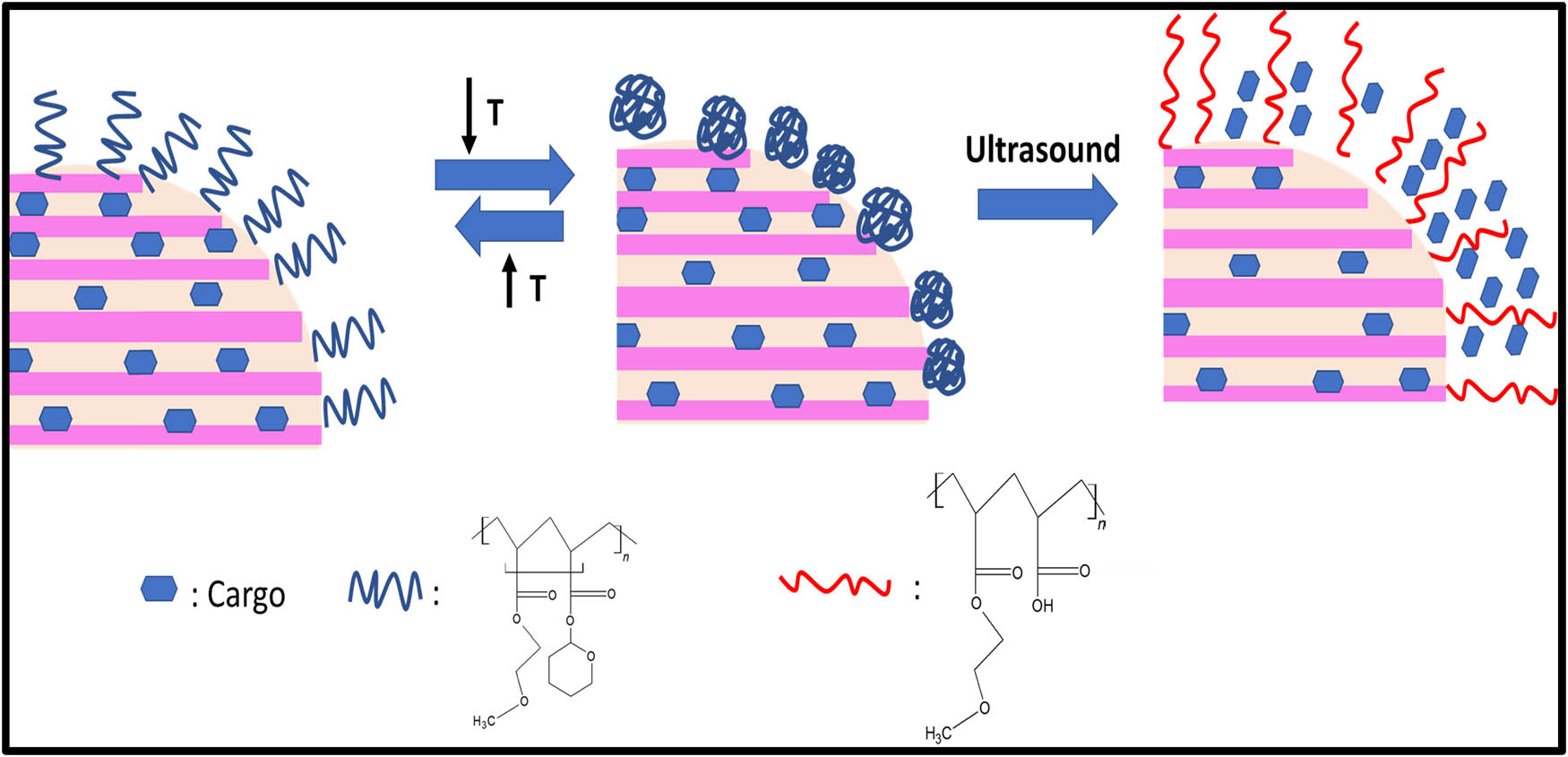
Schematic illustration of Ultrasound responsive systems.
Kim [93] and his group members developed a DOX-loaded titanium nanoparticles surface coated with poly(methyl vinyl ether-alt-maleic anhydride) pPBA through the generation of the boronic ester bond, forming pPBA@TNP-DOX nanoparticle to enhance the tumor-targeting via the synergy between PBA and sialylated epitope of cancer cells. When it is exposed by ultrasound, the drug molecule could be released from pPBA@TNP-DOX on breaking of boronic ester bonding mediated by ultrasound-mediated ROS generation. This system suggests that pPBA@TNP-DOX could be ideal for ultrasound responsive drug delivery (Table 5).
Ultrasound responsive DDS for mono and combination cancer therapy
| Nanoparticles used | Composition | Payload for delivery | Outcome | References |
|---|---|---|---|---|
| Lipid microbubbles | Paclitaxel-loaded lipid microbubbles | Paclitaxel | • Has ultrasound-mediated drug delivery with low-frequency US transducer | [94] |
| • Able to visualize and track in the iliac artery which is good for identification restenosis | ||||
| Micelle | Perfluoropentane or perfluoro-15-crown-5-ether | DOX | • Ultrasound triggered intracellular and nuclear trafficking. Megahertz continuous wave or pulsed ultrasound with 33% duty cycle at 3.4 W cm−2 nominal power density | [95] |
| Alginate hydrogels | Cross-linked polymers with respect to ultrasonic irradiation can stimulate drug release on-demand | Mitoxantrone and macromolecules such as siRNAs | • Ultrasound triggered system enables the digital drug release | [96] |
| • Ultrasound mediated release effectively reduces the tumor growth in vivo | ||||
| Nested nanobubbles- | Nested-nanobubbles containing outer liposomal shell-loaded with drug and couple of nanobubbles | Calcein | • The use of continuous wave and exposure to ultrasound trigger the drug release | [97] |
2.2.3 Temperature responsiveness
There are many exogenous stimuli used for drug delivery systems, among those temperature-responsive drug delivery systems afford potential advantages compared to other counterparts because of their flexibility in design, regulating the phase transition temperatures, passive targeting capability. The localized hyperthermia from 42.5 to 43.5°C helps to evade cancer cells by inducing high temperature to tumor tissues. On the other way, these stimuli on hyperthermia would enlarge the blood vessels and modify the perforation of tumor cell membranes, thereby enhancing the anti-tumor drug delivery. Temperature responsive properties are seen in the polymers such as poly(N-isopropyl acrylamide) (PNIPAM). These temperature-responsive polymers have a LCST factor. When the room temperature is lesser than the LCST, these polymers become soluble and move to the swelling state because of the hydrogen bonds present between the polymer chain and water molecules. As the temperature increases, the hydrogen bonds break, leading to insolubility and collapse of the polymer. This mechanism would help in developing the temperature-responsive drug delivery system [98].
Temperature responsive drug carrier Fe3O4/PNIPAM/5-Fu@mSiO2-CHI/R6G was developed by Shen et al. [99]. They studied a drug carrier using different temperatures in vitro. In this system, Fe3O4 magnetic nanoparticles, as a source of magnetic used for the treatment of hyperthermia. This hyperthermia treatment of Fe3O4, Fe3O4/PNIPAM/5-Fu@mSiO2, and Fe3O4/PNIPAM/5-Fu@mSiO2-CHI/R6G was demonstrated using a magnetic field. The thermoresponsive drug delivery could be controlled by the magnetocaloric function of the magnetic field. These systems would release a drug ideally at 45°C, which was due to the advantage of smart thermoresponsive polymer PNIPAM. Demirci et al. [100] developed an iron oxide nanoparticle coated with dextran to destroy the retinoblastoma cells in a significant manner as compared to normal cell counterparts. Magnetic hyperthermia stimulated apoptosis seen in Y79 cells basically through the intrinsic pathway initiated by FAS and TNF-a signaling. These iron oxides-coated dextran nanoparticles would be a promising strategy for thermo-chemotherapy. Zamora-Mora et al. [101] in 2017 reported tripolyphosphate salt (TPP) cross-linked with chitosan nanoparticles (CSNPs) for combined delivery of MHCT and 5-fluorouracil (5-FU) in human glioblastoma A-172 cells. The dual functionality of these magnetic CSNPs with core-shell morphology showed a dose-dependent reduction in cell viability levels of glioma cells on AMF application as compared to fibroblasts cells counterparts showing no reduction in viability levels. Magnetic core–shell nanoparticle (MCNP) activated drug delivery of a mitochondria-targeting proapoptotic amphipathic tail-anchoring peptide (ATAP) in association with MHCT for malignant brain cancer cells as shown in Figure 8 (Table 6).
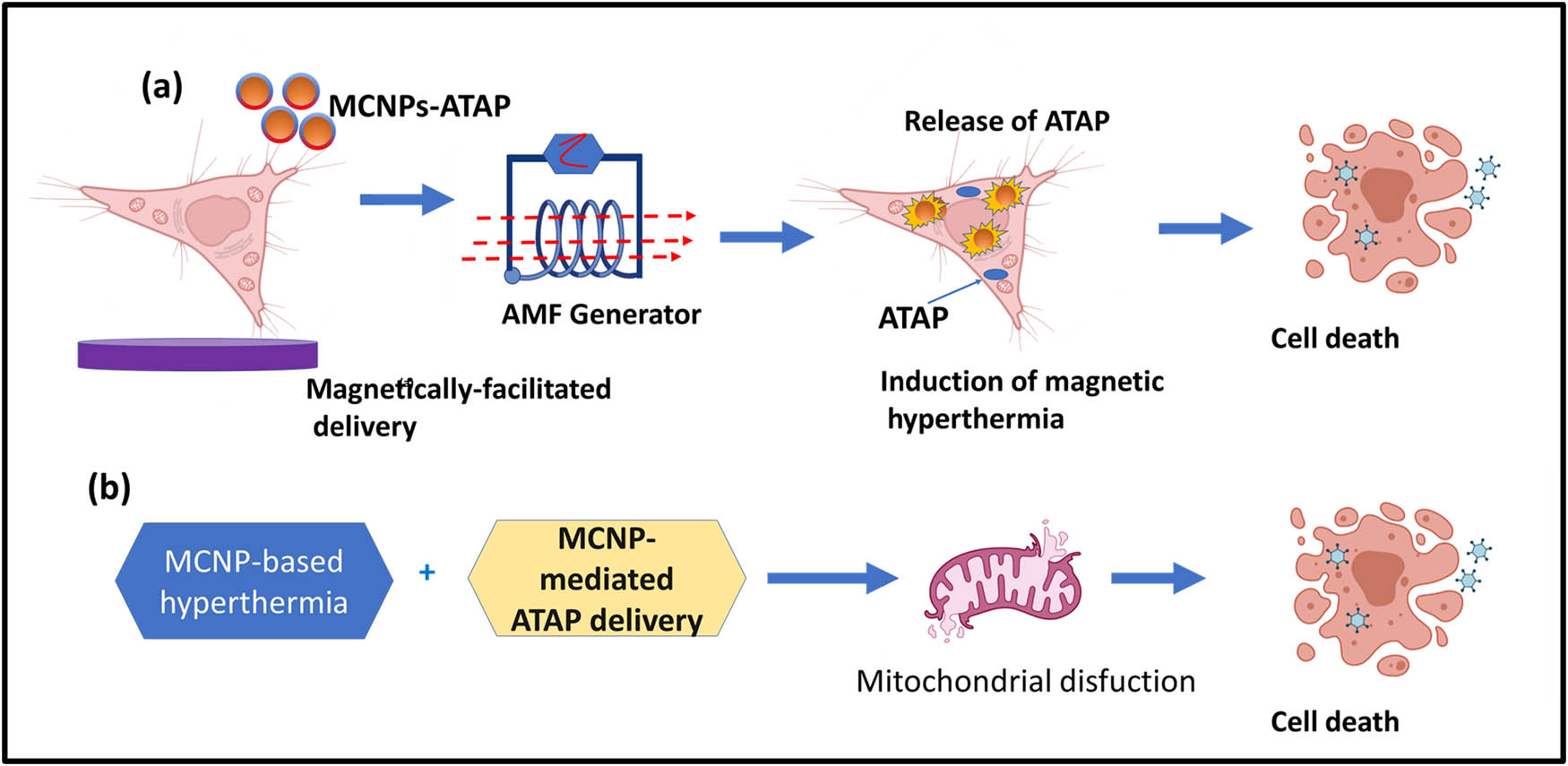
Schematic representation of temperature-responsive systems.
Temperature-responsive DDS for mono and combination cancer therapy
| Nanoparticles used | Composition | Payload for delivery | Outcome | References |
|---|---|---|---|---|
| Polypeptides | Polyaspartamides with isopropylamide and hydroxyalkylamide pendant groups | DOX | • The phase transition temperature (Tp) of the copolymers phase transition temperature could be varied by modulating the pendants composition | [102] |
| • Exhibited a temperature-responsive release | ||||
| Core-shell nanoparticles | Hollow poly(N-isopropylacrylamide) (PNIPAM) nanogels surface modified with silica nanoparticles | DOX | • Lower density of cross-linking showed an elevated controlled release | [103] |
| • Determines a higher toxicity toward Hela cells | ||||
| Thermo-responsive barrier gel | Injectable thermo-gelling poly(diethylaminoethyl methacrylate) (PDEAEM)-Pluronic F127 (PL)-PDEAEM pentablock copolymer (PB) | Paclitaxel, DNA | • Magnified transfection efficiency | [104] |
| • Anti-cancer activity of paclitaxel in vitro |
2.2.4 Light responsiveness
Light-stimulated drug delivery system is one of the exogenous systems, most applied stimulus because of its noninvasive nature [105]. The main impact of light-responsive drug delivery systems is their temporal and spatial property which controls the release behavior accurately upon stimulation light exposure at a specific time and position. There are different lights with different wavelengths. As of now, the drug delivery systems use ultraviolet light, visible light, and near-infra-red light. There are three major mechanisms on how these light-induced drug delivery systems work, such as isomerization, bond cleavage, and disaggregation of the nanocarrier on exposure to light. The wavelength of the ultraviolet ranges from 10 to 400 nm, lower than that of the visible light but greater than that of X-rays. The most commonly used chromophores are azobenzene, coumarin, spiropyran, pyrenylmethyl, o-nitro benzyl are UV light-responsive groups. Wang and Wu [106] demonstrated mesoporous silica nanoparticles nano valves surface capped with tetra-ortho-methoxy-substituted azobenzene (mAzo) and β-CD and loaded with DOX for a controlled release from the mesopores. Azobenzene has the light-inducing property that would trigger the drug release to the tumor cells on red light irradiation as shown in Figure 9 (Table 7).
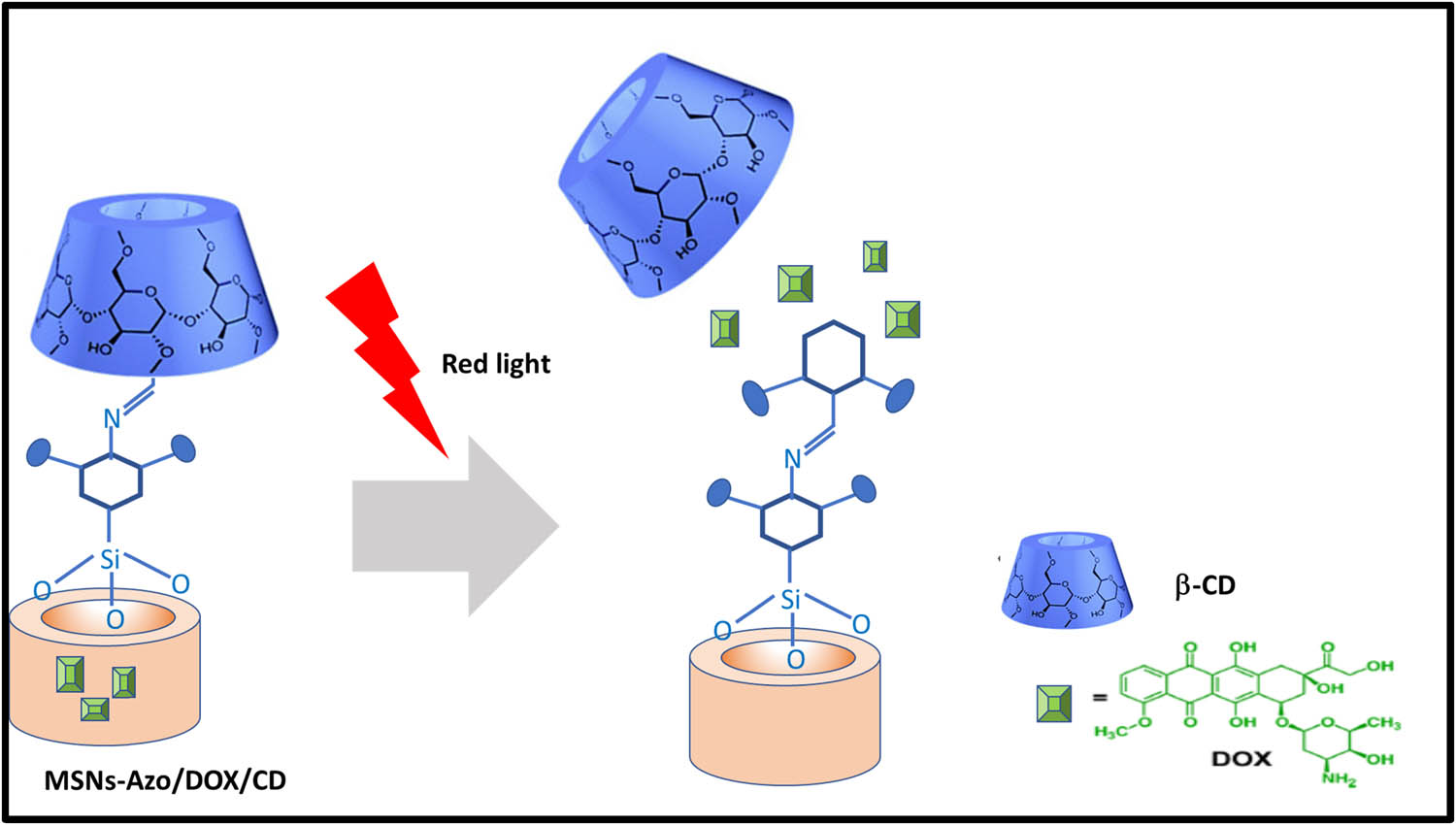
Schematic representation of light-responsive systems.
Light responsive DDS for mono and combination cancer therapy
| Nanoparticles used | Composition | Payload for delivery | Outcome | References |
|---|---|---|---|---|
| Dendritic micellar nanoparticles | Diazonaphthoquinone, light 808 nm; NIR irradiation; 365 nm, UV irradiation | DOX | • Exhibited a light-responsive release of DOX | [107] |
| Polymeric drug conjugates | Porphyrin-based polymeric drug conjugates PSDTD-m | Porphyrins | • Controlled release was achieved through reactive oxygen species cleavable linkage for anti-cancer efficacy and photodynamic therapy | [108] |
| HA-based nanocarriers | HA-photosensitizer conjugate (HA-TK-Ce6) | Ce6-Chlorin e6 | • Laser irradiation at 660 nm produces ROS during a photodynamic (PDT) therapy to cleave the TK linker next to Ce6, demonstrating in light-stimulated TKHCENPDOX breakage and selective DOX release in the tumor cells. | [109] |
2.3 Dual and multiple stimuli-responsiveness
Dual and multiple stimuli stars have been playing a potential role in biomedical materials on controlled drug delivery systems. An et al. [110] developed multistimuli-responsive nanoparticles, mesoporous silica nanoparticles end-capped with PEG-a-PCL-SSP(NIPAM-co-DMA) and loaded with an anti-cancer agent (paclitaxel, PTX) and a photothermal cyanine dye (cypate), which stimulate pH/reduction–responsive drug release upon exposure to disulfide bridges, resulting in the lysis of the acetal bonds, on exposure to NIR-light-irradiation enhance the temperature increase, photostability, and intracellular reactive oxygen species of cypate. Reactive oxygen species induced drug transportation from lysosomes to the cytoplasm, which results in the synergistic effect and causes effective thermo-chemotherapy as shown in Figure 10.
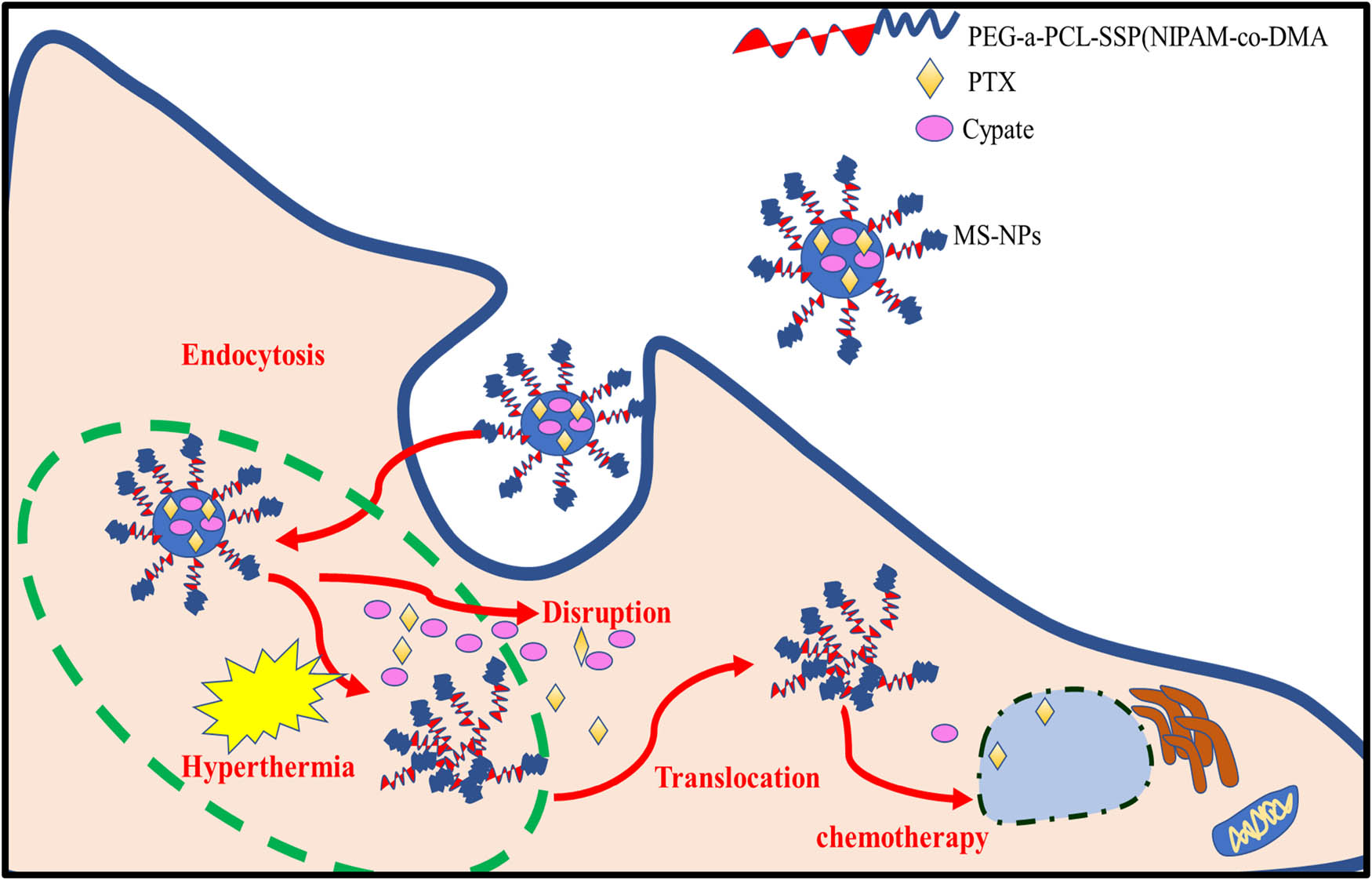
Schematic illustration of multiple stimuli-responsive systems.
They have also developed multistimuli composed of polymer, Dex-g-PpIX-g-PBA-SS-CPT (DPPSC), fabricated using dextran(hydrophilic) grafted protoporphyrin IX (PpIX) and loaded with anticancer drug camptothecin (CPT) and also conjugated with Dextran via the disulfide bridge holds the pH-responsive linker (hydrophobic). Dextran is a polysaccharide that has the property to prolong the blood circulation time of self-assembled micelles of the polymer, increases tumor accumulation through the enhanced permeability and retention effect. After the micelles get inside the tumor tissue, on a short time of light irradiation PpIX gets activated and enhances the endocytosis process. Further, the puncture of the endocytic membrane on exposure to PDT long time irradiation activates reactive oxygen species, causing the micelles to get away from endosomes and lysosomes and get into the cytoplasm. In the acidic lysosomal environment, the hydrolysis of the boric acid ester linkage at low pH enhances the release of the prodrug (PBA-SS-CPT) from the nanoparticles. Once it enters the cytoplasm, the prodrug CPT converts into the free CPT through GSH responsiveness. Therefore, the drug enters the nucleus for an effectively combined chemo phototherapy with minimized side effects [111]. Researchers have also developed a dual stimulus by supramolecular polymer nanocarriers CPAP (CDPEG), hydrophilic and hydrophobic (Azo-PCL). PEG was grafted onto the surface of b-CD through disulfide linkages to form CD-PEG, and Azo-PCL were poly(ε-caprolactone) chains with azobenzene groups grafted at one end. CD-PEG and Azo-PCL were combined by the host–guest interaction between azobenzene and b-CD. The PCL chains with hydrophobic core loaded the hydrophobic drug DOX. The nanocarriers CPAP-loaded DOX detaches and triggers the drug release with an exposure of glutathione and light irradiation at 365 nm. These results demonstrated that CPAP nanocarrier would be the potential for killing the cancer cells by preventing the normal cells with adverse side effects [112]. Ko et al. [113] developed a novel dual stimuli-responsive graphene quantum dots grafted multifunctional carbon nanoparticles (DS-CNPs). These nanoparticles further functionalized with PEG and HER to form the ester/amide bonds (pH-dependent and GSH responsive disulfide bonds). Further DOX was encapsulated in DS-CNPs (DL-CNPs) as the size of the nanoparticles larger than 200 nm are preferred intravenous injection, gathers in the breast cancer region through EPR effect. Once endocytosis of DL-CNPs occurs, HER and DOX get released on exposure to pH and high concentration level of GSH. DOX gets inside the nucleus and causes apoptosis by interrupting DNA. HER also functions to suppress tumor growth.
2.4 Stimuli-responsive nanocarriers in clinical translation
There are few advancements made for stimuli-responsive nanocarrier systems from bench to bedside in the clinical trial and various formulations. They are also the barriers behind the clinical translations along with the progress such as
The differences between animal tumor models and tumors in patients, as tumors in patients, are more heterogeneous and complicated. The most primary factors that should be inscribed are toxicity, biosafety, and biodegradability of the nanocarriers.
In vivo function of the stimuli-responsive should be stable.
The efficacy and accumulation of the tumor of stimuli-responsive nanocarriers must be evaluated in the clinical trial.
The route of administration and dose of the stimuli-responsive nanocarrier must be considered. The most commonly used routes are intravenous injection (i.v.), intraperitoneal injection (i.p.). Hence, the clinical transfer of the stimuli-responsive nanocarriers and formulations should be optimized from sessions of a clinical trial [114,115].
Hence, stimuli-responsive nanoparticles and polymers in clinical trials as presented in Table 8.
Stimuli-responsive nanoparticles in clinical trials
| Stimulus | Nanocarriers | Type of cancer | Drug | Status | Reference |
|---|---|---|---|---|---|
| pH | Polymeric micelles | Solid tumor, sarcoma, and metastatic sarcoma | Epirubicin | Phase I/II | NCT03168061 |
| Thermosensitive liposomal | Thermo DOX | Breast cancer, primary liver cancer | DOX | Phase II/III | NCT00826085 |
| Magnetic | Iron oxide magnetite | Prostate cancer | Iron oxide nanoparticles | Phase I | NCT02033447 |
| Iron and carbon | Hepatocellular carcinoma | DOX | Phase II | NCT00034333 | |
| Genexol®-PM | PEG-PLA | Recurrent breast cancer | PTX | Phase IV | NCT00912639 |
| Breast cancer | Phase III | NCT00876486 | |||
| Ovarian cancer | Phase II | NCT00877253 | |||
| Non-small-cell lung cancer | Phase II | NCT01023347, NCT01770795 | |||
| NK012 | PEG-PGlu | Refractory solid tumors | SN-38 | Phase I | NCT00542958 |
| Nanoxel®M | PEG-PLA | Head and neck squamous cell carcinoma | Docetaxel | Phase II | NCT02639858 |
| NC-6004 | PEG-PGlu | Head and neck neoplasms | DDP | Phase I | NCT02817113 |
3 Conclusion
Very broad research has been carried out on stimuli-responsive drug delivery systems. As we desired to elaborate this review, the design of the stimuli-responsive drug delivery systems is not that easy process and it requires multidisciplinary knowledge of materials, chemistry, and biology. A unique aspect achieved by the stimuli-responsive systems is high drug loading capacity, in vitro drug release profiles, biocompatibility, and safety concerns on the efficacy of small molecule drugs. These stimuli-responsive nanocarriers can be tagged along with antibodies toward tumor immunotherapy [116,117]. A clinical stage of the stimuli-responsive system is arriving at the near future, interdisciplinary cooperation. Finally, the term “nanomedicine” will establish applications for novel and superior diagnostic, therapeutic, and preventive measures.
4 Future perspectives
Nanocarriers are a wonder of nanoscience, playing an important role in anti-cancer delivery and biomedical applications. To minimize the adverse events in conventional chemotherapy, stimuli-responsive drug delivery systems have been developed. Though there are exogenous and endogenous stimuli applied for the controlled drug release system into the tumor-specific release. Still, there are many challenges ahead to overcome in the stimuli-responsive drug delivery system. Therefore, stimuli-responsive research is a promising strategy for cancer treatment in chemotherapy. Hence this responsive system is under ongoing research to overcome the major challenges such as lack of multistimuli systems, immature tumor-targeting effects, biodegradation, and toxicity issues, developing combinational therapy for cancer treatment.
The future challenge is to evolve drug delivery systems acting to biomarkers with small concentration ranges and fabricate drug delivery systems with promptly activating factors. And also integrating multiple functions to drug delivery systems for occurring at the same time such as detection, diagnosis, and therapy of a disease with a nanoparticle. These stimuli-responsive systems - nanomedicine has paid great attention in the scientific community, however toward the academic lab research. The clinical translation of these systems are likely challenging, yet certainly not impossible.
Acknowledgment
The authors would like to acknowledge the University of Malaya.
-
Funding information: The authors would like to acknowledge the financial support provided by a Research University grant from the University of Malaya (RU001-2019, RU001-2020 and RU001-2021).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All relevant data are within the paper.
References
[1] Mahase E. Cancer overtakes CVD to become leading cause of death in high income countries. BMJ. 2019;366:366. 10.1136/bmj.l5368.Suche in Google Scholar PubMed
[2] You W, Henneberg M. Cancer incidence increasing globally: the role of relaxed natural selection. Evol Appl. 2017;11(2):140–52.10.1111/eva.12523Suche in Google Scholar PubMed PubMed Central
[3] Rotello VM. Sniffing out cancer using “chemical nose” sensors. Cell Cycle. 2009;8(22):3615–6.10.4161/cc.8.22.9915Suche in Google Scholar PubMed
[4] Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges, and opportunities. Nat Rev Cancer. 2017;17(1):20–37.10.1038/nrc.2016.108Suche in Google Scholar PubMed PubMed Central
[5] Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100–10. 10.1093/carcin/bgp263.Suche in Google Scholar PubMed PubMed Central
[6] Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA. Cancer J Clin. 2019;69:363–85. 10.3322/caac.21565.Suche in Google Scholar PubMed
[7] Adjiri A. Identifying and targeting the cause of cancer is needed to cure cancer. Oncology and therapy. 2016;4:17–33. 10.1007/s40487-015-0015-6.Suche in Google Scholar PubMed PubMed Central
[8] Bajaj A, Miranda OR, Phillips R, Kim IB, Jerry DJ, Bunz UH, et al. Array-based sensing of normal, cancerous, and metastatic cells using conjugated fluorescent polymers. J Am Chem Soc. 2010;132(3):1018–22.10.1021/ja9061272Suche in Google Scholar PubMed PubMed Central
[9] Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25.10.1016/j.addr.2013.11.009Suche in Google Scholar PubMed PubMed Central
[10] Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410.10.1038/s41467-018-03705-ySuche in Google Scholar PubMed PubMed Central
[11] Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12(11):958–62.10.1038/nmat3792Suche in Google Scholar PubMed PubMed Central
[12] Zhu X, Xue J, Gu X, Chen G, Cao F, Shan H, et al. Neoadjuvant chemotherapy plays an adverse role in the prognosis of grade 2 breast cancer. J Cancer. 2019;10:5661–70. 10.7150/jca.33168.Suche in Google Scholar PubMed PubMed Central
[13] Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–43. 10.18632/oncotarget.16723.Suche in Google Scholar PubMed PubMed Central
[14] Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, Roy P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis-complicating the treatment of cancer. Neoplasia. 2004;6(5):423–31.10.1593/neo.04169Suche in Google Scholar PubMed PubMed Central
[15] Bae YH. Drug targeting and tumor heterogeneity. J Control Rel. 2009;133(1):2–3.10.1016/j.jconrel.2008.09.074Suche in Google Scholar PubMed PubMed Central
[16] Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76.10.1038/nbt994Suche in Google Scholar PubMed
[17] Bae S, Ma K, Kim TH, Lee ES, Oh KT, Park ES, et al. Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials. 2012;33(5):1536–46.10.1016/j.biomaterials.2011.10.050Suche in Google Scholar PubMed
[18] Ke X, Shen L. Molecular targeted therapy of cancer: the progress and future prospect. Front Lab Med. 2017;1:69–75. 10.1016/j.flm.2017.06.001.Suche in Google Scholar
[19] De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–95.10.1038/nm871Suche in Google Scholar PubMed
[20] Bajaj A, Miranda OR, Kim IB, Phillips RL, Jerry DJ, Bunz UH, et al. Detection and differentiation of normal, cancerous, and metastatic cells using nanoparticle-polymer sensor arrays. Proc Natl Acad Sci. 2009;106(27):10912–6.10.1073/pnas.0900975106Suche in Google Scholar PubMed PubMed Central
[21] Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Rel. 2015;200:138–57.10.1016/j.jconrel.2014.12.030Suche in Google Scholar PubMed
[22] Navya PN, Kaphle A, Daima HK. Nanomedicine in sensing, delivery, imaging and tissue engineering: advances, opportunities and challenges. Nanoscience. 2019;5:30–56.10.1039/9781788013871-00030Suche in Google Scholar
[23] Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10(9):3223–30.10.1021/nl102184cSuche in Google Scholar PubMed PubMed Central
[24] Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.10.1201/9780429399039-2Suche in Google Scholar
[25] Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin F-H, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23:20. 10.1186/s40824-019-0166-x.Suche in Google Scholar PubMed PubMed Central
[26] Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today. 2016;19(3):157–68.10.1016/j.mattod.2015.08.022Suche in Google Scholar PubMed PubMed Central
[27] Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60(11):1307–15.10.1016/j.addr.2008.03.016Suche in Google Scholar PubMed
[28] Nosrati H, Salehiabar M, Manjili HK, Davaran S, Danafar H. Theranostic nanoparticles based on magnetic nanoparticles: design, preparation, characterization and evaluation as novel anticancer drug carrier and MRI contrast agent. Drug Dev Ind Pharm. 2018;44:1668–78.10.1080/03639045.2018.1483398Suche in Google Scholar PubMed
[29] Hua X, Yang Q, Dong Z, Zhang J, Zhang W, Wang Q, et al. Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 2017;24(1):511–8.10.1080/10717544.2016.1256001Suche in Google Scholar PubMed PubMed Central
[30] Qiu L, Li JW, Hong CY, Pan CY. Silver nanoparticles covered with pH-sensitive camptothecin-loaded polymer prodrugs: switchable fluorescence “off” or “on” and drug delivery dynamics in living cells. ACS Appl Mater Interfaces. 2017;9(46):40887–97.10.1021/acsami.7b14070Suche in Google Scholar PubMed
[31] Guldris N, Gallo J, García-Hevia L, Rivas J, Bañobre-López M, Salonen LM. Orthogonal clickable iron oxide nanoparticle platform for targeting, imaging, and on-demand release. Chem Eur J. 2018;24:8624–31.10.1002/chem.201800389Suche in Google Scholar PubMed
[32] Siafaka PI, Okur NÜ, Karantas ID, Okur ME, Gündoğdu EA. Current update on nanoplatforms as therapeutic and diagnostic tools: a review for the materials used as nanotheranostics and imaging modalities. Asian J Pharm Sci. 2021;16:24–46. 10.1016/j.ajps.2020.03.003.Suche in Google Scholar PubMed PubMed Central
[33] Navya PN, Madhyastha H, Madhyastha R, Nakajima Y, Maruyama M, Srinivas SP, et al. Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater Sci Eng C. 2019;96:286–94.10.1016/j.msec.2018.11.024Suche in Google Scholar PubMed
[34] Bano S, Nazir S, Munir S, AlAjmi MF, Afzal M, Mazhar K. “Smart” nickel oxide based core–shell nanoparticles for combined chemo and photodynamic cancer therapy. Int J Nanomed. 2016;11:3159–66.10.2147/IJN.S106533Suche in Google Scholar PubMed PubMed Central
[35] Vimala K, Sundarraj S, Paulpandi M, Vengatesan S, Kannan S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 2014;49(1):160–72.10.1016/j.procbio.2013.10.007Suche in Google Scholar
[36] Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:23. 10.1186/s40580-019-0193-2.Suche in Google Scholar PubMed PubMed Central
[37] Navya PN, Daima HK. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016;3(1):1.10.1186/s40580-016-0064-zSuche in Google Scholar PubMed PubMed Central
[38] Sabnis S, Sabnis NA, Raut S, Lacko AG. Superparamagnetic reconstituted high-density lipoprotein nanocarriers for magnetically guided drug delivery. Int J Nanomed. 2017;12:1453–64.10.2147/IJN.S122036Suche in Google Scholar PubMed PubMed Central
[39] Meng L, Huang W, Wang D, Huang X, Zhu X, Yan D. Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules. 2013;14(8):2601–10.10.1021/bm400451vSuche in Google Scholar PubMed
[40] Yeh H-P, DelValle AC, Syu MC, Qian Y, Chang YC, Huang YF. A new photosensitized oxidation-responsive nanoplatform for controlled drug release and photodynamic cancer therapy. ACS Appl Mater Interfaces. 2018;10:21160–72.10.1021/acsami.8b05205Suche in Google Scholar PubMed
[41] Tang Y, Liang J, Wu A, Chen Y, Zhao P, Lin T, et al. Co-delivery of trichosanthin and albendazole by nano-self-assembly for overcoming tumor multidrug-resistance and metastasis. ACS Appl Mater Interfaces. 2017;9(32):26648–64.10.1021/acsami.7b05292Suche in Google Scholar PubMed
[42] Semkina AS, Abakumov MA, Skorikov AS, Abakumova TO, Melnikov PA, Grinenko NF, et al. Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomed Nanotechnol Biol Med. 2018;14(5):1733–42.10.1016/j.nano.2018.04.019Suche in Google Scholar PubMed
[43] Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. 10.1016/j.addr.2015.09.012.Suche in Google Scholar PubMed PubMed Central
[44] Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71:1185–98. 10.1111/jphp.13098.Suche in Google Scholar PubMed
[45] Fu X, Shi Y, Qi T, Qiu S, Huang Y, Zhao X, et al. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct Target Ther. 2020;5:262. 10.1038/s41392-020-00342-0.Suche in Google Scholar PubMed PubMed Central
[46] Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12:137. 10.1186/s13045-019-0833-3.Suche in Google Scholar PubMed PubMed Central
[47] Pham SH, Choi Y, Choi J. Stimuli-Responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics. 2020;12:630. 10.3390/pharmaceutics12070630.Suche in Google Scholar PubMed PubMed Central
[48] Feng Q, Tong R. Anticancer nanoparticulate polymer-drug conjugate. Bioeng Transl Med. 2016;1:277–96. 10.1002/btm2.10033.Suche in Google Scholar PubMed PubMed Central
[49] Cassim S, Vučetić M, Ždralević M, Pouyssegur J. Warburg and beyond: the power of mitochondrial metabolism to collaborate or replace fermentative glycolysis in cancer. Cancers (Basel). 2020;12(5):1119. 10.3390/cancers12051119.Suche in Google Scholar PubMed PubMed Central
[50] Yang K, Luo H, Zeng M, Jiang Y, Li J, Fu X. Intracellular pH-triggered, targeted drug delivery to cancer cells by multifunctional envelope-type mesoporous silica nanocontainers. ACS Appl Mater Interfaces. 2015;7:17399–407. 10.1021/acsami.5b04684.Suche in Google Scholar PubMed
[51] Xu Y, Zi Y, Lei J, Mo X, Shao Z, Wu Y, et al. pH-Responsive nanoparticles based on cholesterol/imidazole modified oxidized-starch for targeted anticancer drug delivery. Carbohydr Polym. 2020;233:115858. 10.1016/j.carbpol.2020.115858.Suche in Google Scholar PubMed
[52] Wang P, Liu W, Liu S, Yang R, Pu Y, Zhang W, et al. pH-responsive nanomicelles of poly(ethylene glycol)-poly(ε-caprolactone)-poly(l-histidine) for targeted drug delivery. J Biomater Sci Polym Ed. 2020;31:277–92. 10.1080/09205063.2019.1687132.Suche in Google Scholar PubMed
[53] Anirudhan TS, Chithra Sekhar V, Athira VS. Graphene oxide based functionalized chitosan polyelectrolyte nanocomposite for targeted and pH responsive drug delivery. Int J Biol Macromol. 2020;150:468–79. 10.1016/j.ijbiomac.2020.02.053.Suche in Google Scholar PubMed
[54] Zhang Y-H, Zhang Y-M, Yu J, Wang J, Liu Y. Boronate-crosslinked polysaccharide conjugates for pH-responsive and targeted drug delivery. Chem Commun. 2019;55:1164–7. 10.1039/C8CC09956A.Suche in Google Scholar
[55] Men W, Zhu P, Dong S, Liu W, Zhou K, Bai Y, et al. Layer-by-layer pH-sensitive nanoparticles for drug delivery and controlled release with improved therapeutic efficacy in-vivo. Drug Deliv. 2020;27:180–90. 10.1080/10717544.2019.1709922.Suche in Google Scholar PubMed PubMed Central
[56] Yang Y, Wang Z, Peng Y, Ding J, Zhou W. A smart pH-sensitive delivery system for enhanced anticancer efficacy via paclitaxel endosomal escape. Front Pharmacol. 2019;10:10. 10.3389/fphar.2019.00010.Suche in Google Scholar PubMed PubMed Central
[57] Palanikumar L, Al-Hosani S, Kalmouni M, Nguyen VP, Ali L, Pasricha R, et al. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun Biol. 2020;3:95. 10.1038/s42003-020-0817-4.Suche in Google Scholar PubMed PubMed Central
[58] Ma X, Zhao Y, Ng KW, Zhao Y. Integrated hollow mesoporous silica nanoparticles for target drug/siRNA co-delivery. Chemistry. 2013;19(46):15593–603. 10.1002/chem.201302736Epub 2013 Oct 2.Suche in Google Scholar PubMed
[59] Gao N, Xing C, Wang H, Feng L, Zeng X, Mei L, et al. pH-Responsive dual drug-loaded nanocarriers based on poly(2-ethyl-2-oxazoline) modified black phosphorus nanosheets for cancer chemo/photothermal therapy. Front Pharmacol. 2019;10:270. 10.3389/fphar.2019.00270.Suche in Google Scholar PubMed PubMed Central
[60] He C, Zhang Z, Ding Y, Xue K, Wang X, Yang R, et al. LRP1-mediated pH-sensitive polymersomes facilitate combination therapy of glioblastoma in vitro and in vivo. J Nanobiotechnol. 2021;19:29. 10.1186/s12951-020-00751-x.Suche in Google Scholar PubMed PubMed Central
[61] Siafaka PI, Üstündağ Okur N, Karavas E, Bikiaris DN. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: current status and uses. Int J Mol Sci. 2016;17(9):1440. 10.3390/ijms17091440.Suche in Google Scholar PubMed PubMed Central
[62] Xiong D, Yao N, Gu H, Wang J, Zhang L. Stimuli-responsive shell cross-linked micelles from amphiphilic four-arm star copolymers as potential nanocarriers for “pH/redox-triggered” anticancer drug release. Polym (Guildf). 2017;114:161–72. 10.1016/j.polymer.2017.03.002.Suche in Google Scholar
[63] Han L, Zhang X-Y, Wang Y-L, Li X, Yang X-H, Huang M, et al. Redox-responsive theranostic nanoplatforms based on inorganic nanomaterials. J Control Rel. 2017;259:40–52. 10.1016/j.jconrel.2017.03.018.Suche in Google Scholar PubMed
[64] Sauraj Kumar V, Kumar B, Priyadarshi R, Deeba F, Kulshreshtha A, et al. Redox responsive xylan-SS-curcumin prodrug nanoparticles for dual drug delivery in cancer therapy. Mater Sci Eng C. 2020;107:110356. 10.1016/j.msec.2019.110356.Suche in Google Scholar PubMed
[65] Jia X, He J, Shen L, Chen J, Wei Z, Qin X, et al. Gradient redox-responsive and two-stage rocket-mimetic drug delivery system for improved tumor accumulation and safe chemotherapy. Nano Lett. 2019;19:8690–700. 10.1021/acs.nanolett.9b03340.Suche in Google Scholar PubMed
[66] Chang S, Wang Y, Zhang T, Pu X, Zong L, Zhu H, et al. Redox-responsive disulfide bond-bridged mPEG-PBLA prodrug micelles for enhanced paclitaxel biosafety and antitumor efficacy. Front Oncol. 2019;9:823. 10.3389/fonc.2019.00823.Suche in Google Scholar PubMed PubMed Central
[67] Chen X, Sun H, Hu J, Han X, Liu H, Hu Y. Transferrin gated mesoporous silica nanoparticles for redox-responsive and targeted drug delivery. Colloids Surf B Biointerfaces. 2017;152:77–84. 10.1016/j.colsurfb.2017.01.010.Suche in Google Scholar PubMed
[68] Žid L, Zeleňák V, Girman V, Bednarčík J, Zeleňáková A, Szűcsová J, et al. Doxorobicin as cargo in a redox-responsive drug delivery system capped with water dispersible ZnS nanoparticles. RSC Adv. 2020;10:15825–35. 10.1039/D0RA02091E.Suche in Google Scholar PubMed PubMed Central
[69] Chibh S, Kour A, Yadav N, Kumar P, Yadav P, Chauhan V, et al. Redox-responsive dipeptide nanostructures toward targeted cancer therapy. ACS Omega. 2020;5(7):3365–75. 10.1021/acsomega.9b03547.Suche in Google Scholar PubMed PubMed Central
[70] Yin Q, Shen J, Chen L, Zhang Z, Gu W, Li Y. Overcoming multidrug resistance by co-delivery of Mdr-1 and survivin-targeting RNA with reduction-responsible cationic poly(β-amino esters). Biomaterials. 2012;33(27):6495–506. 10.1016/j.biomaterials.2012.05.039.Suche in Google Scholar PubMed
[71] Chen M, Hu J, Wang L, Li Y, Zhu C, Chen C, et al. Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy. Sci Rep. 2020;10:14447. 10.1038/s41598-020-71071-1.Suche in Google Scholar PubMed PubMed Central
[72] Ma X, Nguyen KT, Borah P, Ang CY, Zhao Y. Functional silica nanoparticles for redox-triggered drug/ssDNA co-delivery. Adv Healthc Mater. 2012;1(6):690–7. 10.1002/adhm.Suche in Google Scholar
[73] Xu X, Wu J, Liu S, Saw PE, Tao W, Li Y, et al. Redox-responsive nanoparticle-mediated systemic RNAi for effective cancer therapy. Small. 2018;14(41):e1802565. 10.1002/smll.201802565.Suche in Google Scholar PubMed PubMed Central
[74] Ibrahim YH-EY, Regdon G, Hamedelniel EI, Sovány T. Review of recently used techniques and materials to improve the efficiency of orally administered proteins/peptides. DARU J Pharm Sci. 2020;28:403–16. 10.1007/s40199-019-00316-w.Suche in Google Scholar PubMed PubMed Central
[75] Naz S, Wang M, Han Y, Hu B, Teng L, Zhou J, et al. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int J Nanomed. 2019;14:2533–42. 10.2147/IJN.S202210.Suche in Google Scholar PubMed PubMed Central
[76] Sharma A, Kim E-J, Shi H, Lee JY, Chung BG, Kim JS. Development of a theranostic prodrug for colon cancer therapy by combining ligand-targeted delivery and enzyme-stimulated activation. Biomaterials. 2018;155:145–51. 10.1016/j.biomaterials.2017.11.019.Suche in Google Scholar PubMed
[77] Zhou J, Wang M, Ying H, Su D, Zhang H, Lu G, et al. Extracellular matrix component shelled nanoparticles as dual enzyme-responsive drug delivery vehicles for cancer therapy. ACS Biomater Sci Eng. 2018;4:2404–11. 10.1021/acsbiomaterials.8b00327.Suche in Google Scholar PubMed
[78] Qin SY, Feng J, Rong L, Jia HZ, Chen S, Liu XJ, et al. Theranostic GO-based nanohybrid for tumor induced imaging and potential combinational tumor therapy. Small. 2014;10(3):599–608. 10.1002/smll.201301613.Suche in Google Scholar PubMed
[79] Wang G, Zhou Z, Zhao Z, Li Q, Wu Y, Yan S, et al. Enzyme-triggered transcytosis of dendrimer-drug conjugate for deep penetration into pancreatic tumors. ACS Nano. 2020;14(4):4890–4904. 10.1021/acsnano.0c00974.Suche in Google Scholar PubMed
[80] Liu R, Hu C, Yang Y, Zhang J, Gao H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharmaceutica Sin B. 2019;9(2):410–20. 10.1016/j.apsb.2018.09.001.Suche in Google Scholar PubMed PubMed Central
[81] Calderón M, Graeser R, Kratz F, Haag R. Development of enzymatically cleavable prodrugs derived from dendritic polyglycerol. Bioorganic & Medicinal Chem Lett. 2009;19(14):3725–8. 10.1016/j.bmcl.2009.05.058.Suche in Google Scholar PubMed
[82] Hua M-Y, Liu H-L, Yang H-W, Chen P-Y, Tsai R-Y, Huang C-Y, et al. The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials. 2011;32:516–27. 10.1016/j.biomaterials.2010.09.065.Suche in Google Scholar PubMed
[83] Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres M, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71. 10.1186/s12951-018-0392-8.Suche in Google Scholar PubMed PubMed Central
[84] Kong SD, Sartor M, Hu C-MJ, Zhang W, Zhang L, Jin S. Magnetic field activated lipid-polymer hybrid nanoparticles for stimuli-responsive drug release. Acta Biomater. 2013;9:5447–52. 10.1016/j.actbio.2012.11.006.Suche in Google Scholar PubMed PubMed Central
[85] Guisasola E, Baeza A, Talelli M, Arcos D, Moros M, de la Fuente JM, et al. Magnetic-responsive release controlled by hot spot effect. Langmuir. 2015;31:12777–82. 10.1021/acs.langmuir.5b03470.Suche in Google Scholar PubMed
[86] Chen P-J, Hu S-H, Hsiao C-S, Chen Y-Y, Liu D-M, Chen S-Y. Multifunctional magnetically removable nanogated lids of Fe3O4–capped mesoporous silica nanoparticles for intracellular controlled release and MR imaging. J Mater Chem. 2011;21:2535–43. 10.1039/C0JM02590A.Suche in Google Scholar
[87] Hu SH, Liao BJ, Chiang CS, Chen PJ, Chen IW, Chen SY. Core-shell nanocapsules stabilized by single-component polymer and nanoparticles for magneto-chemotherapy/hyperthermia with multiple drugs. Adv Mater. 2012;24(27):3627–32. 10.1002/adma.201201251.Suche in Google Scholar PubMed
[88] Hua X, Yang Q, Dong Z, Zhang J, Zhang W, Wang Q, et al. Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 2017;24(1):511–8. 10.1080/10717544.2016.1256001.Suche in Google Scholar PubMed PubMed Central
[89] Oliveira H, Pérez-Andrés E, Thevenot J, Sandre O, Berra E, Lecommandoux S. Magnetic field triggered drug release from polymersomes for cancer therapeutics. J Control Rel. 2013;169(3):165–70. 10.1016/j.jconrel.2013.01.013.Suche in Google Scholar PubMed
[90] Hongmei B, Xiaojun H. Magnetic field triggered drug release from lipid microcapsule containing lipid-coated magnetic nanoparticles. Chem Phys Lett. 2018;706:455–60. 10.1016/j.cplett.2018.06.051.Suche in Google Scholar
[91] Zhou L, Wang H, Li Y. Stimuli-responsive nanomedicines for overcoming cancer multidrug resistance. Theranostics. 2018;8:1059–74. 10.7150/thno.22679.Suche in Google Scholar PubMed PubMed Central
[92] Paris JL, Cabañas MV, Manzano M, Vallet-Regí M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano. 2015;9:11023–33. 10.1021/acsnano.5b04378.Suche in Google Scholar PubMed
[93] Kim S, Im S, Park E-Y, Lee J, Kim C, Kim T, et al. Drug-loaded titanium dioxide nanoparticle coated with tumor targeting polymer as a sonodynamic chemotherapeutic agent for anti-cancer therapy. Nanomed Nanotechnology, Biol Med. 2020;24:102110. 10.1016/j.nano.2019.102110.Suche in Google Scholar PubMed
[94] Zhu X, Guo J, He C, Geng H, Yu G, Li J, et al. Ultrasound triggered image-guided drug delivery to inhibit vascular reconstruction via paclitaxel-loaded microbubbles. Sci Rep. 2016;6:21683. 10.1038/srep21683.Suche in Google Scholar PubMed PubMed Central
[95] Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharm. 2010;7(6):1959–73. 10.1021/mp100269f.Suche in Google Scholar PubMed PubMed Central
[96] Huebsch N, Kearney CJ, Zhao X, Kim J, Cezar C A, Suo Z, et al. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. PNAS. 2014;111(27):9762–7. 10.1073/pnas.1405469111.Suche in Google Scholar PubMed PubMed Central
[97] Batchelor DVB, Abou-Saleh RH, Coletta PL, McLaughlan JR, Peyman SA, Evans SD. Nested nanobubbles for ultrasound-triggered drug release. ACS Appl Mater Interfaces. 2020;12(26):29085–93. 10.1021/acsami.0c07022.Suche in Google Scholar PubMed PubMed Central
[98] Roussakow S. The history of hyperthermia rise and decline. Conf Pap Med. 2013;2013:428027–40. 10.1155/2013/428027.Suche in Google Scholar
[99] Shen B, Ma Y, Yu S, Ji C. Smart multifunctional magnetic nanoparticle-based drug delivery system for cancer thermo-chemotherapy and intracellular imaging. ACS Appl Mater Interfaces. 2016;8:24502–08. 10.1021/acsami.6b09772.Suche in Google Scholar PubMed
[100] Demirci H, Slimani N, Pawar M, Kumon RE, Vaishnava P, Besirli CG. Magnetic hyperthermia in Y79 retinoblastoma and ARPE-19 retinal epithelial cells: tumor selective apoptotic activity of iron oxide nanoparticle. Transl Vis Sci Technol. 2019;8:18. 10.1167/tvst.8.5.18.Suche in Google Scholar PubMed PubMed Central
[101] Zamora-Mora V, Fernández-Gutiérrez M, González-Gómez Á, Sanz B, Román JS, Goya GF, et al. Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: from preparation to in-vitro studies. Carbohydr Polym. 2017;157:361–70. 10.1016/j.carbpol.2016.09.084.Suche in Google Scholar PubMed
[102] Gu X, Wang J, Liu X, Zhao D, Wang Y, Gaob H, et al. Temperature-responsive drug delivery systems based on polyaspartamides with isopropylamine pendant groups. Soft Matter. 2013;9:7267–73. 10.1039/C3SM50904D.Suche in Google Scholar
[103] Hajebi S, Abdollahi A, Roghani-Mamaqani H, Salami-Kalajahi M. Temperature-responsive poly(N-isopropylacrylamide) nanogels: the role of hollow cavities and different shell cross-linking densities on doxorubicin loading and release. Langmuir. 2020;36(10):2683–94. 10.1021/acs.langmuir.9b03892.Suche in Google Scholar PubMed
[104] Zhang B, Jia F, Fleming MQ, Mallapragada SK. Injectable self-assembled block copolymers for sustained gene and drug co-delivery: an in vitro study. Int J Pharm. 2012;427(1):88–96. 10.1016/j.ijpharm.2011.10.018.Suche in Google Scholar PubMed
[105] Shah BP, Pasquale N, De G, Tan T, Ma J, Lee K-B. Core–Shell nanoparticle-based peptide therapeutics and combined hyperthermia for enhanced cancer cell apoptosis. ACS Nano. 2014;8:9379–87. 10.1021/nn503431x.Suche in Google Scholar PubMed PubMed Central
[106] Wang D, Wu Si. Red-Light-Responsive Supramolecular Valves for Photocontrolled Drug Release from Mesoporous Nanoparticles. Langmuir. 2016;32(2):632–6. 10.1021/acs.langmuir.5b04399.Suche in Google Scholar PubMed
[107] Sun L, Yang Y, Dong CM, Wei Y. Two-photon-sensitive and sugar-targeted nanocarriers from degradable and dendritic amphiphiles. Small. 2011;7(3):401–6. 10.1002/smll.201001729.Suche in Google Scholar PubMed
[108] Liu L, Wang R, Wang C, Wang J, Chen L, Cheng J. Light-triggered release of drug conjugates for an efficient combination of chemotherapy and photodynamic therapy. Biomater Sci. 2018;6(5):997–1001. 10.1039/c7bm01114h.Suche in Google Scholar PubMed
[109] Chun-Yang S, Bei-Bei Z, Zhou JY. Light-activated drug release from a hyaluronic acid targeted nanoconjugate for cancer therapy. J Mater Chem B. 2019;7:4843–53. 10.1039/C9TB01115C.Suche in Google Scholar PubMed
[110] An X, Zhu A, Luo H, Ke H, Chen H, Zhao Y. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano. 2016;10:5947–58. 10.1021/acsnano.6b01296.Suche in Google Scholar PubMed
[111] Wang Y, Wei G, Zhang X, Xu F, Xiong X, Zhou S. A step-by-step multiple stimuli-responsive nanoplatform for enhancing combined chemo-photodynamic therapy. Adv Mater. 2017;29:29. 10.1002/adma.201605357.Suche in Google Scholar PubMed
[112] Li J, Li X, Liu H, Ren T, Huang L, Deng Z, et al. GSH and light dual stimuli-responsive supramolecular polymer drug carriers for cancer therapy. Polym Degrad Stab. 2019;168:108956. 10.1016/j.polymdegradstab.2019.108956.Suche in Google Scholar
[113] Ko NR, Van SY, Hong SH, Kim S-Y, Kim M, Lee JS, et al. Dual pH- and GSH-responsive degradable PEGylated graphene quantum dot-based nanoparticles for enhanced HER2-positive breast cancer therapy. Nanomater. (Basel, Switzerland). 2020;10(1):91. 10.3390/nano10010091.Suche in Google Scholar PubMed PubMed Central
[114] Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557–88. 10.7150/thno.38069.Suche in Google Scholar PubMed PubMed Central
[115] Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Rel. 2014;190:465–76.10.1016/j.jconrel.2014.06.042Suche in Google Scholar PubMed
[116] Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Kiessling F, et al. Nanomedicine and macroscale materials in immuno-oncology. Chem Soc Rev. 2019;48:351–81.10.1039/C8CS00473KSuche in Google Scholar
[117] Shi Y, Lammers T. Combining nanomedicine and immunotherapy. Acc Chem Res. 2019;52:1543–54.10.1021/acs.accounts.9b00148Suche in Google Scholar PubMed PubMed Central
© 2021 Baranya Murugan et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

