Abstract
Graphene is a single-atom-thick sheet of sp2 hybridized carbon atoms that are packed in a hexagonal honeycomb crystalline structure. This promising structure has endowed graphene with advantages in electrical, thermal, and mechanical properties such as room-temperature quantum Hall effect, long-range ballistic transport with around 10 times higher electron mobility than in Si and thermal conductivity in the order of 5,000 W/mK, and high electron mobility at room temperature (250,000 cm2/V s). Another promising characteristic of graphene is large surface area (2,630 m2/g) which has emerged so far with its utilization as novel electronic devices especially for ultrasensitive chemical sensor and reinforcement for the structural component applications. The application of graphene is challenged by concerns of synthesis techniques, and the modifications involved to improve the usability of graphene have attracted extensive attention. Therefore, in this review, the research progress conducted in the previous decades with graphene and its derivatives for chemical detection and the novelty in performance enhancement of the chemical sensor towards the specific gases and their mechanism have been reviewed. The challenges faced by the current graphene-based sensors along with some of the probable solutions and their future improvements are also being included.
Graphical abstract
1 Introduction
Graphene and its derivatives have been emerging materials for modern chemistry and physics owing to its fascinating properties profile since Andre Geim and Konstantin Novoselov (Nobel Prize winners for Physics in 2010) achieved groundbreaking experiments regarding the two-dimensional (2D) material graphene in 2004 [1]. Since its first isolation, the scientific development involving its synthesis methodologies and related applications has been inspiringly progressive, suggesting that graphene would revolutionize the industry with its superlative and promising properties [2,3]. Graphene has a high basal plane elastic modulus with 1 TPa, ultimate strength about 130 GPa, and room temperature charge carrier mobility by 10,000 cm2/V s [3]. Graphene could be defined as a single layer of carbon atoms that are tightly packed to form a 2D honeycomb structure of sp2 hybridized carbon [4]. Further extension of honeycomb network is the basic building block of other important allotropes, such as it can be stacked to form 3D graphite, rolled to form one-dimensional (1D) nanotubes, and wrapped to form 0D fullerenes [5]. The use of three π-electrons in carbon-carbon bonding results in a system of delocalized π-electrons perpendicular to the honeycomb plane giving rise to graphene’s exceptional electrical properties.
Long-range of π-conjugation in graphene produces extraordinary thermal, mechanical, and electrical properties, which have long been the interest of many theoretical studies and more recently became an exciting area for wide range of applications [6,7]. In the applied field of research, among its wide range of applications, graphene has been largely used in batteries and cells as anodes and in supercapacitors due to its low charging time, high strength to weight ratio, and large surface area. Due to its unique properties which include a distinctive nano-porous structure, high mechanical strength, and high electrical and thermal conductivity, it has also found a large number of applications in areas like sensors, biomedical engineering, nano and flexible electronics, catalysis, and cement-based and geopolymer materials [8]. Besides that, a single atomic sheet of graphite has ignited intense research activities to clarify the electronic properties of this novel 2D electronic system [9]. However, charge transport in graphene is substantially different from that of conventional 2D electronic systems as a consequence of the linear energy dispersion relation near the charge neutrality point (Dirac point) in the electronic band gap structure [10,11]. This unique band gap structure is fundamentally responsible for the distinct electronic properties of carbon nanotubular graphene and carbon nanotubes (CNTs) [12].
Referring to Yavari and Koratkar (2012), the development of graphene-based chemical sensors presents the possibility of ultrahigh sensitivity detection of a range of gas species in air at room temperature and atmospheric pressure [13]. Before the beginning of graphene, there had been an extensive research on the starts of CNTs-based chemical sensors [14,15,16]. Graphene offers some important advantages compared to CNTs which is (1) a free-standing or suspended graphene sheet has both of its sides exposed to the chemical environment, thereby maximizing its sensitivity towards the analytes. Like multiwalled nanotubes, the inner cylinders are shielded from the chemical environment and even for single-walled nanotubes (SWCNTs), the ends may be closed (e.g. for tubes grown by chemical vapour deposition (CVD)), or the metal contact pads might cap the tubes and prevent the inside of the tube from participating in gas adsorption. (2) graphene exhibits inherently low electrical noise at room temperature [17], which arises from its unique 2D crystal lattice and high electron mobility. For these reasons, the sensitivity of graphene-based devices for molecular sensing is superior to that of CNTs. In truth, Schedin et al. (2007) have revealed that even the adsorption of single molecules could be detected using graphene [17].
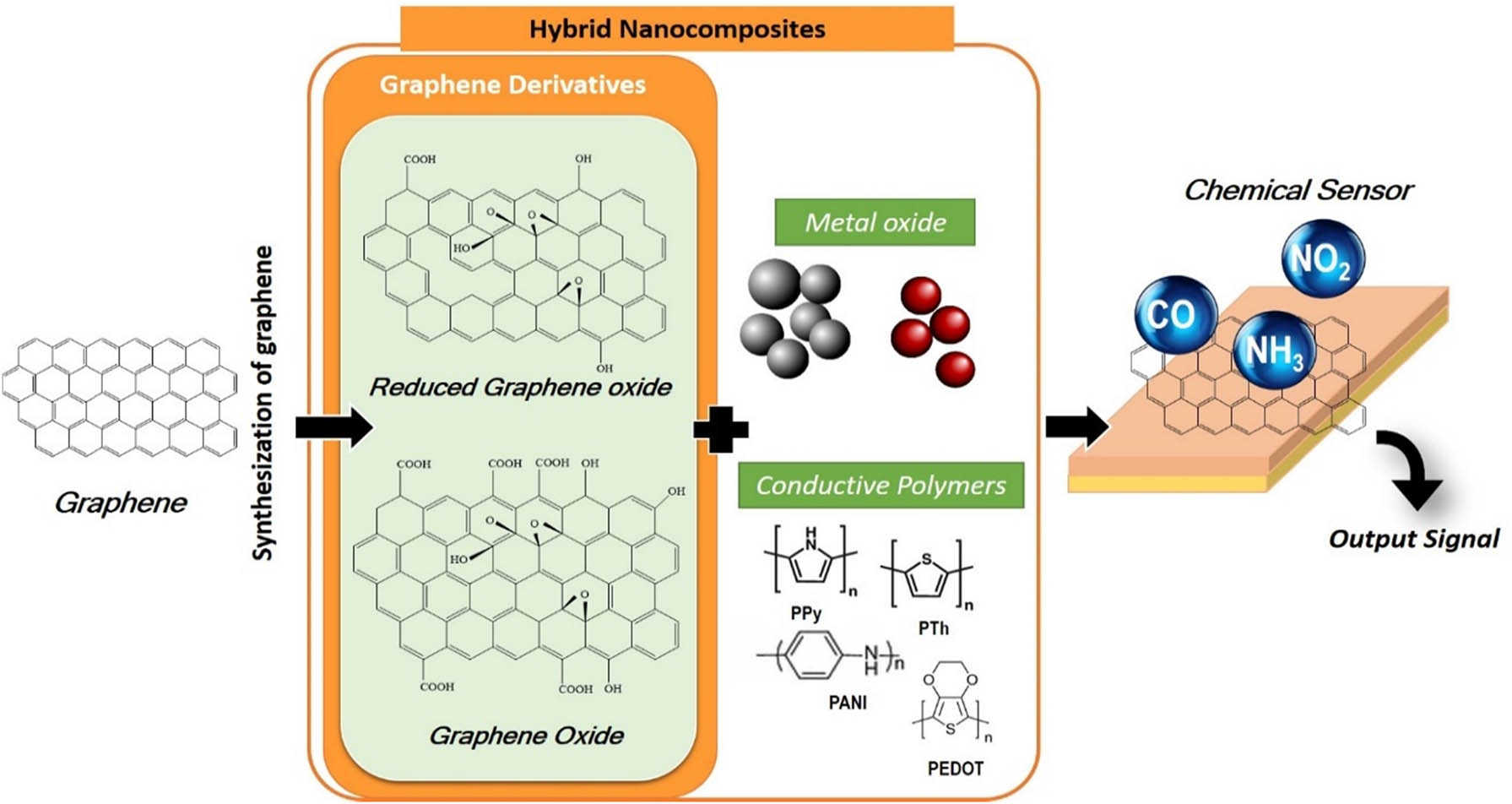
This article provides a state-of-the-art review on performance of graphene’s, graphene oxide (GO), and reduced graphene oxide (rGO) for chemical detection hybridized with metal oxide and conductive polymers have been reviewed. Their mechanism towards the specific gases also has been highlighted. The graphical abstract of the paper.
1.1 Graphene’s characteristic
Cao et al. (2018) reported that arranging two layers of atom-thick graphene so that the pattern of their carbon atoms is offset by an angle of 1.1° makes the material a superconductor [18]. And although the system still needed to be cooled to 1.7° above absolute zero, the results suggest that it may conduct electricity much like known high-temperature superconductors [19]. Graphene already has impressive properties. It has shown superconductivity before, but it occurred when in contact with other materials, and the behaviour could be explained by conventional superconductivity [20]. Figure 1 shows a hexagon made up of two layers of graphene, twisted at an angle of 1.1°, had shown superconducting properties. Although graphene shows superconductivity at a very low temperature, it does so with just one-ten-thousandth of the electron density of conventional superconductors that gain the ability at the same temperature [21]. In conventional superconductors, the phenomenon is thought to arise when vibrations allow electrons to form pairs, which stabilize their path and allow them to flow without resistance. But with so few available electrons in graphene, it can somehow pair up which suggests that the interaction at play in this system should be much stronger than what happens in conventional superconductors like niobium-titanium alloy (type-II) with a superconducting critical material of 11 K [22].
In a global market, there are several types of graphene in a powder form material, such as GO, graphene nanoplatelets, graphene nanoribbons, and graphene quantum dots as well as graphene enabled products such as graphene ink or graphene masterbatches. Referring to Hernaez et al. (2017), the development of a method for the production of high-quality graphene in large quantities is essential to further exploit its full potential [23]. Hence, the use of GO and rGO has gained widespread consideration, as a compromise between the interesting properties of pristine graphene, the synthesization complexity, and cost. Figure 2 shows the schematic illustration of routes for preparation of GO and rGO from graphene flakes [24], whereas Table 1 shows the physical, mechanical, and electrical properties of the graphene materials [25,26,27,28,29,30].
![Figure 2
Schematic illustration of routes for preparation of GO and rGO. Adapted from ref. [24].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_002.jpg)
Schematic illustration of routes for preparation of GO and rGO. Adapted from ref. [24].
Physical, mechanical, and electrical properties of graphene
| Properties | Graphene | GO | rGO |
|---|---|---|---|
| Carbon (C), Oxygen (O) elemental ratio (%) | C (99) | C (62–65) | C (77–87) |
| O (–) | O (35–48) | O (13–22) | |
| Crystal size (nm) | 175.49 | 21.14 | 15.13–15.95 |
| d spacing (nm) | 0.33 | 0.93 | 0.36 |
| Plane size (μm) | 0.5–5 | 1–2 | 1–7 |
| Number of layers | 3–5 | 1–3 | 1–3 |
| Layer thickness (nm) | 0.34 | 0.76–0.84 | 0.35–0.36 |
| Stack thickness in water dispersion (nm) | 180–230 | 1.00–1.20 | 125–175 |
| Raman intensity ratio | 0.25 | 0.79 | 1.10–1.16 |
| Dispersibility in water | Not dispersible | Highly dispersible | Moderately dispersible |
| Tensile strength(GPa) | 130 | ∼0.13 | — |
| Elastic modulus (GPa) | 1,000 | 23–42 | 3.0–9.5 |
| Elongation at break (%) | 80 | 0.6 | — |
| Electrical conductivity (S/m) | ∼1,000 | Non conductive | ∼667 |
GO can be synthesized by functionalizing with hydroxyl (−OH) or carboxyl (C═O) groups covalently bonded to a planar carbon network of graphite, via treatment with oxidizing agents such as sulphuric acid (H2SO4) and nitric acid (HNO3). It is then exfoliated into few-layer or even monolayer GO, which nevertheless contains a high density of defects [31]. Small size of graphene sheets can be obtained by subsequent reduction of GO, which can eliminate most of its oxygen-containing functional groups and partially recover its sp2-bonded carbon network [32,33]. The abundance of functional groups in GO results in a hydrophilic behaviour which is strongly dependant on the level of oxidation. GO sheets show good dispersibility as a result of their strongly charged nature and hydrophilicity and form stable aqueous dispersions in a wide range of concentrations from 0.0125 to 0.05 wt% [34]. Additionally, GO are well-dispersed in organic solvents such as ethylene glycol, dimethylformamide, n-methyl-2-pyrrolidone (NMP), and tetrahydrofuran (THF) by forming hydrogen bonds between the surface and solvent interface.
1.2 rGO characteristic
In comparison to non-oxidized graphene nanoflakes, rGO is highly disordered with a relatively inferior quality due to the presence of many vacancy defects and Stone-Wales defects. Field emission electron microscope (FESEM) image presented at Figure 3 captured by Sharma et al. (2017) shows highly wrinkled and corrugated structure of rGO compared with GO, but shows liner (exhibit ohmic) I–V result for both GO and rGO. This outcomes present hint that both GO and rGO have the potential of being good gas sensing materials [35]. From FESEM in Figure 3(a) and (b), it is evident that surface of the sample gets corrugated upon reduction from GO to rGO due to reduction of oxygen-containing groups from the surface, while Figure 3(c) and (d) shows GO and rGO under high resolution transmission electron microscope (HRTEM) [36].
![Figure 3
(a and b) FESEM images and (c and d) HRTEM image of GO and rGO–Au. Adapted from ref. [36].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_003.jpg)
(a and b) FESEM images and (c and d) HRTEM image of GO and rGO–Au. Adapted from ref. [36].
Furthermore, hydrogenated, fluorinated, and oxidized graphenes which are called fluorinated graphene or fluorographene are expected to have remarkable applications in coating, batteries, separation technologies, and electrochemical sensing. In another theoretical study, porous fluorinated graphene is suggested to modulate the heat of adsorption of molecules, enhancing the binding of dipolar ones (H2O, SO2, H2S, and CO2) over N2, O2, and CH4 [37]. Therefore, applications are projected on separation of CO2 and SO2 from flue gases, purification of natural gas, and removal of H2O from air. A significant benefit is expected for porous fluorinated graphene, related to the fact that gas molecule separation is not dependent on size-exclusion mechanism, but on interaction strengths.
The introduction of either acetylenic or diacetylenic chains between carbon hexagons is experimentally shown to give a layer of single-atom thickness, which is flat like graphene and is predicted to have interesting properties like graphene; the former is named graphyne and the latter graphdiyne [38]. Graphyne and graphdiyne cannot be prepared directly from graphene, but they are compared and discussed with graphene with respect to their structure and properties. Therefore, they are classified as graphene derivatives, together with graphane, fluorographene, and GO. Graphene-related nanomaterials, including doped graphene, graphene nanoribbons, and porous graphene, in addition to five nanomaterials, are classified as graphene derivatives. Figure 4 proposed by Inagaki and Kang (2014) shows the development of graphyne and graphdiyne which suggests us a new carbon family composed of two kinds of C–C bonds, sp2 and sp1. However, graphane, fluorographene, and GO have to be included as new members of the sp3 system. These carbon materials described in the present review can be derived by chemical reactions or theoretical considerations from graphene [38].
![Figure 4
Schematic diagram of the development of graphene and its derivatives. Adapted from ref. [38].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_004.jpg)
Schematic diagram of the development of graphene and its derivatives. Adapted from ref. [38].
1.3 The discovery of graphenes as chemical sensors
These days, emissions of harmful by-products and pollutants, such as nitrogen oxides (NO x ), carbon oxides (CO x ), sulfur oxides (SO x ), and ammonia (NH3), had increased significantly and endangered our health and environment over the long term. To monitor the chemical materials that are harmful to human health and the environment, chemical sensing devices have been extensively developed and explored. Improvement and optimization of present chemical sensors, including gas sensors as well as the development of new sensors that possess higher sensing performance with higher sensitivity but lower cost, are still necessary for not only industrial sectors but also indoor health and safety, environmental monitoring, and beyond.
The nanostructures of metal oxides like titanium oxide (TiO2), Iron(iii) oxide (Fe2O3), zinc oxide (ZnO), stannous oxide (SnO2), tungsten oxide (WO3), cuprous oxide (Cu2O), etc. have been intensively explored for sensing applications. This is mainly due to their proven characteristics such as large specific surface area, excellent mechanical flexibility, good chemical stability, and better sensitivity [39,40,41,42]. However, metal oxide-based sensor materials hold certain limitations of high operating temperature (100–500°C), resulting in high power consumption, which in turn adversely affects the integration and long-term stability. In addition, metal oxide gas sensors are used since many decades to detect a gas species at high working temperature that is needed to promote gas reaction with the oxygen ionosorbed over the semiconductor, inducing a variation in the resistance of the material. As a matter of fact, high-temperature operation could raise the problem of ignition of fuels when detecting high explosive gases. Hydrogen, for example, can explode when mixed with atmospheric oxygen at concentration of 4% of lower explosive limit. Thus, room temperature detection is very important [43].
Besides that, common commercial gas sensors are based on semiconductor, polymer materials, and the methods used for sensing are optical methods, chemiresistive, calorimetric methods, gas chromatography, and acoustic methods, etc. [44]. The limitations of these gas sensors can be one or more of the following: costly, rare sensitivity in parts per billion (ppb), poor selectivity, limited life time, poor repeatability, difficult in miniaturization, and high power consumption [45,46].
As an alternative, nanomaterial-based gas sensing materials have gained significant momentum due to many promising electrical, optical, and thermal properties combined with high surface to volume ratio, short response and recovery times, high sensitivity, selectivity, reversibility, and stability [47,48]. Other than metal oxide, different carbonaceous materials, such as CNT, charcoal, and carbon black, have been shown to be useful as chemical and biosensors due to the ease in tailoring their sensitivity through functionalization. Chatterjee et al. (2015) [49] had highlighted the other sides of graphene that can be considered the limitations. The problems related to intrinsic graphene are: (1) it is not producible in large scale, (2) it has no functional groups (required for gas/vapour adsorption), and (3) it has no band gap. The main performance enhancement techniques in graphene-based sensors are found to be doping [50], hybridization [51], functionalization [52], nano mesh formation [53], and field-effect transistor modulation [54]. In the context of rGO, a form of graphene, produced by reduction of GO which contains many functional groups and defects, has offered great potential as it is easy and cheap to produce in large scale. In addition, it can be easily functionalized, thereby generating and tuning the band gap energy. It is therefore no wonder that researchers have shown a great deal of interest in exploring rGO as a gas sensor candidate [55,56].
It was reported that the first graphene-based gas sensor was pioneering in 2007 by Schedin’s and team [17]; demonstrated micrometre size sensors made from graphene are capable of detecting individual gas molecules that attach to or detach from the graphene surface. They showed that the adsorbed molecules change the local carrier concentration in graphene one electron by one electron, which leads to step-like changes in resistance. The achieved sensitivity is due to the fact that graphene is remarkably low-noise material electronically, which makes it a promising material not only for chemical detectors but also for other applications where local probes sensitive to external charge, magnetic field, or mechanical strain are required [17]. The gas-induced changes in resistivity had different magnitudes for different gases, and the sign of the change indicated whether the gas was an electron acceptor (e.g. NO, NO2, O3,) or an electron donor (e.g. CO, NH3, C2H5OH). This research has created new possibilities for researchers to develop graphene-based gas sensors [57]. The interaction between graphene sheets and gas could vary from weak van der Waals to strong covalent bonding. All these interactions transform the electronic structure of graphene, which can be readily monitored by convenient electronic methods. Booth et al. (2008) and Hill (2011) indicated that the presence of interaction between target gas/vapour molecules could reach the lower limit of even a single molecule, i.e. high sensitivity even at low gas concentrations [58,59].
Recently, the use of graphene and its derivatives like GO, rGO, etc. [60,61,62,63] has been reported to show a promising sensing characteristic application due to its countless exceptional properties such as good thermal stability, ballistic conductivity, high carrier mobility at room temperature, low electrical noise due to its unique 2D honeycomb lattice as well as large surface area (theoretical surface area of 2,630 m2/g) [17]. In addition, 2D materials can screen charge fluctuations better than 1D materials like CNT, etc. [64]. Referring to Ratinac et al. (2010), the most important reason graphene has been considered as a promising gas sensing material is that its electronic properties are strongly affected by the adsorption of gas molecules. Based on their discoveries, the planar structure of graphene eases Hall pattern fabrication and four probe measurements, limiting the contact resistance impact, and helps to focus only on the active area compared to its 1D counterpart, CNT [65,66].
Lu et al. (2011) reported that under a positive gate potential (n-type conductance), rGO exhibits sudden response and fast recovery for ammonia (NH3) detection, far superior to the performance in p-mode at zero or negative gate potential [67]. In order to enhance the gas sensing performance of graphene, researchers have further explored chemical and physical functionalization of graphene with nanomaterials and in particular, conducting polymers, metals, and metal oxides such that a sizable energy gap can be opened up in graphene through the quantum confinement effect [68,69]. Russo et al. (2012) reported fabrication of room temperature hydrogen gas sensor from rGO, tin oxide (SnO₂), and platinum (Pt) with fast response and recovery times [70]. Paul et al. (2012) fabricated a sensor based on a nano mesh patterned from a CVD-grown large area graphene which exhibits sensitivities of about 4.32%/ppm of nitrogen dioxide (NO2) and 0.71%/ppm of NH3 with limits of detection of 15 and 160 ppb, respectively [71].
Furthermore, the involvement of graphene as GO and rGO in the developed sensors is attributed to some of the distinct advantages like the large surface-to-volume ratio, unique optical properties, excellent carrier mobility, and exceptional electrical and thermal properties compared to the other allotropes of carbon [72]. These properties are constant for the double and multilayered graphene structures. Apart from the difference in the structure and working conditions, the use of these advantages in graphene sensors lies mainly in their capability to adjust according to the application. For example, in strain sensors, properties like the detection limit, maximum sensing range, signal response, and reproducibility of the response hold a pivotal role to determine the quality of the sensor. These characteristics are attributed to the electrical and mechanical properties of graphene. In electrochemical sensors, its large surface area helps the loading of the desired biomolecules, resulting in the interaction between the analyte molecule and electrode surface due to the high ballistic transport capability and the very small band gap. Another advantage of graphene materials is its low environmental impact, making it more popular for sensing purposes than other nanostructured metal oxides [49].
Chemical sensor is particularly interesting as it hybridizes the nanostructure of graphene with metal oxides to form hybrid nanostructures. This is because not only do they display the individual properties of the nanoparticles and of graphene, but may also exhibit additional synergistic properties that are desirable and advantageous for gas sensing applications; in other word, compliment the limitations on each for the desired detection and applications. One of the major advantages of such nanocomposite sensor is that graphene has near metallic conductivity with a possible inherent-amplified sensing configuration [49]. The high specific surface area of graphene may cause synergetic effects in achieving good gas response at room temperature when blended with metal oxides, especially on sensitivity and selectivity. Table 2 shows the structural and electrical properties of various types of carbonaceous materials.
The structural and electrical properties of various carbonaceous materials including graphene
| Carbon materials | Advantages | Limitations | Structural | Electrical | Reference |
|---|---|---|---|---|---|
| Amorphous porous carbon | High surface area, advanced porous system, abundant defective sites, superior chemical inertness | Relative low conductivity, poor adhesion with fluorine-doped tin oxide (FTO) | Consists of an outer spherical shell with porous interior structure | High electronic conductivity and high surface area | [74] |
| A covalent random network composed of sp3 and sp2 hybridized carbons without grain boundaries | Electronic conductivity and ionic conductivity, with specific capacities of 212 mA h g−1 and 162 mA h g−1 at 0.5C and 1C, respectively. | ||||
| Non-crystalline | |||||
| Graphene | Excellent conductivity, fast charged carrier mobility, good mechanical strength, high optical transparency, good mechanical inertness, large surface area | Low surface area arising from the easy aggregation, low quantities of defective sites, high-quality thin film still lacking reproducibility, difficult to control bi-layer, tri-layer graphene, sheet resistance of tens or hundreds of nm thick layer is quite high | Crystalline carbon materials | Conducts both electricity and heat | [75] |
| Monolayers of carbon atoms arranged in a honeycomb network | Thermal conductivity and mechanical stiffness (3,000 W m−1 K−1 and 1,060 GPa, respectively) | ||||
| Giant aromatic macromolecule | |||||
| Graphite | Good conductivity, corrosion resistance, excellent thermal stability | Poor porous system, low surface area | Stacks of graphene layers | High electrical and thermal conductivity | [76,77] |
| Weak interactions that hold the graphene sheets together | Thermal conductivity 25–470 W m−1 K−1 | ||||
| Electrical resistivity 5 × 10−4–30 × 10−4 Ωcm | |||||
| Carbon black | Plentiful defective sites, good chemical inertness | Low surface area, inappropriate pore size, inadequate conductivity | Typical particle sizes range from around 8–100 nm for furnace blacks | Highly structured carbon blacks provide higher viscosity | [78] |
| Greater electrical conductivity and easier dispersion for specialty carbon blacks | |||||
| Electrical volume resistivity between 1 and 106 Ω cm | |||||
| Carbon nanofibre | Excellent mechanical strength, high thermal conductivity, good chemical inertness | Insufficient conductivity, low surface area, inferior porous system | Cylindrical nanostructures with graphene layers arranged as stacked cones, cups, or plates | High electrical conductivity, and high thermal conductivity | [79,80] |
| Diameters from 50 to 200 nm | Intrinsic conductivity, at room temperature to be 5 × 10−5 Ωcm | ||||
| Carbon nanotube | Large surface area, high electrical conductivity, good chemical inertness, shorter response and recovery times, reversibility and stability for raw CNT, ageing and thermal process increase the sensitivity of the sensors | Low quantities of defective sites, additional purifications and functionalizations required, lack of selectivity for raw CNT | Crystalline carbon materials | High electrical conductivity | [80,81] |
| Most of the physical properties of carbon nanotubes derived from graphene | High thermal conductivity | ||||
| Carbon atoms are densely organized in a regular sp2-bonded atomic-scale honeycomb (hexagonal) pattern | Resistivity of the SWCNT is 10−4 Ωcm at 27°C | ||||
| sp2 hybridization of carbon builds a layered construction with weak out-of-plane bonding of the van der Waals form and strong in-plane bounds | The SWCNT ropes able to sustain much higher stable current densities, as high as 1013 A/cm2 |
While graphene and rGO exhibit ambipolar and almost symmetric behaviour in the electron and hole doping regions, they show p (hole)-dominant conducting properties because of the adsorbed water and oxygen molecules [73]. The strategy of hybridization of graphene with metal oxide will be discussed in the last part. To date, the unique optical, chemical, and morphological properties of graphene functionalized with conductive polymers, metal oxides, etc. are attracting a growing interest in the scholar’s league. A closer look at Figure 5 in the number of publications reveals the rapid progress on the research of graphene and graphene composite-based gas sensors in the last decade. For the past ten years, about 886,000 number of publications related to the keyword “graphene” and about 41,700 number of publications related to the keyword “graphene chemical sensor” were found in Google Scholar (28th November 2020). In addition, about 25,500 number of publications related to “graphene composite chemical sensor” were found. These number indicate that graphene is becoming an interesting subject to be studied, and many more explorations can be done that could lead to huge impacts to the nation. This paper discussed the fundamentals of graphene and derivatives, the synthesization of graphene involved, the chemical modification of graphene involved, chemical sensor characteristics, and the performance of functionalized graphene for chemical sensor which includes hybrid of graphene with metal oxide and conductive polymers. This review ends with a conclusion and future perspectives of graphene materials in chemical sensors.

Progress of graphene-based chemical sensor in the last decade.
2 Chemical sensor characteristics and the basic mechanism
Chemical sensors are attracting tremendous interest because of the demand of sensitive, fast response, reversibility, and stable at low temperature sensor for public safety, space exploration, biomedicine, pharmaceuticals, for leakage detections of explosive gases such as hydrogen, for a real-time detection of toxic or carcinogenic gases in industries, and for the military purposes especially at the airport or at public area. Recently, extensive interest in improving reliable graphene-based chemical sensors has been rising as innumerable fields have been expanding. The key aspects expected for the development of a chemical sensor include sensitivity in the parts per million (ppm) to billion (ppb) range where the trace levels are involved, absolute discrimination, mild operation temperature, low power consumption, practical size, volume and mass, and low cost for large-scale applications [82,83]. Figure 6 explains a brief description of the four major important aspects for chemical sensor [84]. To satisfy these critical aspects and enhance the sensing performance, miscellaneous detection techniques for gas sensing have been explored and studied in the following section. This review begins with the sensing mechanism and working principles of the most prevalent gas sensing methods.

Four major important aspects for chemical sensor.
Sensor response traits of n-type and p-type gas sensors to different gases are summarized in Table 3 [85]. Because of these different characteristics, sensor response is defined according to resistivity of the active layer and in different ways depending on the type of measurements. Nevertheless, it is easily and commonly described as the ratio of resistance in air (R a) to resistance in the presence of analyte (R g) (R a/R g) for an n-type material with a reducing analyte. The response is expressed otherwise (R g/R a) for a n-type material with an oxidizing analyte [86,87]. It is vice versa for p-type sensors [88,89]. Although several compiled sensors make up a p–n heterojunction, they are decidedly sorted into their single dominant charge carrier trait of either n-type or p-type behaviour based on how resistivity decreases or increases in the augmentations of analyte concentration. This is to guarantee a straightforward understanding and an effective assessment of analyte sensing characteristics for both n-type and p-type gas sensors. The sensing response of p-type graphene-based chemical sensor over the chemical or analytes detection was determined using equation (1).
Where R o and R g are the electrical resistance of graphene-based sensor before and after the exposure to analytes (e.g. NH3) at specific time and temperatures, respectively.
Sensing response behaviour of p-type and n-type sensors to reducing and oxidizing analytes
| Sensing response behaviour | Examples of analytes | p-type sensor | n-type sensor |
|---|---|---|---|
| Reducing analytes | CO, NH3, C2H5OH, SO2 | Resistance increases | Resistance decreases |
| Oxidizing analytes | NO2, H2O, F2, Cl2, Br2, I2, and O2 | Resistance decreases | Resistance increases |
| Dominant charge carrier | — | Holes (h+) | Electrons (e−) |
Pristine graphene, GO and rGO, have presented a distinct gas sensing capability based on its structural characteristics. The 2D structure of graphene makes the electron transport through the graphene highly sensitive to the adsorption of gas molecules [90]. The adsorption of gas molecules on graphene’s surface leads to changes in its electrical conductivity attributed to the change in the local carrier concentration. That change in the local carrier concentration is induced by the surface adsorbates which act as electron donors or acceptors [91]. All these materials have different electrical conductivity and surface functional groups, which play an important role in the gas sensing mechanism. For example, due to absence of functional groups in pristine graphene, the interaction occurs through the defects, and it possesses low intrinsic noise and high electrical conductivity even in absence of charge carriers; few charge carriers induced by the gas adsorbates lead to notable changes in charge carrier density resulting in detectable changes in electrical conductivity. In the following sections, we summarized the theoretical aspects of the sensing performance and related mechanisms on the detected gas of graphene-based materials followed by a detailed discussion on the state of the novel research work on applications of pristine graphene, GO, rGO, and functionalized graphene for gas sensing.
Referring to Jeevitha et al. (2019), the vapour sensing mechanism of rGO/metal oxide nanocomposites is governed by several factors like sensor porosity, specific surface area, and heterojunction formation. Metal oxide semiconductor-based sensors work on the principle of the change in resistance owing to the reaction among gas molecules and the sensitive surface. In the case of rGO/WO3 nanocomposites, WO3 is an n-type semiconductor and rGO behaves like p-type. It is well-known that the n and p type materials are dominated by electrons and holes, respectively. Once they come into contact with each other, a depletion layer is formed at the interface which is a p–n heterojunction. The rGO/WO3 sensor shows p-type behaviour towards NH3 detection. rGO possesses a higher work function and defects in the prepared nanocomposite surface, which will provide many adsorption centers for NH3. Therefore, when the sensor surface is exposed to NH3, the NH3 molecules are adsorbed on the composite surface, and the interaction between adsorbed
The sensing behaviour of the polypyrrole (PPy)/rGO sensor is attributed to the electron transfer between NH3 molecule and the rGO/PPy nanocomposite. As described at the equations (2) and (3), the PPy behaves like a p-type semiconductor. When the electron-donating NH3 molecules adsorb onto the PPy surface, electrons transfer from NH3 to the π backbone of the PPy [93]. This neutralizes holes in the PPy, thereby increasing the PPy resistance. For desorption of analytes, the electrons come back from the PPy to NH3 and then the neutralized PPy becomes p-type, recovering the PPy resistance to its original value. The resistance changes from the electron transfer occurring on the surface of the PPy can be effectively transferred to the interdigitated electrodes (IDEs) through the rGO. It is worth noting that the electron transfers between the PPy and rGO is feasible due to the good interfacial affinity
Besides that, the rGO includes a high density of sp2-bonded carbons, vacancies, structural defects, and residual oxygen groups for a hole-transporting matrix, which behaves as a p-type semiconductor. The adsorbed NH3 may transfer to the rGO matrix through the PPy layer and donating electrons to the rGO. The electrons transfer depletes holes in the matrix, also increased the rGO resistance [94]. The synergistic effect of the PPy and rGO explains that the PPy/rGO sensor exhibits significantly higher sensitivity than the pristine rGO sensor. Although pristine PPy is a sensing material for NH3 at room temperature, the response and recovery time is very long. The PPy layer allows electrons to quickly transfer between NH3 molecules and the PPy/rGO nanocomposite. Besides, the presence of the PPy molecules on the surface of the rGO allows the complete interaction between NH3 and the binding sites; the adsorbed NH3 molecules can bind on the rGO through the PPy layer for the electron transfer. Therefore, the ultrathin PPy layer plays an important role in the PPy/rGO sensor response [95].
3 Performance of functionalized graphene in chemical sensor
3.1 Performance of graphene nanocomposite-based sensor
Graphene is highly sensitive towards changing chemical environment. This is because the suspended graphene has extremely high electron mobility at room temperature, and the electron transport in graphene remains increasing up to 0.3 μm at 300 K. Besides, in every carbon atom in graphene, there is a surface atom that provides the highest possible surface area per unit volume which leads the electron transport through the graphene and is highly sensitive to the adsorbed molecular species. The other factor is graphene has characteristically low electrical noise due to the quality of its crystal lattice and its high electron mobility [13]. These properties make graphene the best candidate for the ultrahigh sensitivity detection of different gases existing in various environments. High levels of sensitivity in detection processes are important for different industrial, environmental, public safety, and military applications [96].
The working principle for most of the graphene-based gas sensor is based on changes in their electrical conductivity due to the adsorption of gas molecules on the graphene’s surface. These gas molecules act as donors or acceptors on graphene, similar to other solid-state sensors [97]. In 2007, Schedin and team were the first researchers team who fabricated a microscopic sensor made from graphene that is capable of detecting individual gas molecules such as NO2, NH3, H2O, and CO. From the experiment, their sensor is able to respond as soon as a gas molecule attaches to or detaches from graphene’s surface and the adsorbed molecules change the local carrier concentration in graphene. This leads to step-like changes in resistance.
The device shows concentration-dependent changes in electrical resistivity by adsorption of gases after which the sensor is regenerated by annealing at 150°C under vacuum. This ultrahigh sensitivity stems from the fact that pristine graphene is an exceptionally low-noise material. The detection limit for solid-state gas sensors is usually defined as the minimal concentration that causes a signal exceeding sensors’ intrinsic noise. In Schedin et al. (2007) graphene sensor, a typical noise level in their devices is ∆ρ/ρ ≈ 10−4 that translates into the detection limit of the order of 1 ppb. As a result, this places graphene on parity with other materials used for most sensitive gas sensors [98]. The lowest level of noise was found in their devices with the highest mobility (>10,000 cm2/V s) and the lowest contact resistance. Sensors made from few-layer graphene (3 to 5 layers) were most electrically quiet, probably because their contact resistance could be as low as 50 Ω, as compared with typically 1,000 Ω for single-layer of graphene devices.
Different gases have different effects on the resistivity. The magnitudes and the sign of the change in the resistivity indicate whether the gas is an electron acceptor (e.g. NO2, H2O, and I2) or an electron donor (e.g. CO, ethanol, and NH3) [99]. Since conductivity is proportional to the product of number of charge carriers and mobility, the change in conductivity must be due to changes in the number density or mobility of carriers, or both. From the results, Hall effect measurement showed extra charge carriers being formed during gas adsorption on their device. That means that gas adsorption can increase the number of holes if the gas is an acceptor or increase the number of electrons if the gas is a donor. This change in the carrier concentration is the basic mechanism that governs the operation of all electrical conductivity-based graphene gas sensor devices towards the increased or decreased of resistance.
Graphene-based chemical sensors offer the possibility of ultrahigh sensitivity detection of a range of gas type in mixtures with air at room temperature and atmospheric pressure. Referring to Yavari and Koratkar (2012), comparing graphene to CNTs, it is concluded that a free-standing or suspended graphene sheet has both of its sides exposed to the chemical environment, thereby maximizing its sensitivity. But, for the multiwalled CNTs, the inner cylinders are shielded from the chemical environment. Even for SWCNTs, the ends may be closed (e.g. for tubes grown by CVD), or the metal contact pads might cap the tubes and prevent the inside of the tube from participating in gas adsorption. Graphene also exhibits inherently low electrical noise at room temperature, which arises from its unique 2D crystal lattice and high electron mobility [100].
Chu et al. (2011) investigated the characteristics of hydrogen detection using epitaxial graphene covered with a thin layer of platinum as a catalyst. The multilayered graphene was grown by CVD on a Si-polar 4H-SiC substrate. Graphene covered with a thin film of Pt showed reduced resistance in response to exposure to 1% hydrogen at various temperatures. This sensor works based on splitting of the H2 molecule in the presence of the catalytic metal. Dissociated hydrogen atoms will accumulate at the surface of Pt and diffuse into the graphene/Pt boundary, causing the hydrogen atoms to form covalent bonds with graphene. This hydrogenated form of graphene will have an increased work function and the separation distance increase between graphene and Pt can also cause the Fermi-level shift to become larger. Thus, the free carrier concentrations will increase and raise the conductance of the graphene/Pt device [101].
Chen et al. (2011) investigated the electrical resistivity of monolayer CVD-grown graphene that exhibits significant changes upon exposure to oxygen (O2) at room temperature. Results showed that O2 can be detected at concentrations of around 1.25% in volume ratio. When O2 molecules are attached to the surface of graphene, they form epoxide and carboxylic groups that are electron-withdrawing and increase hole concentration of the conduction band which generates a significant decrease in resistance [102].
Unlike graphene with zero band gap, which greatly hinders its applications, penta-graphene (PG), a new 2D allotrope of carbon based on Cairo pentagonal tiling pattern, is a material with individual atomic layer exclusively consisting of pentagons (a mixture of sp2- and sp3-coordinated carbon atoms) in a planar sheet geometry [103]. The sensing capabilities of monolayer of PG using first-principles and non-equilibrium Green’s function (NEGF) calculations towards small gas molecules, such as CO, CO2, NH3, NO, and NO2, have been conducted by Cheng et al. (2019) [104]. The results proved that monolayer PG is most preferred for the NO x (x = 1, 2) molecules with suitable adsorption strength and apparent charge transfer. Moreover, the current–voltage (I–V) curves of PG display a tremendous reduction of 88% (90%) in current after NO2 (NO) adsorption. The superior sensing performance of PG rivals or even surpasses that of other 2D materials such as graphene and phosphorene. The ultrahigh sensitivity and selectivity to nitrogen oxides make PG a superior gas sensor that promises wide-ranging applications. Figure 7(a) shows that the a 3 × 3 PG supercell with and without gas adsorption is used for each of the left and right electrodes and the centre scattering region, respectively, and Figure 7(b) and (c) shows the I–V curves of PG with and without the NO x gas adsorption.
![Figure 7
(a) Illustration of the two-probe systems of semi-infinite left and right electrode regions (red shaded region) which are in contact with the central scattering region of PG (b) and (c) display the I–V curves of PG and PG with the NO and NO2 adsorption. Reproduced from ref. [104].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_007.jpg)
(a) Illustration of the two-probe systems of semi-infinite left and right electrode regions (red shaded region) which are in contact with the central scattering region of PG (b) and (c) display the I–V curves of PG and PG with the NO and NO2 adsorption. Reproduced from ref. [104].
Lee et al. (2016) investigated the characteristic of defect-engineered graphene chemical sensors on NO2 and NH3 detection using commercial graphene [105]. Theoretically, a graphene defect engineering strategy was proposed to tailor the interface and mechanical properties of graphene nanocomposites. From the study, the defects were formed from the reaction of oxygen radicals and graphene via diffusion because the direct ion bombardment was blocked by the silicon wafer. During oxidation, oxygen radicals react with the carbon atoms in the graphene, forming sp3-type defects and vacancies formed by the detachment of carbon atoms in the form of CO or CO2. In this work, defects were created in graphene using oxygen plasma, with a conventional reactive ion etching system. The graphene was grown on copper foil by the CVD technique and the monolayered graphene (0.5 × 0.5 cm2) on the copper foil was wet-transferred to the (11 cm2) SiO2/Si substrate. Result shows that the defect-engineered graphene chemical sensors exhibited optimized sensitivities of 53 and 25% to 200 ppm of NO2 and NH3, respectively. The density functional theory simulations showed that the graphene sensors can be activated and enhanced by the presence of defects. Besides that, vacancy defect, whose density can be precisely controlled by defect engineering, is the main factor contributing to sensitivity. From the experiment, the pristine graphene exhibited sensitivity towards NH3 molecules, suggesting that vacancies are already present even in pristine graphene. The lower adsorption strength of NH3 compared with NO2 can explain a higher sensitivity of the latter than the former. However, the increase in the sensitivity of NH3 due to defect engineering was much higher than that in the sensitivity of NO2 (increases of sensitivity for NO2 and NH3 gases were 33 and 614%, respectively) because there was no charge transfer between defect-free graphene and NH3 molecules. Figures 8 and 9 show the molecular adsorption on defective graphene which has higher adsorption strength than pristine graphene for NO2 and NH3.
![Figure 8
Optimized structures of the NO2 molecule adsorbed on (a) pristine graphene, (b) sp3-type defect (epoxy group)-graphene, (c) sp3-type defect (carbonyl group)-graphene, (d) sp3-type defect (ether group)-graphene, and (e) single vacancy of graphene. The red, brown, and grey colours represent O, C, and N atoms, respectively. Reproduced from ref. [105].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_008.jpg)
Optimized structures of the NO2 molecule adsorbed on (a) pristine graphene, (b) sp3-type defect (epoxy group)-graphene, (c) sp3-type defect (carbonyl group)-graphene, (d) sp3-type defect (ether group)-graphene, and (e) single vacancy of graphene. The red, brown, and grey colours represent O, C, and N atoms, respectively. Reproduced from ref. [105].
![Figure 9
Optimized structure of the NH3 molecule adsorbed on (a) pristine graphene, (b) sp3-type defect (epoxy group)-graphene, (c) sp3-type defect (carbonyl group)-graphene, (d) sp3-type defect (ether group)-graphene, and (e) single vacancy of graphene. The red, brown, grey, and light pink colours represent O, C, N, and H atoms, respectively. Reproduced from ref. [105].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_009.jpg)
Optimized structure of the NH3 molecule adsorbed on (a) pristine graphene, (b) sp3-type defect (epoxy group)-graphene, (c) sp3-type defect (carbonyl group)-graphene, (d) sp3-type defect (ether group)-graphene, and (e) single vacancy of graphene. The red, brown, grey, and light pink colours represent O, C, N, and H atoms, respectively. Reproduced from ref. [105].
A developed graphene-based resistive gas sensor fabricated by transfer of CVD-grown graphene on a smooth paper showed low detection limit of 300 parts per trillion (ppt) for NO2 at room temperature which is comparable to or better than those from other paper-based sensors [106]. The overall sensor response of the graphene paper sensor was around 118% ppm−1 of NO2 and by ultraviolet exposure for 10 min, and the response is increased by a factor of 2.5. Referring to Kumar et al. (2015), large strain can generate cracks in graphene paper and results in large effect on its electrical and sensing properties. When graphene paper is subjected with different values of strain, the conductance (S) increased by a factor of 2 over the unstrained sample [106]. For large strains applied here (radius of curvature about 12 mm or lower), the resistance of the sample changes irreversibly to higher value of strains. Large strains can produce cracks and other defects in graphene layer, so this behaviour is expected. The cracks and defects formation provides more sites for adsorption of test gas, as well as bear large effect on resistance when they are bridged. As a result, the response improves as presented in the inset of Figure 11(c). Figure 10(a) and (b) shows the schematic diagram of preparation of graphene paper and the transfer process on the paper. Figure 11(a) shows the conductance change of graphene paper strips when 2.5 ppm of NO2 exposure, while Figure 11(b) shows the change in conductance of a graphene paper as concentration of NO2 increases from 0.5 pm up to 2.5 ppm.
![Figure 10
(a) and (b) Schematic diagram of graphene transfer process on to the paper. Starting with (1) graphene on Cu foil, (2) a layer of poly(methyl methacrylate) (PMMA) is spin-coated and Cu is etched to get graphene supported by PMMA in water, (3) PMMA-graphene film is then dredged on to paper and PMMA is dissolved with acetone and (4) normal paper yields patchy coverage compared to transfer on smooth paper (5). The smooth paper at (5) shows two layers of graphene transferred on smooth paper. (b) Schematic of a G-paper strip in action as a gas sensor. The circuit had sufficient current to make an LED glow ((bottom left). Reproduced from ref. [106].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_010.jpg)
(a) and (b) Schematic diagram of graphene transfer process on to the paper. Starting with (1) graphene on Cu foil, (2) a layer of poly(methyl methacrylate) (PMMA) is spin-coated and Cu is etched to get graphene supported by PMMA in water, (3) PMMA-graphene film is then dredged on to paper and PMMA is dissolved with acetone and (4) normal paper yields patchy coverage compared to transfer on smooth paper (5). The smooth paper at (5) shows two layers of graphene transferred on smooth paper. (b) Schematic of a G-paper strip in action as a gas sensor. The circuit had sufficient current to make an LED glow ((bottom left). Reproduced from ref. [106].
![Figure 11
(a) Change in conductance of a graphene paper strip to 2.5 ppm of NO2. The inset shows a fit of double exponential function to the temporal response for 2.5 ppm of NO2, (b) change in conductance of a graphene paper as concentration of NO2 increases starts from 0.5 to 2.5 ppm. The inset shows the plot of response at t = 1,000 s vs the concentration of NO2, which has a slope of 167 ppm−1 at the start (indicated by dashed line) and (c) change in conductance of a graphene paper as strain is applied at 2.5 ppm of N NO2 flow. The inset shows that both the baseline resistance and response of the strip increase with increasing strain. Reproduced from ref. [106].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_011.jpg)
(a) Change in conductance of a graphene paper strip to 2.5 ppm of NO2. The inset shows a fit of double exponential function to the temporal response for 2.5 ppm of NO2, (b) change in conductance of a graphene paper as concentration of NO2 increases starts from 0.5 to 2.5 ppm. The inset shows the plot of response at t = 1,000 s vs the concentration of NO2, which has a slope of 167 ppm−1 at the start (indicated by dashed line) and (c) change in conductance of a graphene paper as strain is applied at 2.5 ppm of N NO2 flow. The inset shows that both the baseline resistance and response of the strip increase with increasing strain. Reproduced from ref. [106].
Graphene gas sensors with different number of graphene layers were successfully fabricated by the facile transfer of monolayer CVD graphene sheets grown on Cu foil for NO2 sensing detection [107]. Results on gas sensing show that all graphene sensors demonstrated the p-type sensing behaviours under the adsorption of NO2 molecules. The highest response and the highest sensitivity towards NO2 at room temperature were shown by the bilayer graphene gas sensor. The increase is linearly with NO2 concentration over the range of 1 to 25 ppm and had a high linear sensitivity of 1.409 ppm−1. The bilayer graphene gas sensor also exhibited high selectivity to NO2 against CO, CO2, NH3, C2H5OH, and H2 at room temperature.
For the NO2 sensing mechanism, p-type gas sensing characteristics and bilayer graphene display considerably higher NO2 response than monolayer, 3-layer, and 4-layer of graphene. The NO2 sensing mechanism of graphene at low temperature may generally be explained based on (1) reducing reaction and (2) direct change transfer processes. In the case of the reducing reaction process, chemisorbed oxygen species (
Before the NO2 gas adsorption, the monolayer of graphene at Figure 12(a) exhibits the unique massless conical band electronic structure, while bilayer and multilayer graphene structures have typical parabolic bands associated with the finite charge carrier effective mass, which increases with increasing number of graphene layers [107]. In air,
![Figure 12
Schematic diagram of band diagrams of NO2-sensing mechanism of (a) monolayer, (b) bilayer, and (c) multilayer of graphene gas sensors proposed by Seekaew et al. Reproduced from ref. [107].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_012.jpg)
Schematic diagram of band diagrams of NO2-sensing mechanism of (a) monolayer, (b) bilayer, and (c) multilayer of graphene gas sensors proposed by Seekaew et al. Reproduced from ref. [107].
For monolayer graphene at Figure 12(a), the amount of charge transfer to NO2 molecules may be quite limited due to less available electronic state at high energy in valence band compared with bilayer one. Thus, the bilayer graphene, which has comparable accessible active surface area as monolayer one, could exhibit more charge transfer due to N
3.2 Performance of GO nanocomposite-based sensor
As discussed in synthesis of graphene in Section 2, the chemical oxidation of graphene and simultaneous reduction of resulting GO is one of the popular approaches used for graphene synthesis. GO has a layered structure parallel to graphene, but it is functionalized with oxygen-containing groups such as hydroxyls, epoxies, carboxyl, and lactones. These functional groups not only expand the interlayer spacing, but also make the atomic-thick layers more hydrophilic, enabling these oxidized layers to exfoliate in water under ultra-sonication or mechanical stirring [110]. Choi et al. (2015) studied the role of oxygen functional groups in GO for reversible room-temperature NO2 sensing [111]. Based on the results, graphene-based materials defects such as oxygen functional groups, Stone-Wales defects, and holes from the basal plane can act as active sites for interaction with molecules [112]. Owing to its oxygen-rich functional groups, GO could be considered as an ideal material for gas sensing. But the numerous oxygen functional groups of GO make it too electrically insulating for use as an active material for chemiresistive sensors. Choi et al. (2015) also stated that the major drawback of rGO-based sensors is the extremely sluggish and irreversible recovery to the initial state after a sensing event, which makes them incapable of producing repeatable and reliable sensing signals.
Based on study by Peng and Li (2013), GO shows better detection of NH3 than pristine graphene, since the active surface of defective sites of GO such as the epoxy and hydroxyl groups promotes the interactions between the NH3 molecules and GO [113]. Based on ab initio studies [114], the presence of hydroxyl and carbonyl groups attached on the carbon atoms on GO surface results in large binding energies and enhanced charge transfers from nitrogen oxides NO x (x = 1, 2, 3). This leads to chemisorption of gas molecules on GO surface [115]. Alam et al. (2018) have studied the development of GO-gold nanocrystals (AuNCs) nanocomposite-modified glassy carbon electrode (GCE) for the sensitive detection of dopamine (DA), uric acid (UA), and 4-aminophenol (4-AP) [116]. The GO was synthesized through modified Hummer’s method, which was utilized to prepare GO-AuNCs nanocomposites by in situ synthesis method using sodium L(−) malate as a reducing agent. It was observed that the sensor showed interference-free and selective detection of DA and UA with sensitivities of ca. 30.3 and 17.28 μA/cm2/μM, respectively, and detection limits of ca. 28 and 50 nM, respectively, with wider dynamic ranges, measured by differential pulse voltammetry (DPV) technique. It also shows a sensitivity and detection limit of ca. 5.70 μA/cm2/mM and 0.017 nM, respectively, for the detection of 4-AP, using current density (J)-voltage (V) measurement method. The sensor revealed an excellent stability, reproducibility, and recoveries of DA, UA, and 4-AP in real samples.
Yu et al. (2018) had fabricated Pt nanoparticle-incorporated GO nanocomposite-based microfiber sensor with high sensitivity for NH3 sensing [117]. The Pt-decorated sensor displayed a sensitivity of 10.2 pm/ppm, 3 times higher than the sensitivity without Pt-decorated nanoparticles. These results indicate the sensor has the optimal sensing sensitivity when the Pt nanoparticle concentration is 185.2 mg/L and exhibits a linear response with NH3 gas concentration below 80 ppm. This nanocomposite film-based passive optical fibre sensor provides an approach for highly sensitive NH3 sensing in a limited space, flammable or in explosive environment.
An excellent gas sensing characteristic of GO is contributed by the dangling bonds attached on its surface, which are essential for detecting the gas molecules. Yu and team (2018) made GO sheets that have higher adsorption efficiency of gas molecules by the presence of Pt nanoparticles. More NH3 gas molecules absorb to the surface of GO because the NH3 gas molecule can bind strongly to the bulk surfaces of platinum [118]. This incidentally improves the absorption ability of GO to NH3 gas molecules, resulting in the improvement in sensitivity for NH3 sensing. From their experiment, the different concentrations of Pt with the same GO content were tested for NH3 sensing. The result shows that the sensitivity for NH3 sensing is raised as the Pt concentration increases in the range from 0 mg/L to 185.2 mg/L. The sensing sensitivity was not improved significantly when the Pt concentration exceeds 185.2 mg/L. This could be because the refractive index change of GO is saturated with more gas molecules absorbed onto the surface of GO.
NH3 gas sensor characteristics based on fluorinated-GO (f-GO) have been investigated by Park et al. (2016) [119]. Gas sensor was fabricated by dropping dispersion solution (5 μL) of f-GO onto SiO2/Si wafers patterned with Pt electrodes. The fluorination treatment was carried out at partial fluorine gas (98%) pressures of 0.1 (GO-F1N9), 0.3 (GO-F3N7), and 0.5 (GO-F5N5) bar with an injection time of 5 min. The total pressure was adjusted to 1 bar with nitrogen gas, and the reaction time was 10 min. After the reaction, the residual gas was expelled and then purged 2 times with nitrogen gas. The gas sensor fabricated using f-GO (GO-F1N9) shows an approximately 7% change in the resistive response, whereas the non-treated GO does not exhibit any sensing ability to NH3 gas [119]. This change in the response is attributed to the lower Fermi level of GO and the increased number of holes in the GO after fluorination, which is helpful for effectively attracting NH3 gas. However, all samples do not exhibit recovery at room temperature and the presence of a higher number of fluorine-carbon bonds caused a decrease in the electrical resistance change.
For these reasons, fluorine bonded to GO surface leads to change in the electronic structure of GO. In Park et al. (2016) study, f-GO acts as an electron acceptor showing an increase of resistance when the reducing gas like NH3 is detected (Figure 13). GO decorated with fluorine functional group has a lower Fermi level than raw GO and makes electrons in the valence band move to the lowest unoccupied molecular orbital (LUMO) due to high electronegativity of fluorine. This fluorine doped on the GO surface creates a higher number of holes in the valence band. The absorption of NH3 gas on f-GO results in the electron lone pair of the NH3 molecules being transferred to LUMO of f-GO, leading to increases of Fermi level. Holes are not created in the valence band any more, thus the current flow is limited by the increase of resistance due to the decreased number of hole carriers. However, the GO-F5N5 (the higher fluorine gas pressure bar) showed a lower sensitivity to NH3 relative to those of GO-F1N9 and GO-F3N7. This result is supported by the loss of sp2 bonds by excess fluorination in GO. Figure 14 shows the sensing behaviour of f-GO under 100 ppm of NH3 gas at room temperature.

Adsorption phenomenon of NH3 with increase of resistance when the reducing gas like NH3 is detected.
![Figure 14
Sensing behaviour of f-GO under 100 ppm of NH3 gas at room temperature. Reproduced from ref. [119].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_014.jpg)
Sensing behaviour of f-GO under 100 ppm of NH3 gas at room temperature. Reproduced from ref. [119].
Prezioso et al. (2013) had investigated a number of forms of GO to optimize the sensing sensitivity and efficiency [120]. The fabricated sensing device by drop-casting water-dispersed single-layer GO flakes on standard 30 µm spaced interdigitated Pt electrodes. A practical p-type gas sensor using GO drop-casted shows a stable operating conditions, has a lifetime larger than 1,000s h, and exhibits a very low detection limit (20ppb). This excellent performance is due to the high quality of GO which have large and highly oxidized flakes. With correspondence to other carbonaceous materials such as rGO and CNTs, GO has the advantage to have a much larger number of active sites but the limitation being poorly conductive. Because of this, GO is considered as a good choice for applications that do not require fast responses, but require high sensitivities.
Dielectrophoresis (DEP) process for GO nanostructures for hydrogen gas sensor at room temperature was investigated by Wang et al. (2014) [121]. The GO nanostructures synthesized using Hummer’s method was assembled into gold electrodes using DEP process by varying parameters such as frequency, peak-to-peak voltage (Vpp), and processing time (t). Results show that an optimum DEP parameter required for hydrogen gas sensing using GO nanostructures is observed to be Vpp = 10 V, frequency = 500 kHz, and t = 30 s. The optimized device was found more effective and better hydrogen sensor with a good sensing response of 5%, fast response time (<90 s), and fast recovery time (<60 s) for 100 ppm hydrogen gas concentration at room temperature.
Some et al. (2013) had studied the highly sensitive and selective gas sensor using hydrophilic and hydrophobic graphenes coated on polymer optical fibre (POF) [122]. The high oxygenated functionalities on GO surface were observed to maintain the high sensitivity in highly unfavourable environments (extremely high humidity, strong acidic or basic). The GO-based sensor displayed faster sensing and higher sensitivity when compared to rGO even under extreme environments of over 90% humidity, making it the best choice for an environmentally friendly gas sensor. Furthermore, according to the experimental results, the sensitivity of GO to VOCs (mainly nitro and amine containing compounds) is much higher than that of rGO due to the presence of numerous polar functional groups like hydroxyl and carboxylic acid [122].
Shown in Figure 15 are the comparison of sensing responses for GO and rGO to eight different chemicals (hydrazine, ethanol, methanol, dichloromethane, acetone, THF, nitromethane, and diethylamine) at a 500 ppb of concentration level. The result shows that only GO and only rGO-coated POF sensors showed different sensitivities toward the various vapours. The intensity of the reflected optical response for the only GO and only rGO POF sensor was highest for hydrazine, diethylamine, and nitromethane vapours at the same concentration, respectively and was lowest for methanol and dichloromethane vapours, respectively. The experimental result shows the sensitivity of GO to VOCs (mainly nitro and amine containing compounds) is much higher than that of rGO due to the presence of numerous polar functional groups.
![Figure 15
Comparative of sensing behaviour towards the different chemical gas by GO and rGO coated on POF. Reproduced from ref. [122].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_015.jpg)
Comparative of sensing behaviour towards the different chemical gas by GO and rGO coated on POF. Reproduced from ref. [122].
3.3 Performance rGO nanocomposite-based sensor
Referring to Yavari and Koratkar (2012), even though GO is electrically insulating due to plentiful oxygen functional groups, the conductivity can be restored to several orders of magnitude. This is by the removal of oxygen groups using chemical or thermal reduction or can be partially reduced to graphene-like sheets. By removing the oxygen-containing groups with the recovery of a conjugated structure termed as rGO, the conductivity can be possessing up to several orders of magnitude. Even so, this process does not lead to pure graphene, and some residual oxygen groups remain even after the reduction process. Consequently, rGO has both high electrical conductivity and chemically active defect sites, making it a promising candidate for gas sensing. This sensor is able to reversibly and selectively detect chemically aggressive vapors such as NO2, Cl2, and so forth down to concentrations ranging from 100 to 500 ppb [13].
Exploitation of a new concept for enhancing the performance of rGO gas sensors by combining the structural engineering techniques of 3D microstructuring has been explored by Duy et al. (2015) [123]. From that, a high performance of three-dimensional chemical sensor platform using rGO for NH3 and NO2 detection was achieved. The performance of a chemical sensing device was enhanced from a simple three-dimensional (3D) chemiresistor-based gas sensor platform with an increased surface area by forming networked, self‐assembled rGO nanosheets on 3D SU8 micro‐pillar arrays. The 3D rGO sensor is highly responsive at low concentration of NH3 and NO2 diluted at room temperature. Compared to the two-dimensional (2D) planar rGO sensor structure, as the result of the increase in sensing area and interaction cross‐section of R‐GO on the same device area, the 3D rGO gas sensors show improved sensing performance with faster response (about 2%/s exposure), higher sensitivity, and even a possibly lower limit of detection towards NH3 at room temperature. Figure 16 shows the schematic diagram of structure for 2D and 3D devices and sensing response of rGO sensors towards 5 ppm of NO2 and 40 ppm of NH3.
![Figure 16
(a) Schematic diagram of structure for 2D and 3D devices of SU8 micro‐pillar arrays with different heights of 40 and 70 μm for 3D devices, (b) sensing response of rGO sensors towards a) 5 ppm NO2 (exposure time about 15 min) and (c) 40 ppm NH3 (exposure time about 30 min). Adapted from ref. [123].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_016.jpg)
(a) Schematic diagram of structure for 2D and 3D devices of SU8 micro‐pillar arrays with different heights of 40 and 70 μm for 3D devices, (b) sensing response of rGO sensors towards a) 5 ppm NO2 (exposure time about 15 min) and (c) 40 ppm NH3 (exposure time about 30 min). Adapted from ref. [123].
A sono-synthesis method to produce rGO nanosheets for NH3 vapour detection at room temperature was studied by Veluswamy et al. (2015). From their study, the polyethylenimine (PEI) was used as reducing agent to reduce the GO, which increases the conducting nature of the GO. The synthesis of GO and reduced rGO was prepared by Hummer’s method and sonication method with low-frequency ultrasound under ambient condition, respectively. The rGO-based chemiresistive sensors showed a good sensing response (3,500%), high sensitivity (38.85 kΩ/ppm), low level detection (1 ppm), wide range of detection (1 –100 ppm), quick response (6 s), recovery time (75 s), good reparability, better selectivity, and stability to be operated at room temperature for NH3 detection. Figure 17(a) shows the sensing response of various test vapours, and Figure 17(b) and (c) shows the sensing response of rGO film towards 1–5 ppm, and 10–100 ppm range of NH3 vapour [124].
![Figure 17
(a) Sensing response of various test vapours, (b) and (c) show the sensing response of rGO film towards 1–5 ppm and 10–100 ppm range of NH3 vapour. Adapted from ref. [124].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_017.jpg)
(a) Sensing response of various test vapours, (b) and (c) show the sensing response of rGO film towards 1–5 ppm and 10–100 ppm range of NH3 vapour. Adapted from ref. [124].
The NH3 sensing response of GO and rGO also has been investigated by Veluswamy et al. (2018) and shown in Figure 18. The result indicates that the NH3 response increases for rGO drastically (nearly 3–9 times), increases from 16 to 45% for 10 ppm, and from 403 to 3,400% for 100 ppm for rGO. The oxidation process from graphene to the highly oxygenated GO depends on various factors such as the size of GO sheets, the content of oxygen functional groups, and thus greater defect intensity of GO, which is confirmed by Raman results [125]. The electrical resistance of GO is high because of the disturbance of the conjugated electronic structure by these oxygen-containing groups. However, chemical reduction of GO can effectively bring back its conductivity; meanwhile, a fewer amount of oxygen-containing functional groups still remain in rGO, because of its incomplete reduction. The reduction process may introduce some vacancies and structural defects which can also act as adsorption sites. From that, the interaction of chemical molecules with high-energy defects in graphene differed dramatically from those with conjugated carbon structure. Therefore, optimization of defect density and its kind may be an effective way to manage the response, sensitivity, and selectivity of rGO-based chemical sensor [125].
![Figure 18
Comparative ammonia sensing response of GO and rGO film. Adapted from ref. [124].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_018.jpg)
Comparative ammonia sensing response of GO and rGO film. Adapted from ref. [124].
A highly selective detection of carbon monoxide (CO) gas by rGO-based chemical sensor at room temperature has been investigated by Panda et al. (2016) [126]. The sensing performances of rGO-based chemical sensor against CO were studied in terms of percent sensitivity (sensor response), response and recovery times, and I/V characteristics at room temperature. Gas sensing experiments exhibit about 71% sensitivity at room temperature at 30 ppm of CO. The selectivity of the sensor using different n-type reducing gases was negligible cross-sensitivity against NH3, CH4, and H2 at different concentrations. Thus, the rGO is shown as highly potential material for development of CO gas sensor with high degree of sensitivity, selectivity, and reliability, proving it selective to CO gas at room temperature within permissible exposure limits.
The research on rGO-based gas sensor functionalized with a peptide receptor to detect explosives, dinitrotoluene (DNT), which is a by-product of trinitrotoluene (TNT) was investigated by Lee et al. (2019) [127]. The rGO-based sensor was fabricated using DNT-specific binding peptide functionalized rGO. The sensitivity was calculated by measuring the resistance change using the differential signals between DNT-BP (binding peptide) and DNT-NBP (non-binding peptide) to function as highly specific and highly non-specific (for the control experiment) peptide receptors.
The rGO-based sensor showed an excellent linear sensitivity of (0.27 ± 0.02) × 10−4 ppb with an approximate limit of detection (LOD) of 2.43 ppb. The multi-arrayed rGO sensor was fabricated using spin coating and a standard microfabrication technique. The result was sensitivity of 27 ± 2 × 10−6 part per billion (ppb) for the slope of resistance change versus DNT gas concentration of 80, 160, 240, 320, and 480 ppm, respectively. By sequentially flowing DNT vapour (320 ppb), acetone (100 ppm), toluene (1 ppm), and ethanol (100 ppm) onto the rGO sensors, the change in the signal of rGO in the presence of DNT gas is 6,400 × 10−6 per ppb (Figure 19(c)). However, the signals from the other gases show no changes and represent highly selective performance. Figure 19(a) and (b) shows the rGO-based sensor for DNT detection and location of the rGO sensor patterns (200 μm × 100 μm) between the Au electrodes, respectively.
![Figure 19
(a) Enlarged rGO chemical sensor with eight multiple arrays for DNT detection, (b) location of the rGO sensor patterns (200 μm × 100 μm) between the Au electrodes, and (c) resistance change on five different gases: the differential values (black bar) of DNT-BP and DNT-NBP confirm the high selectivity. Adapted from ref. [127].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_019.jpg)
(a) Enlarged rGO chemical sensor with eight multiple arrays for DNT detection, (b) location of the rGO sensor patterns (200 μm × 100 μm) between the Au electrodes, and (c) resistance change on five different gases: the differential values (black bar) of DNT-BP and DNT-NBP confirm the high selectivity. Adapted from ref. [127].
A rGO-based sensor was synthesized and fabricated under H2/Ar treatment 100 to 900°C for H2 detection [128]. Before that, the GO samples were prepared according to a modified Hummers’ method. The prepared GO was then treated at different temperature to obtain a series rGO samples (rGO-100 to rGO-900); numbers denote the treating temperature. From the results obtained, the rGO-100 and rGO-200 showed very weak responses. Further responses of rGO-300 to rGO-900 against 500 ppm of H2 at room temperature were presented in Figure 20(a). From that, rGO-300 had exhibited the best sensitivity towards the H2 detection. Other than that, CO and CH4 also produced signals on rGO-300-based sensors. The balance between the chemical adsorption capacity and electronic conductivity and the dominance of either electrons or holes are the key factors of the high sensitivity from the rGO-300.
![Figure 20
(a) rGO structure with the attached carbonyl, carboxylic, hydroxyl, and epoxide groups considered as defective sites and (b) oxygen content, conductivity, and sensitivity of rGO samples with different treatment temperature. Adapted from ref. [128].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_020.jpg)
(a) rGO structure with the attached carbonyl, carboxylic, hydroxyl, and epoxide groups considered as defective sites and (b) oxygen content, conductivity, and sensitivity of rGO samples with different treatment temperature. Adapted from ref. [128].
Zhang et al. (2011) stated that in order to generate a strong sensing signals, adsorption of target gas molecules was necessary. It required relatively strong binding force between the sensing materials and the target gas molecules. As example, it was reported that the hydrogen molecules tended to be adsorbed on the defect site of the rGO (Figure 20(a)). Thus, the binding force between H2 and the sidewall of rGO was rather weak. This was the same for rGO-700 and rGO-900 samples because these two samples had few defect sites. For rGO, the oxygenate groups could be considered as the defect sites on the graphene sheets. The relationship among sensing properties, conductivity, and the oxygen content could be further described in Figure 20(b). The oxygen content decreased from GO to rGO-900 as determined from XPS, leading to increased conductivity. During the sensing process, the gas molecule is adsorbed on the rGO surface with the oxygenated groups through hydrogen-bonding, then electron is transferred to the rGO resulting in a change of the resistance. In order to generate a sufficient sensing signal, a sufficient amount of functional groups is needed to act as an adsorption site. However, too many functional groups also would significantly reduce the conductivity of the rGO sample as observed on rGO-100 and rGO-200. The amount of hydrogen adsorption may also affect the response pattern of rGO-300 and rGO-900. Therefore, a delicate balance between chemical adsorption capacity and the conductivity of the rGO samples was the key factor for good sensing responses’ properties realized on rGO-300.
Lipatov et al. (2013) had fabricated an array of thermally rGO-based integrated gas sensors [129]. The result shows the definitive identification of chemically similar analytes such as ethanol, methanol, and isopropanol by making use of the significant device-to-device variations of rGO-based sensors. Each rGO device used in the integrated gas sensing system has a unique sensor response due to the irregular structure and electronic properties of the rGO flakes produced from the same fabrication process. The sensing behaviour for rGO-based sensor arrays demonstrated a high selectivity that was sufficient to discriminate between different alcohols, such as methanol, ethanol, and isopropanol, at a 100% success rate (Figure 21). According to Robinson et al. (2008), the fast response for a certain gas or chemicals could be attributed to the adsorption of molecules at the low-energy binding sites, such as sp2 carbon domains, while the slow response was mainly caused by interactions between gas molecules with high-energy binding sites, such as vacancies, defects, and oxygen-containing functionalities [130].
![Figure 21
Scatterings of gas responses from twenty samples of rGO-based sensor to different analytes with 100% success rate at 1,000 rpm. Adapted from ref. [129].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_021.jpg)
Scatterings of gas responses from twenty samples of rGO-based sensor to different analytes with 100% success rate at 1,000 rpm. Adapted from ref. [129].
3.4 Performance of hybrid graphene/metal oxide nanocomposites-based sensor
Widely used commercial gas sensors are primarily focused on the semiconductor of metal oxide, polymer materials, and various sensing methods: optical, calorimetric and acoustic methods, and gas chromatography approaches. The shortcomings of these gas sensors can be one or more: expensive, rare ppb sensitivity, poor selectiveness, low endurance, poor reproducibility, difficult miniaturization, and high power consumption [45]. However, higher operating temperatures of these sensors obviously negatively affect the integration and long-term stability of the device, resulting in high power consumption as well as environment pollution as explosive gases may be emitted. Thus, a combination of graphene with metal oxide is one of improved strategies in order to overcome the hindrances.
To date, owing to their superior stability and as their electrical resistance to adsorbates is relatively highly sensitive, various kinds of metal oxides like n-type zinc oxide (ZnO) [131], iron(iii) oxide (Fe2O3) [132], stannous oxide (SnO2) [133], Indium oxide (In2O3) [134], tungsten oxide (WO3) [135], and p-type metal oxide like copper(ii) oxide (CuO) [136], nickel(ii) oxide (NiO) [137], chromium(iii) oxide (Cr2O3) [138], cobalt(ii,iii) oxide (Co3O4) [139], etc. have been extensively explored as gas sensing materials. Figure 22(a) shows classification of n-type and p-type of metal oxide used as chemical sensor and Figure 22(b) shows results of a search study on metal oxides semiconductor used as sensing materials for chemoresistive gas sensors, including both the n-type and p-type oxides [140].
![Figure 22
(a) Classification of n-type and p-type of metal oxide used as chemical sensor and (b) shows results of a search study on metal oxides semiconductor used as sensing materials for chemoresistive gas sensors. Reproduced from ref. [140].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_022.jpg)
(a) Classification of n-type and p-type of metal oxide used as chemical sensor and (b) shows results of a search study on metal oxides semiconductor used as sensing materials for chemoresistive gas sensors. Reproduced from ref. [140].
Due to their intrinsic large surface area, high electron mobility, and excellent conductivity under ambient conditions, rGO as derivatives of graphene are considered as ideal candidate in developing a room temperature gas like NO2 gas sensors. rGO are more practically applied to realize room-temperature gas sensing in view of their cost-effective mass production and the introduced oxygen functional groups and surface defects, which act as active sites for interaction with gas molecules. Thus, it is especially important to combine graphene and metal oxides to form hybrid nanostructures, since they do not only show the different properties of nanoparticles and graphene, but also display additional synergistic effect of being attractive, favourable for gas sensor applications, and foremost for the detection of gas at room temperature [141].
Due to adsorption of contaminants such as water and oxygen molecules, graphene and rGO comprised a bipolar and nearly symmetrical behaviour in the electron and hole doping regions. Hence, they show p-dominant (hole carriers) conducting properties. In addition, the decoration of graphene sheets with an n-type metal oxide can result in forming of a p–n junction, which exhibits better performances than the individual materials, resulting in new nanostructure. Researchers have therefore paid a great deal of attention in recent years to graphene hybridized with metal oxide or hybrid rGO architectures that function at room temperature for very sensitive, selective, and cost-effective gas sensors [142–145].
Spinel Co3O4 is a type of metal oxide consisting of CoO and Co2O3 which are rich in oxygen content, and thus comprised semiconductor features of p-type have been studied as a potential gas sensing material. The gas sensing characteristics have been investigated, where the sensing is normally based on the catalytic properties of the oxide surface and typically operating sensors at temperature over 200°C [146–148]. Chen et al. (2013) observed that the rGO-based NO2 gas sensors could considerably improve their reaction at room temperature when the Co3O4 nanocrystals are intercalated [149]. Two particular mechanisms clarified the improved response of Co3O4 graphene sensor in Co3O4 bonds efficiently to a single rGO layer surfactant, resulting in stronger reaction than pure rGO, with an expanded surface area. In addition, the Co3O4 nanocrystals act like nanopillars resulting in an extra macroporous arrangement between the layers of rGO, thereby further improving the gas diffusion to the surface of rGO, driven by the capillary forces [150]. Second is the hybridizing effect between the Co element and GNR as proposed by Liang et al. [151], suggested to help in improving the ability to reduce oxygen. The strong coupling in the GNR matrix between Co and oxygen ions increases the ionic nature of Co-O. As a result, Co3+ centres will serve as the additional NO2 and electron adsorption centres indirectly removed from the p-type GNR through oxygen bridging, contributing to such an additional decrease in resistance in the presence of NO2.
Another study on hybrid rGO with Co3O4 for NO2 gas at room temperature was done by Zhang et al. (2018). The results indicated that the optimal hybrid exhibited a response of 26.8% to 5 ppm of NO2 at room temperature, which was 2.27 times higher than that of undoped Co3O4 at 100°C. The hybrid sensor also showed fast response, excellent selectivity, long-term stability, and extremely low detection limit toward NO2 at room temperature. The enhancement of sensing characteristics to NO2 contributed to larger specific area, more chemisorbed oxygen species, and the hybridizing effect between Co3O4 and graphene in the hybrid [152].
Hybridizing Cu2O and rGO-based sensor for enhancement of low concentration of NO2 sensing at room temperature has been investigated by Pan et al. (2018). The sphere like of Cu2O and the hybrids with rGO have been successfully synthesized by a facile solution-based self-assembly method. The gas sensing properties to 1 ppm of NO2 at room temperature indicate that the 1 wt% of rGO/Cu2O composite not only exhibits 2.8 times higher response than that of pristine Cu2O and excellent selectivity, but also exhibit a rapid response and recovery at room temperature. The enhanced sensing characteristics mainly are attributed to increased gas adsorption active sites and the fast carriers transport due to the incorporating of rGO in the hybrid nanocomposites. Figure 23(a) shows FESEM images of GO nanocomposites and Cu2O and Figure 23(b) shows response of sensor based on Cu2O and 1 wt% rGO/Cu2O to 1 ppm of different tested gases at room temperatures [153].
![Figure 23
(a) FESEM images of (a) GO, (b) Cu2O, and (c and d) 1 wt% of rGO/Cu2O nanocomposite and (b) response of sensor based on Cu2O and 1 wt% rGO/Cu2O to 1 ppm of different tested gases at room temperatures. Reproduced from ref. [153].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_023.jpg)
(a) FESEM images of (a) GO, (b) Cu2O, and (c and d) 1 wt% of rGO/Cu2O nanocomposite and (b) response of sensor based on Cu2O and 1 wt% rGO/Cu2O to 1 ppm of different tested gases at room temperatures. Reproduced from ref. [153].
SnO2 is one among the foremost promising inorganic n-type semiconductors with band gap of 3.62 eV at 298 K and exhibits excellent gas sensing properties having good response to varied sorts of toxic gases and organic vapours [154–158]. The hybrid rGO with SnO2 nanomaterials-based gas sensors had obtained great attentions from the researchers [125,159–161]. Mao et al. (2012) had fabricated SnO2 nano crystal incorporated with rGO sheet which was decorated onto Au IDEs, as a novel gas sensing device [125]. The sensors’ manufactured performance demonstrated an optimum response to target gases at room temperature (detection limit 1 part per million (ppm) for NO2) and a SnO2 nanocrystal rGO strengthened the sensor signal to NO2 but had weaken the sensor response to NH3. Figure 24 shows comparison of gas sensing signals of NO2 and NH3 from pristine rGO sensors and rGO with SnO2 nanocomposites.
![Figure 24
Gas sensing signals of NO2 and NH3 from pristine rGO sensors (blue line) and SnO2 nanocomposites (red line). Reproduced from ref. [125].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_024.jpg)
Gas sensing signals of NO2 and NH3 from pristine rGO sensors (blue line) and SnO2 nanocomposites (red line). Reproduced from ref. [125].
Previous research by Zhang et al. (2014) has shown that the rGO/SnO2 nanocomposites show an efficient sensing material for detection of NO2 at temperature 50°C. The fabricated sensors shown in Figure 25(b) were by dropping the aqueous dispersion of products on a ceramic plate, which was previously coated with gold electrodes and ruthenium oxides as heater on frontal and back sides by screen printing technique followed by drying at room temperature. It is found that rGO/SnO2 nanocomposites exhibit high response of 3.31 at 5 ppm NO2, which is much higher than that of rGO (1.13), rapid response, and good selectivity and reproducibility. The enhanced gas sensing of the rGO/SnO2 sensors was due to the formation of heterojunctions at the interface between SnO2 and rGO and the effective electronic interaction between SnO2 nanocrystals and rGO. This results in facilitating the detection of gases through the change in the electrical conductivity of the hybrid nanostructure. In addition, the binding of SnO2 nanoparticles onto rGO contributes to more active sites (such as vacancies, defects, oxygen functional groups, and sp2-bonded carbon) for the adsorption of NO2 molecules; finally contributes to a higher sensitivity than pure rGO [160]. Figure 25(c) shows the sensing mechanism of the adsorption behaviour of NO2 molecules towards the rGO/SnO2 nanocomposite proposed by Zhang et al. (2014).
![Figure 25
(a) Images of blank sensor used to detect NO2 analytes, (b) images of the coated sensor with rGO/SnO2 nanocomposites as sensing materials, and (c) as sensing mechanism on the adsorption behaviour of NO2 molecules on the rGO/SnO2 nanocomposite. Reproduced from ref. [160].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_025.jpg)
(a) Images of blank sensor used to detect NO2 analytes, (b) images of the coated sensor with rGO/SnO2 nanocomposites as sensing materials, and (c) as sensing mechanism on the adsorption behaviour of NO2 molecules on the rGO/SnO2 nanocomposite. Reproduced from ref. [160].
For the sensing mechanism between the SnO2 and rGO, the improvement of gas sensing performances on NO2 sensor based on SnO2–rGO-2 (SnCl4·5H2O added is 0.024 g) could be attributed to introduction of SnO2 nanoparticles into the rGO matrix. This improvement also due to heterojunction has formed at the interface between SnO2 and rGO. In open air, the first depletion layer is due to the adsorption of ionized oxygen (
Cui et al. (2013) studies show indium (In) and ruthenium (Ru) doping in SnO2 not only increases the sensing properties against NO2, but also decreases the working temperature. Cui et al. (2013) had reported low-cost manufacturing hybrid nanomaterials in which In-doped SnO2 (IDTO) nanoparticles, 2 to 3 nm in size, were uniformly distributed to rGO. The improved sensing efficiency of rGO/In-SnO2 (rGO–IDTO) against NO2 was demonstrated at room temperature hitting a detection limit as low as 0.3 ppm. In addition, excellent selectivity was also attained, as confirmed by other common gases tested such as NH3, H2, CO, and H2S. Higher rGO–IDTO nanomaterials sensitivity indicates more NO2 adsorption and more electron transfer from nanoparticles to NO2. The higher sensitivity is also attributed by the improvement of higher surface-to-volume ratio of the nanoparticles exposed, which contributes to sufficient absorption sites in the sensing phase and thus increased sensitivity [161].
Another important n-type semiconductor, the WO3, exhibits distinct physical and chemical properties, viz. has small band gap (about 2.585 eV) and stable physiochemical properties, which attracted considerable interest such as an active layer for chemical sensors [162,163]. An ultrafine rGO/WO3 nanowire nanocomposite for highly sensitive toxic NH3 gas sensors has been studied by Hung et al. (2020). The nanocomposites composed of single crystal WO3 nanowires with an average size of 10 nm entangled by thin rGO layers. Sensing measurements confirmed that the rGO/WO3 nanocomposite-based sensor can detect highly toxic NH3 gas at low concentrations ranging from 20 to 500 ppm with detection limit of 138 ppb [164].
The development of rGO/WO3 nanolamellae nanocomposites with different contents of rGO (0.5, 1, 2, 4 wt%) was synthesized via controlled hydrothermal method by Jiang et al. (2018) for acetylene (C2H2) gas detection at low operating temperature. Among four contents of prepared samples, sensing materials with 1 wt% rGO nanocomposite exhibited the best C2H2 sensing performance with lower optimal working temperature at 150°C, higher sensor response with 15.0 toward 50 ppm, faster response-recovery time at 52 and 27 s, lower detection limitation (1.3 ppm), long-term stability, and excellent repeatability. The enhancement of gas sensing performance of nanocomposite is possibly attributed to the formation of p–n heterojunction and the active interaction between WO3 nanolamellae and rGO sheets. Besides, the introduction of rGO nanosheets leads to the impurity of synthesized materials, which creates more defects and promotes larger specific area for gas adsorption, outstanding conductivity, and faster carrier transport [165].
A room-temperature NO2 gas sensor based on rGO/WO3 nanocomposite films was fabricated using one-pot polyol process [166]. The sensor based on a nanocomposite film of rGO/WO3 found that the introduction of rGO was effective for increasing the conductance of rGO/WO3 nanocomposite film especially at strong response low concentration of NO2 gas. When the amount of rGO added was below 3.2 wt%, the response (S) of the sensors dramatically increased and the concentration of NO2 gas sensing increased with increasing the amount of rGO added. However, the amount of rGO added exceeded the percolation threshold, no variation in response versus concentration of NO2 gas was clearly observed. The NO2 gas sensor was very sensitive with acceptable linearity between 0.5 and 5 ppm, good reversibility, and long-term stability (at least 45 days) when used at room temperature.
Hydrothermally synthesized rGO/WO3 nanocomposites-based gas sensor with interdigitated chromium electrode for NH3 detection has been investigated by Punetha and Pandey (2019). The sensor shows the best performance at 150°C operating temperature; for 10 and 100 ppm of NH3 gas concentration. The sensor response achieved 10.89 and 27.7 with 11/17 and 7/19 s response/recovery time, respectively. However, at room temperature, the sensor depicts the sensing response 4.35 with 13/20 s response/recovery time for 10 ppm of NH3 gas concentration. The performance and stability of the device have been reported and analysed for different time intervals. This study paves a new approach to design and fabricate the gas sensing electronic device with high-performance parameters for semiconducting applications [167].
ZnO with a direct wide band gap (3.37 eV) and large exciton binding energy (60 meV) has been widely studied for gas sensing application due to its good response to a variety of reducing or oxidizing gases, low cost, and being friendly to the environment [168,169]. A highly sensitive room temperature of hydrogen gas sensor-based rGO/ZnO nanocomposites has been reported by Das et al. (2020). The ZnO nanoparticles were grown by chemical deposition method, while the rGO layer was produced by the electrochemical exfoliation using tetramethyle ammonium hydroxide (TMAH) as organic solvent and then drop-casted on the ZnO nanoparticles layer. The hybrid rGO–ZnO nanocomposite sensor with a Pd–Ag (70%) catalytic contact was tested for five different hydrogen concentrations (e.g. 100, 500, 1,000, 5,000, and 10,000 ppm) in synthetic air at room temperature. The sensor showed 484.1% response magnitude with 21.04 and 47.09 s response and recovery time at 100 ppm H2, respectively [170].
Hierarchical rGO/ZnO hybrids with a flower-like morphology of ZnO and flexible rGO sheets were synthesized by a facile solution-processed method [171]. The gas sensing properties of hierarchical rGO/ZnO hybrids toward NO2 were studied via a static system. The response of rGO/ZnO hybrids to 50 ppb NO2 was 12, which was seven times higher than that of pristine ZnO at 100°C. The limit of detection achieved as low as 5 ppb. The enhanced sensor response was attributed to the presence of local p–n heterojunctions between rGO sheets and hierarchical structure of ZnO.
Referring to Das et al. (2020), an increased number of gas-interaction sites are probably directed to a high response magnitude of the sensor nanocomposites. The existence of band bending at the ZnO–rGO interface will accelerate more electron flow from ZnO to rGO. Additionally, high carrier mobility of rGO acted as an efficient cross-linkage among the neighbouring ZnO nanoparticles, resulting in faster response time. Besides that, the higher response magnitude is mainly due to the existence of more number of p–n heterojunction with additional oxygen adsorption site available at the rGO–ZnO interface. Such supportive hybridization of two prosperously sensing elements, ZnO nanoparticles and rGO, will aim at the creation of the next generation nanohybrid gas sensor devices with ever-increasing performance.
A sensitive and robust chemiresistive NO2 gas sensing approach with the detection range of 5 ppb to 5 ppm using rGO/ZnO nanocomposite has been reported by Cao et al. (2020). It is investigated that the sensing response of rGO/ZnO sensor is significantly higher than ZnO sensor at 110°C working temperature. The rGO/ZnO sensor shows very low detection limit down to 5 ppb and high sensing bandwidth from 5 ppb to 5 ppm, indicating its potential use for gas sensing. The sensor exhibits high repeatability and long-term stability over the period. The sensitivity of NO2 detection is due to large surface area and ultrahigh carrier mobility of rGO alongside high adsorption capability of ZnO nanospheres. This easily modulates depletion layer through fast electron transfer at the interface of heterojunction [172]. Figure 26(a) shows the schematic diagram for the synthesization of ZnO nanospheres by solvothermal method and the formation of rGO/ZnO heterostructure and Figure 26(b) shows the FESEM image of rGO/ZnO.
![Figure 26
(a) schematic diagram for the synthesization of ZnO nanospheres by solvothermal method and the formation of rGO/ZnO heterostructure, and (b) rGO/ZnO image under FESEM at 500 nm. Reproduced from ref. [172].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_026.jpg)
(a) schematic diagram for the synthesization of ZnO nanospheres by solvothermal method and the formation of rGO/ZnO heterostructure, and (b) rGO/ZnO image under FESEM at 500 nm. Reproduced from ref. [172].
The MoO3 nanoflakes coupled rGO with enhanced ethanol sensing performance and their mechanism has been investigated by Tang et al. (2019). The rGO/MoO3 nanocomposites were fabricated through annealing process, and the rGO/MoO3 shows high sensitivity, fast response, and good selectivity to ethanol. The response towards 100, 200, 500, and 8,000 ppm of ethanol is 53, 68.98, 117.0, and 702, respectively, which are 5.4, 4.83, 5.05, and 3.64 times higher than the MoO3 at the working temperature of 310°C. In addition, the rGO/MoO3 had shown good selectivity towards the ethanol over n-propanol, methanol, isopropanol, xylene, acetone, and benzene. Mo5+ also plays an important role in the sensing mechanism. The possible mechanism of the rGO/MoO3 to ethanol is different from the traditional n-type semiconductors; both of the adsorbed oxygen and lattice oxygen participants in the reaction catalytically oxidize the ethanol into H2O and CO2 and result in the change of resistance. As a result, this indicates that the Mo5 + also plays an important role in the sensing process [173]. Figure 27 shows the schematic diagram of the sensing mechanism of rGO/MoO3 to ethanol.
![Figure 27
Schematic diagram of the sensing mechanism of rGO/MoO3 to ethanol. Reproduced from ref. [173].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_027.jpg)
Schematic diagram of the sensing mechanism of rGO/MoO3 to ethanol. Reproduced from ref. [173].
Bai et al. (2015) had used in situ microwave hydrothermal method in order to fabricate the heterojunction hybrids of rGO/α-MoO3. With different rGO contents (2.5, 5, and 10 wt%), the sensing performance of 5 wt% of rGO/α-MoO3 nanocomposites-based sensor to H2S is significantly preferable. The nanocomposite demonstrates improved sensitivity, strong selectivity, rapid response, and recovery, as well as exhibits stability and repeatability in ppm-level H2S at operating temperatures of 110°C, relative to those of pure MoO3 as shown in Figure 28(a). The change in sensing efficiency can be due to the formation of heterojunction at the hybrid interface, which increases n-type surface area in MoO3, thus promoting electron migration through the addition of rGO [174]. Figure 28(b) shows the response of 5 wt% of rGO/α-MoO3 nanocomposites-based sensor towards different gases analysis at 100°C operating temperature.
![Figure 28
(a) Sensor response to 40 ppm of H2S and (b) response of 5 wt% of rGO/α-MoO3 nanocomposites-based sensor towards different gases analysis 100°C operating temperature. Reproduced from ref. [174].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_028.jpg)
(a) Sensor response to 40 ppm of H2S and (b) response of 5 wt% of rGO/α-MoO3 nanocomposites-based sensor towards different gases analysis 100°C operating temperature. Reproduced from ref. [174].
Furthermore, referring to Bai et al. (2015), the improvement in sensing response could be attributed by these two factors: (1) the sensing response of semiconductor sensors involves adsorption and desorption phenomena and reactions at the interface; the surface accessibility of nanocrystals is crucial to maintain their high sensitivity. In particular, the 2D rGO sheets have created large 3D network architectures which enhance the interconnection of the MoO3 and rGO. The rGO substrates are available in greater surface accessibility as well as rapid carrier transport to promote molecular adsorption, gas diffusion, and mass transport. (2) The enhancement of hybrid gas sensing can be due to electrons migrating at the interface between the MoO3 nanorods and rGO nanosheets because of the disparity in their work function. In addition, the transfer of electrons at the interface further increases hybrid resistance in air because the depletion layer becomes thick. However, when the hybrid is subjected to reducing gas like H2S, the H2S will interact with oxygen ions absorbed onto the MoO3 surface and releases the electrons to MoO3, which results in a decrease in resistance [174].
Ye et al. (2016) had worked on rGO/TiO2 layered film deposition on the interdigital electrode substrate via thermal treatment to trace detection of formaldehyde (CH2O) at ambient temperature. The sensing performances of rGO/TiO2 nanocomposites investigated over low detection concentrations from 0.1 to 0.5 ppmv revealed that the rGO/TiO2 sensor exhibited rapid response, excellent selectivity, good reproducibility, and remarkable sensitivity of 0.8 ppm v−1, which was higher than that of pristine rGO-based sensor (0.5 ppm v−1) [175]. Another study on rGO/TiO2 was done by Li et al. (2016) on the rGO-decorated TiO2 microspheres nanocomposites-based chemiresistive-type sensor. The fabricated chemiresistive-type sensor using hydrothermal method shows good sensitivity and excellent selectivity to different concentrations of NH3 from 5 to 50 ppm at room temperature. However, the response and recovery speeds of the sensor to NH3 are slow and need further optimization [176]
The detection of NH3 sensor working at room temperature has been successfully developed using rGO/TiO2 [177]. rGO/TiO2 hybrid through simple hydrothermal method and the sensor devices is easily fabricated through spray method to create conductive sensing network on the surface of IDEs. The sensing properties of the hybrid sensor suggest that introduction of TiO2 into rGO significantly enhances the sensing performance. The main contributing factor for improved performance of rGO/TiO2 hybrids in comparison to the pristine rGO is ascribed to the supporting function of TiO2, which ameliorates the surface structure and enriches the active adsorption sites.
3.5 Performance of hybrid graphene/conductive polymer nanocomposites-based sensor
The development of highly sensitive and selective gas and vapour sensors based on graphene and their polymer nanocomposites has been attracted by the aforementioned properties. This section would address recent progress in the use of conductive polymer/graphene nanocomposites in sensors [178]. Many different types of organic materials have been used for gas sensing. The simplest organic compounds that can be electrically conductive are polymers, based on carbon and hydrogen [179]. Organic conductive polymers including PPy [180], polyaniline (PANI) [181], polythiophene (PTh) [182 and poly(3,4-ethylenedioxythiophene) (PEDOT) [183] are examples of materials for fabricating gas sensors. Some conducting polymers can behave like semiconductors due to their heterocyclic compounds which display physicochemical characteristics. As a result, reversible changes in the sensing layer’s conductivity can be detected upon polar chemicals’ adsorption on the surfaces at room temperature [184]. This effect is believed to be caused by the charge transfer between gas molecules and the polymer or swelling of the polymer films [118]. This sensing response has intensively increased motivation to develop high sensitive and selective chemical sensors by tailoring the compounds of different organic polymers with functionalized graphene.
Due to adsorption of interested chemical and gases, there are volumetric changes of the matrix polymer. This leads to a distinct change in percolation-type conductivity around a critical composition of the material, which is known as “percolation threshold”. Generally, the percolation threshold is dependent on the shape of the conducting particle. Conductive polymer consisting of particles with higher aspect ratio shows lower threshold and higher sensitivity [185]. Consequently, by using conductive polymers, the sensitivity and selectivity of graphene-based chemical sensors can be enhanced. There have been numerous efforts in order to incorporate graphene or its derivatives with polymers.
Andre et al. (2017) had fabricated hybrid layer-by-layer (LbL) films of PANI/GO/ZnO gas sensors operating at room temperature which used to monitor the environment for hazardous pollutants like NH3 gas. Because of synergistic effect in the materials properties, the films with 3 tetra layers were found to be the most adequate for detecting NH3 in the range from 25 to 500 ppm with a response time of 30 s [186].
According to Huang et al. (2012), the hybrid rGO/PANI exhibited much rapid increase in resistance of 59.2% upon exposure to 50 ppm NH3 gas as compared to a resistance change of about 5.2 and 13.4% for pristine rGO and pristine PANI nanofibre-based sensor, respectively. The rGO/PANI nanofibre hybrid sensitivity to NH3 gas is 3.5 times higher than the sensitivity of pristine PANI nanofibre sensor and 10.4 times higher than the sensitivity of bare rGO. This much better sensitivity is due to the combined effect of rGO sheets and the decorated PANI nanoparticles. However, owing to the high surface ratio of rGO sheets and PANI nanoparticles, a long recovery time of 4 min for the sensing device based on rGO/PANI nanocomposites was observed. These hybrids have exhibited strong reversibility, long-term stable sensing efficiency under standard operating conditions, and high NH3 gas selectivity in the presence of different analytes such as DMMP, methanol, dichloromethane, cyclohexane, and chloroform [187].
Li et al. (2018) studied about a sensitive sensor used for NH3 detection at room temperature comprised of PANI nanosphere and GO-rambutan-PANIHs hybrids nanocomposites, with different weight percentages of GO (0.2–2 wt%). The nanocomposite was prepared by in situ chemical oxidative polymerization method and assembled on flexible polyethylene terephthalate (PET) substrate with no extra electrode. The efficiency of the gas sensor on the basis of 0.5 GO-rambutan-PANIHs nanocomposites shows the most sensitivity to the 10 ppm of NH3 at room temperature. It exhibits response of approximately 31.8 for 100 ppm NH3 and a rapid response time and recovery time of 102 and 186 s, respectively. This nanocomposite-based gas sensor achieved an amazing selectivity and an ultra-low detection limit of 50 ppb of NH3 at room temperature [188]. Figure 29(a) and (b) shows the TEM image of rambutan-like PANIHs nanocomposites and Figure 29(c) the characteristic of the responses of sensors based on pure 0.2, 0.5, 1, and 2 wt% GO-PANIHs to 10 ppm NH3 at room temperature.
![Figure 29
(a and b) TEM image of rambutan-like PANIHs and the nanocomposites, and (c) sensor response on the effect of weight percentages of GO. Reproduced from ref. [188].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_029.jpg)
(a and b) TEM image of rambutan-like PANIHs and the nanocomposites, and (c) sensor response on the effect of weight percentages of GO. Reproduced from ref. [188].
A room temperature sub-ppb H2S gas sensors based on SnO2/rGO/PANI nanocomposites was synthesized by in situ polymerization technique by Zhang et al. [189]. The SnO2/rGO/PANI nanocomposite film was manufactured using a polyethylene terephthalate (PET) substrate with interdigital electrodes (IDEs). The SnO2/rGO/PANI sensor has excellent sensing characteristics such as high sensitivity, rapid response and recovery time, robust repeatability, excellent selectivity, and long-term stability. The response of the nanocomposite film sensor produced by in situ polymerization is 23.9 to 200 ppb H2S, which is twice as high than that of physical doping method, and the detection limit is 50 ppb. The experimental result reveals that the SnO2/rGO/PANI sensor is an excellent candidate to detect H2S gas in exhaled human breath for early diagnosis of halitosis. The underlying sensing mechanism of the SnO2/rGO/PANI sensing device towards H2S gas is due to the high surface area of SnO2/rGO/PANI film, chemisorption of oxygen on surface of SnO2 hollow spheres, and the special role of heterojunction.
Xie et al. (2014) had reported the NO2 detection behaviour of organic thin film transistor (OTFT)-based gas sensors employing pure P3HT film and rGO/P3HT bilayer films were compared. The results demonstrated an 80% improvement in the sensing response of OTFT gas sensor based on rGO/P3HT bilayer film which was due to the deposited rGO as the bottom layer of the bilayer film. The larger sensitivity of rGO/P3HT-based OTFT gas sensor is due to the peculiar properties of rGO such as large surface area due to 2D structure and availability of many graphitic carbon atoms as active sites for NO2 adsorption. On exposure to other gases such as NH3, SO2, CO, CO2, and H2S, the sensing response of rGO/P3HT-based sensor was two orders of magnitude lower than that of NO2, owing to the presence of P3HT layer that prevents the interference gases from contacting rGO. This work suggests that the low selectivity issue of graphene could get benefit from the functionalization of graphene with polymers [190].
Ye et al. (2014) have used rGO/P3HT nanocomposite films for fabricating NH3 gas sensors and the rGO/P3HT films showed better sensitivity than rGO film sensor, where the sensor was prepared by the spray process. The improved sensor reaction has resulted from superior surface morphology in the composite films and the π–π interactions of the P3HT and rGO films. Sensitivity (7.15% for rGO/P3HT, 5.37% for rGO), reaction time (141 s for rGO/P3HT thin film, 637 s for rGO thin film), and recovery time (488 s for rGO/P3HT thin film; 609 s for rGO film) are all sensor parameters recorded [191].
Yang et al. (2014) had fabricated an efficient chemiresistive sensing platform using rGO-based nanocomposite with porous conductive polymer like PEDOT showed great promise for high performance gas sensing due to the enhanced sensitivity and selectivity of the gas sensor to NH3 gas at ppb-level. The gas sensing performance indicated that, in comparison to bare rGO and standard PEDOT, the porous rGO/PEDOT-based gas sensor displayed an apparent improvement in sensitivity and response/recovery performance (Figure 30). The vast area of the PEDOT and its open structure contributed to an excellent synergistic impact during the gas sensing phase between PEDOT and rGO. This nanocomposite-based sensor also demonstrated higher selectivity to NH3 in comparison to other reduction analyte gases as a result of a uniform distribution of the PEDOT porous network in the rGO sheets [192].
![Figure 30
Gas selectivity of bare rGO and PEDOT/rGO-based sensing device towards 1 ppm various analyte gases. Reproduced from ref. [192].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_030.jpg)
Gas selectivity of bare rGO and PEDOT/rGO-based sensing device towards 1 ppm various analyte gases. Reproduced from ref. [192].
A gas sensor device was developed using rGO-doped PEDOT-polystyrene sulfonate (PSS) organic thin film for the detection of NH3 gas at room temperature [193]. The doping of rGO was used in PEDOT-PSS targeted to increase the conductivity of pristine PEDOT-PSS thin films by threefold. The gases such as NH3, CO, NO2, and nitrogen were used to test the sensing performance in the prepared thin films. The best gas sensitivity was achieved at 10 wt% of rGO-doped PEDOT-PSS thin film of about 87% for NH3 gas with fast response and recovery times on exposing the sensor device to ammonia gas compared to other test gases such as CO, NO2, and nitrogen. The sensor stability test shows the prepared sensor is highly stable even after a period of 1 month. Due to improved sensitivity, stability, and improved response and recovery times, these rGO-doped PEDOT-PSS organic thin films possibly will be used to detect NH3 gas at low concentrations at room temperature.
The effect combination of PPy with 3D rGO to construct bioinspired nanocomposite for NH3 sensing enhancement was conducted by Qin et al. (2019). The 3D-rGO, pre-prepared by a facile hydrothermal reduction method, serves as 3D skeleton to provide solid support for sensitive PPy nanoparticles attachment. The NH3 sensing properties of the PPy/3D-rGO nanocomposite and the single components evaluated at room temperature found that the bioinspired PPy/3D-rGO nanocomposite displays a 4 to 5 times enhancement in gas response compared with pure PPy or pure rGO and can rapidly respond to NH3 with sub-ppm level. Pure PPy is known as a kind of sensing material operating at room temperature; the response values to 1 to 5 ppm of NH3 are calculated to be 1.3 to 3.4. It is now proven that the response capability of PPy can be enhanced considerably after fabricated with 3D rGO. The enhanced sensing performances of PPy/3D-rGO nanocomposite are analysed reasonably based on its unique microstructure and the effective nanocompositeization between rGO and PPy nanoparticles [194].
For this reason, the ultrafast charge transfer occurring between the conjugated PPy and rGO can be explained by the π–π stacking that can also consequently promote the sensing performance of nanocomposite film. In other words, the strongly coupled hetero-interface between PPy and rGO due to vdW-bonded nanocompositeization endows the ultrafast charge transfer between each other and then also contributes to the enhanced sensing performance of the nanocomposite. Once the PPy/3D-rGO nanocomposite is exposed to NH3, NH3 molecules adsorb on the surface of the nanocomposite, and the H-bonding may take place between the adsorbed NH3 and PPy or between NH3 and rGO [93]. This could break the current H-bonding between rGO and PPy, encouraging an additional increase in resistance for PPy/3D-rGO nanocomposite sensor [195]. Table 4 shows the summary of hybrid and non-hybrid graphene-based chemical sensor performance towards their chemical detection.
Summary of hybrid and non-hybrid graphene-based chemical sensor performance
| Type of graphene | Metal oxide | Polymer | Chemical detection | Concentration | Sensor response | Response time | Recovery time | Reference |
|---|---|---|---|---|---|---|---|---|
| Graphene | Cu2O | — | H2S | 5 ppb | 11% | — | — | [196] |
| Pd | PMMA | H2 | — | 66.37% | 1.8 min | 5.5 min | [197] | |
| — | PANI | H2 | 1 ppm | 16.57% | 2 min | 3 min | [198] | |
| — | PANI | Toluene | 100 ppm | 35.5% | ∼11 min | 54 min | [199] | |
| TiO2 | PPy | NH3 | 50 ppm | 36% | 36 s | 16 s | [200] | |
| — | PEDOT:PSS | NH3 | 500 ppm | 9.6% | 3 min | 5 min | [201] | |
| CuTsPc/N | PEDOT-PSS | NH3 | 200 ppm | 20% | 2.5 min | 1 min | [202] | |
| Graphene quantum dots | S, N | PANI | NH3 | 100 ppm | 42.3% | 1.92 min | 0.73 min | [203] |
| N | PANI | NH3 | 1,500 ppm | 110.92 | 7 min | 0.083 min | [204] | |
| GO | WO3 | — | NO2 | 1 ppm | 61 | — | — | [205] |
| ZnO | — | NH3 | 1 ppm | 24% | 6 min | 3 min | [206] | |
| ZnO | — | CO | 22 ppm | 24.3% | 5 min | 4 min | [206] | |
| ZnO | — | NO | 5 ppm | −3.5% | 25 min | — | [206] | |
| ZnO | PANI | NH3 | 100 ppm | 38.31% | 30 s | — | [186] | |
| — | PANI | NH3 | 100 ppm | 11.33% | 50 s | 23 s | [207] | |
| TiO2 | PANI | NH3 | 100 ppm | 110% | 32 s | 17 s | [208] | |
| — | PANIH | NH3 | 10 ppm | 31.8 | 2 min | 3 min | [188] | |
| rGO | — | — | NO2 | 100 ppm | 1.41% | 15 min | 35 min | [209] |
| — | — | NO2 | 5 ppm | 30% | >10 min | 10 min | [210] | |
| Co3O4 | — | NO2 | 5 ppm | 26.8% | 1.5 min | 40 min | [152] | |
| Co3O4 | — | NO2 | 60 ppm | 80% | 1 min | 2 min | [149] | |
| Cu2O | — | NO2 | 2 ppm | 67.8% | — | — | [145] | |
| SnO2 | — | NO2 | 1 ppm | 2.87 | — | — | [125] | |
| SnO2 | — | NO2 | 50 ppm | 6.5% | 3.17 min | 3.73 min | [211] | |
| SnO2 | — | NO2 | 8 ppm | ∼1,000% | 5 min | 8 min | [159] | |
| SnO2 | — | NO2 | 5 ppm | 3.31 | 2.25 min | 3.33 min | [160] | |
| In-SnO2 | — | NO2 | 100 ppm | 11 | 11 min | — | [161] | |
| SnO2 | — | NO2 | 1 ppm | 700 | 11 min | 6 min | [212] | |
| WO3 | — | NO2 | 1 ppm | 1.26 | 0.4–3 min | 0.4–3 min | [213] | |
| WO3 | — | NO2 | 56 ppm | 40.8% | — | — | [214] | |
| Fe3O4 | — | NO2 | 400 ppm | 24.2% | 4.58 min | 12.3 min | [215] | |
| — | P3HT (OTFT) | NO2 | 2 ppm | 7.5% | 5 min | 5 min | [190] | |
| ZnO | — | NO2 | 50 ppm | 8% | 2.2 min | 2.73 min | [216] | |
| ZnO | — | NO2 | 5 ppm | 119 | 2.3 min | 4.3 min | [217] | |
| ZnO | — | CO | 5 ppm | 22.6 | — | — | [217] | |
| ZnO | — | C6H6 | 5 ppm | 19.1 | — | — | [217] | |
| ZnO | — | C2H5OH | 5 ppm | 19.1 | — | — | [217] | |
| Pd–WO3 | — | H2 | 500 ppm | ∼102 | <1 min | <1 min | [206] | |
| TiO2 | — | HCHO | 1 ppmv | 0.64 | 1 min | 2 min | [175] | |
| TiO2 | — | NH3 | 50 ppm | ∼5% | — | — | [176] | |
| — | — | NH3 | 5 ppm | 10% | >10 min | 10 min | [210] | |
| — | P3HT | NH3 | 10 ppm | 7.15% | 2 min | 8 min | [191] | |
| — | PEDOT | NH3 | 1 ppm | 20% | 1.5 min | 2 min | [192] | |
| — | PANI | NH3 | 50 ppm | 59.2% | 18 min | ∼4 min | [187] | |
| — | PANI | NH3 | 50 ppm | 47.6% | 18 min | 4 min | [218] | |
| — | PANI | NH3 | 100 ppm | 344.2 | 20 s | 27 s | [219] | |
| SnO2 | PANI | NH3 | 20 ppm | 160% | 13 min | 30 min | [220] | |
| SnO2 | — | NH3 | 1 ppm | 1.12 | — | — | [125] | |
| SnO2 | — | NH3 | 50 ppm | 15.9% | <1 min | <1 min | [221] | |
| SnO2 | PANI | H2S | 10 ppm | 91.11% | — | — | [189] | |
| MoO3 | — | H2S | 20 ppm | 23 | 9 s | 17 s | [174] | |
| — | PANI | DMMP | 50 ppm | 22% | — | — | [218] | |
| Co3O4 | — | Methanol | 300 ppm | 4% | 4 min | 6 min | [149] | |
| — | PEI | CO2 | 1,000 ppm | 0.7% | 5 min | 10 min | [222] | |
| Na-X | PPy | CO | 1,000 ppm | 77.4% | 5.1 min | — | [223] | |
| Graphene-carbon nanotubes | — | PMMA | Methane | 10 ppm | 0.55 | — | — | [224] |
4 Conclusions and future perspective
One of the nanocomposites applications highlighted is graphene-based chemical sensor. Graphene-based materials have been used as new sensing elements in developing gas and chemical sensors for wearable electronics due to their unique optical, electrical, mechanical, and thermal properties. The different types of sensors discussed here indicate that the right combination with doping materials, conductive polymer, and metal oxides device architectures, synergistically contributing interfacial effects, is important for high-performance of sensors. The detection performance of sensor is influenced by the chemical composition and structure, conductivity and charge transport surface area of morphological nanostructures, defects in graphene layered structures, functionalization sites, graphene interlayer spacing, concentration of analytes, intermolecular π–π interactions, along with its adsorption sites ability. At this stage, the parameters need to be considered for sensor are sensitivity, analyte concentration, operation temperature, limit of detection, recovery time, response time, response, and selectivity.
Further studies are paramount to discover each property and interaction on graphene-based nanocomposites. From the recent discoveries, chemical modifications of graphene would help to challenge the loops regarding its production, storage, handling, and processing as the modified graphene nanocomposites can have synergistic properties resulting from both graphene and the modifiers. Graphene nanocomposites can be tailored to have desired solubility and stability, tunable electrical, thermal and mechanical properties, and enhanced catalytic and biological properties. This can be achieved via either covalent or non-covalent chemical modifications of graphene. The former can be used to form graphene composites with strong affinity which finally will maximally keep the graphene’s intrinsic properties. π–π stacking, hydrogen-bonding, van der Waals force, and coordination are the main interactions for non-covalent modifications. As a result, graphene nanocomposites modified via these methods, particularly generated via the π–π stacking process, would offer good mediums for any adaptable applications. In addition, through the chemical modification, either covalent bonding or non-covalent interactions can be realized. For the covalent modifications, it is usually achieved by a few techniques, such as atom doping or reaction with residual functional groups on graphene formed during the production or destruction of graphene’s unsaturated structure, via destruction of conjugated structures, and modifications via residual structures.
In light of this, to be competitive with commercial sensors, graphene-based chemical sensor devices must be mass reproducible at low cost. Unlike CNTs, which do not exist in nature, graphene sheets are already present in graphite. It is worth to mention that GO is highly ranked functional substrate owing to its oxygen, which sometimes also leads to taking a part in the surface modification interactions and sometimes indirect detection of reactive gases. Therefore, top-down methods such as exfoliation of GO could be possible to be used for mass production graphene nanosheets at low cost. Such top-down techniques do not exist for most other categories of nanomaterials. Likewise, CVD synthesis of macro-GFs and roll-to-roll deposition of graphene on large area substrates by CVD could substantially lower the cost of graphene-based chemical sensors. These properties make them desired materials characteristics for the fabrication of intelligent and ultrasensitive sensors and green energy devices and competitive with commercial sensor technologies with mass production at low cost.
Acknowledgments
The authors would like to thank Department of Higher Education (JPT), Ministry of Higher Education Malaysia and The Science and Technology Facilities Council, United Kingdom (STFC), under Newton fund’s Programand Malaysia Partnership and Alliances in Research (MyPAiR) for research grant ISIS-NEWTON/2019/SG/01 and Chemical Defence Research Centre (CHEMDEF), and National Defence University of Malaysia for a research grant UPNM/ 2018/CHEMDEFF/ST/3 are gratefully acknowledged.
-
Funding information: Newton fund’s research grant ISIS-NEWTON/2019/SG/01 and Chemical Defence Research Centre (CHEMDEF) research grant UPNM/ 2018/CHEMDEFF/ST/3 are gratefully acknowledged.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Geim A, Novoselov K. The nobel prize in physics 2010. Sweden: Nobelprize org Nobel Media AB; 2010.Suche in Google Scholar
[2] Novoselov KS, Fal VI, Colombo L, Gellert PR, Schwab MG, Kim K. A roadmap for graphene. Nature. 2012;490(7419):192–200.10.1038/nature11458Suche in Google Scholar PubMed
[3] Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–9.10.1126/science.1102896Suche in Google Scholar PubMed
[4] Geim AK, Novoselov KS. The rise of graphene, in nanoscience and technology: a collection of reviews from nature journals. World Sci. 2010;11–9.10.1142/9789814287005_0002Suche in Google Scholar
[5] Georgakilas V, Perman JA, Tucek J, Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev. 2015;115(11):4744–822.10.1021/cr500304fSuche in Google Scholar PubMed
[6] Allen MJ, Tung VC, Kaner RB. Honeycomb carbon: a review of graphene. Chem Rev. 2010;110(1):132–45.10.1021/cr900070dSuche in Google Scholar PubMed
[7] Chandrasekar M, Senthil MKT, Senthilkumar K, Mohd Nurazzi N, Sanjay MR, Rajini N, et al. Inorganic. Nanofillers-based thermoplastic and thermosetting composites. Lightweight polymer composite structures. CRC Press; 2020. p. 309–30.10.1201/9780429244087-11Suche in Google Scholar
[8] Liu C, Huang X, Wu YY, Deng X, Liu J, Zheng Z, et al. Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide. Nanotechnol Rev. 2020;9(1):155–69.10.1515/ntrev-2020-0014Suche in Google Scholar
[9] Sang M, Shin J, Kim K, Yu KJ. Electronic and thermal properties of graphene and recent advances in graphene based electronics applications. Nanomater. 2019;9(3):374.10.3390/nano9030374Suche in Google Scholar PubMed PubMed Central
[10] Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva I, et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature. 2005;438(7065):197–200.10.1038/nature04233Suche in Google Scholar PubMed
[11] Zhang Y, Tan YW, Stormer HL, Kim P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature. 2005;438(7065):201–4.10.1038/nature04235Suche in Google Scholar PubMed
[12] Fathi D. A review of electronic band structure of graphene and carbon nanotubes using tight binding. J Nanotechnol. 2011;1–6.10.1155/2011/471241Suche in Google Scholar
[13] Yavari F, Koratkar N. Graphene-based chemical sensors. J Phys Chem Lett. 2012;3(13):1746–53.10.1021/jz300358tSuche in Google Scholar PubMed
[14] Collins PG, Bradley K, Ishigami M, Zettl DA. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science. 2000;287(5459):1801–4.10.1126/science.287.5459.1801Suche in Google Scholar PubMed
[15] Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, et al. Nanotube molecular wires as chemical sensors. Science. 2000;287(5453):622–5.10.1126/science.287.5453.622Suche in Google Scholar PubMed
[16] Modi A, Koratkar N, Lass E, Wei B, Ajayan PM. Miniaturized gas ionization sensors using carbon nanotubes. Nature. 2003;424(6945):171–4.10.1038/nature01777Suche in Google Scholar PubMed
[17] Schedin F, Geim AK, Morozov SV, Hill EW, Blake P, Katsnelson MI, et al. Detection of individual gas molecules adsorbed on graphene. Nat Mater. 2007;6(9):652–5.10.1038/nmat1967Suche in Google Scholar PubMed
[18] Cao Y, Fatemi V, Demir A, Fang S, Tomarken SL, Luo JY, et al. Correlated insulator behaviour at half-filling in magic-angle graphene superlattices. Nature. 2018;556(7699):80–4.10.1038/nature26154Suche in Google Scholar PubMed
[19] Cao Y, Fatemi V, Fang S, Watanabe K, Taniguchi T, Kaxiras E, et al. Unconventional superconductivity in magic-angle graphene superlattices. Nature. 2018;556(7699):43–50.10.1038/nature26160Suche in Google Scholar PubMed
[20] Ichinokura S, Sugawara K, Takayama A, Takahashi T, Hasegawa S. Superconducting calcium-intercalated bilayer graphene. ACS Nano. 2016;10(2):2761–5.10.1021/acsnano.5b07848Suche in Google Scholar PubMed
[21] Gibney E. Surprise graphene discovery could unlock secrets of superconductivity. Nature. 2018;555:151–2.10.1038/d41586-018-02773-wSuche in Google Scholar PubMed
[22] Mele EJ. Novel electronic states seen in graphene. Nature. 2018;556:37–8.10.1038/d41586-018-02660-4Suche in Google Scholar
[23] Hernaez M, Zamarreño CR, Melendi-Espina S, Bird LR, Mayes AG, Arregui FJ. Optical fibre sensors using graphene-based materials: a review. Sensors. 2017;17(1):1–24.10.3390/s17010155Suche in Google Scholar
[24] Bai RG, Muthoosamy K, Manickam S, Hilal-Alnaqbi A. Graphene-based 3D scaffolds in tissue engineering: fabrication, applications, and future scope in liver tissue engineering. Int J Nanomed. 2019;14:5753–83.10.2147/IJN.S192779Suche in Google Scholar
[25] Qureshi TS, Panesar DK. A comparison of graphene oxide, reduced graphene oxide and pure graphene: early age properties of cement composites. 2nd RILEM Spring Convention & International Conference on Sustainable Materials, Systems and Structures. Rovinj, Croatia: 2019.Suche in Google Scholar
[26] Chuah S, Pan Z, Sanjayan JG, Wang CM, Duan WH. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr Build Mater. 2014;73:113–24.10.1016/j.conbuildmat.2014.09.040Suche in Google Scholar
[27] Lv W, Li Z, Deng Y, Yang QH, Kang F. Graphene-based materials for electrochemical energy storage devices: opportunities and challenges. Energy Storage Mater. 2016;2:107–38.10.1016/j.ensm.2015.10.002Suche in Google Scholar
[28] Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater. 2010;22(35):3906–24.10.1002/adma.201001068Suche in Google Scholar
[29] Compton OC, Nguyen ST. Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon‐based materials. Small. 2010;6(6):711–23.10.1002/smll.200901934Suche in Google Scholar
[30] Wan S, Chen Y, Wang Y, Li G, Wang G, Liu L, et al. Ultrastrong graphene films via long-chain π-bridging. Matter. 2019;1(2):389–401.10.1016/j.matt.2019.04.006Suche in Google Scholar
[31] Bonaccorso F, Lombardo A, Hasan T, Sun Z, Colombo L, Ferrari AC. Production and processing of graphene and 2d crystals. Mater Today. 2012;15(12):564–89.10.1016/S1369-7021(13)70014-2Suche in Google Scholar
[32] Krishnamoorthy K, Veerapandian M, Yun K, Kim SJ. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon. 2013;53:38–49.10.1016/j.carbon.2012.10.013Suche in Google Scholar
[33] Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45(7):1558–65.10.1016/j.carbon.2007.02.034Suche in Google Scholar
[34] Kazi SN, Badarudin A, Zubir MN, Ming HN, Misran M, Sadeghinezhad E, et al. Investigation on the use of graphene oxide as novel surfactant to stabilize weakly charged graphene nanoplatelets. Nanoscale Res Lett. 2015;10(1):1–15.10.1186/s11671-015-0882-7Suche in Google Scholar PubMed PubMed Central
[35] Sharma N, Sharma V, Jain Y, Kumari M, Gupta R, Sharma SK, et al. Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromolecular Symposia. Germany: Wiley Online Library; 2017.10.1002/masy.201700006Suche in Google Scholar
[36] Yang H, Zhou W, Yu B, Wang Y, Cong C, Yu T. Uniform decoration of reduced graphene oxide sheets with gold nanoparticles. J Nanotechnol. 2012;2012:328565.10.1155/2012/328565Suche in Google Scholar
[37] Schrier J. Fluorinated and nanoporous graphene materials as sorbents for gas separations. ACS Appl Mater Interfaces. 2011;3(11):4451–8.10.1021/am2011349Suche in Google Scholar PubMed
[38] Inagaki M, Kang F. Graphene derivatives: graphane, fluorographene, graphene oxide, graphyne and graphdiyne. J Mater Chem A. 2014;2(33):13193–206.10.1039/C4TA01183JSuche in Google Scholar
[39] Dey A. Semiconductor metal oxide gas sensors: a review. Mater Sci Eng B. 2018;229:206–17.10.1016/j.mseb.2017.12.036Suche in Google Scholar
[40] Rzaij JM, Abass AM. Review on: TiO2 thin film as a metal oxide gas sensor. Chem Rev. 2020;2(2):114–21.10.33945/SAMI/JCR.2020.2.4Suche in Google Scholar
[41] Tyagi P, Sharma A, Tomar M, Gupta V. Metal oxide catalyst assisted SnO2 thin film based SO2 gas sensor. Sens Actuators B Chem. 2016;224:282–9.10.1016/j.snb.2015.10.050Suche in Google Scholar
[42] Shendage SS, Patil VL, Vanalakar SA, Patil SP, Harale NS, Bhosale JL, et al. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens Actuators B Chem. 2017;240:426–33.10.1016/j.snb.2016.08.177Suche in Google Scholar
[43] Ponzoni A, Baratto C, Cattabiani N, Falasconi M, Galstyan V, Nunez-Carmona E, et al. Metal oxide gas sensors, a survey of selectivity issues addressed at the SENSOR Lab, Brescia (Italy). Sensors. 2017;17(4):1–27.10.3390/s17040714Suche in Google Scholar PubMed PubMed Central
[44] Demon SZ, Kamisan AI, Abdullah N, Noor SA, Khim OK, Kasim NA, et al. Graphene-based materials in gas sensor applications: a review. Sens Mater. 2020;32:759–77.10.18494/SAM.2020.2492Suche in Google Scholar
[45] Liu X, Cheng S, Liu H, Hu S, Zhang D, Ning H. A survey on gas sensing technology. Sensors. 2012;12(7):9635–65.10.3390/s120709635Suche in Google Scholar PubMed PubMed Central
[46] Nurazzi NM, Harussani MM, Zulaikha NS, Norhana AH, Syakir MI, Norli A. Composites based on conductive polymer with carbon nanotubes in DMMP gas sensors–an overview. Polimery. 2021;66(2):85–97.10.14314/polimery.2021.2.1Suche in Google Scholar
[47] Vilanova X. Special issue “Advanced nanomaterials based gas sensors”. Sensors. 2020;20(5):1–5.10.3390/s20051373Suche in Google Scholar PubMed PubMed Central
[48] Norizan MN, Moklis MH, Demon SZ, Halim NA, Samsuri A, Mohamad IS, et al. Carbon nanotubes: functionalisation and their application in chemical sensors. RSC Adv. 2020;10(71):43704–32.10.1039/D0RA09438BSuche in Google Scholar PubMed PubMed Central
[49] Chatterjee SG, Chatterjee S, Ray AK, Chakraborty AK. Graphene–metal oxide nanohybrids for toxic gas sensor: a review. Sens Actuators B Chem. 2015;221:1170–81.10.1016/j.snb.2015.07.070Suche in Google Scholar
[50] Mondal TK, Dinda D, Saha SK. Nitrogen, sulphur co-doped graphene quantum dot: an excellent sensor for nitroexplosives. Sens Actuators B Chem. 2018;257:586–93.10.1016/j.snb.2017.11.012Suche in Google Scholar
[51] Tung TT, Tran MT, Feller JF, Castro M, Van Ngo T, Hassan K, et al. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon. 2020;159:333–44.10.1016/j.carbon.2019.12.010Suche in Google Scholar
[52] Bottari G, Herranz MÁ, Wibmer L, Volland M, Rodríguez-Pérez L, Guldi DM, et al. Chemical functionalization and characterization of graphene-based materials. Chem Soc Rev. 2017;46(15):4464–500.10.1039/C7CS00229GSuche in Google Scholar
[53] Jiang L, Fan Z. Design of advanced porous graphene materials: from graphene nanomesh to 3D architectures. Nanoscale. 2014;6(4):1922–45.10.1039/C3NR04555BSuche in Google Scholar
[54] Rajesh GZ, Charlie Johnson AT, Puri N, Mulchandani A, Aswal DK. Scalable chemical vapor deposited graphene field-effect transistors for bio/chemical assay. Appl Phys Rev. 2021;8(1):011311.10.1063/5.0024508Suche in Google Scholar
[55] Yun YJ, Hong WG, Choi NJ, Park HJ, Moon SE, Kim BH, et al. A 3D scaffold for ultra-sensitive reduced graphene oxide gas sensors. Nanoscale. 2014;6(12):6511–4.10.1039/C4NR00332BSuche in Google Scholar PubMed
[56] Mishra SK, Tripathi SN, Choudhary V, Gupta BD. SPR based fibre optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced graphene oxide prepared by in situ polymerization. Sens Actuators B Chem. 2014;199:190–200.10.1016/j.snb.2014.03.109Suche in Google Scholar
[57] Lin YM, Avouris P. Strong suppression of electrical noise in bilayer graphene nanodevices. Nano Lett. 2008;8(8):2119–25.10.1021/nl080241lSuche in Google Scholar PubMed
[58] Hill EW, Vijayaragahvan A, Novoselov K. Graphene sensors. IEEE Sens J. 2011;11(12):3161–70.10.1109/JSEN.2011.2167608Suche in Google Scholar
[59] Booth TJ, Blake P, Nair RR, Jiang D, Hill EW, Bangert U, et al. Macroscopic graphene membranes and their extraordinary stiffness. Nano Lett. 2008;8(8):2442–6.10.1021/nl801412ySuche in Google Scholar PubMed
[60] Li W, Geng X, Guo Y, Rong J, Gong Y, Wu L, et al. Reduced graphene oxide electrically contacted graphene sensor for highly sensitive nitric oxide detection. ACS Nano. 2011;5(9):6955–61.10.1021/nn201433rSuche in Google Scholar PubMed
[61] Chung MG, Lee HM, Kim T, Choi JH, Kyun Seo D, Yoo JB, et al. Highly sensitive NO2 gas sensor based on ozone treated graphene. Sens Actuators B Chem. 2012;166:172–6.10.1016/j.snb.2012.02.036Suche in Google Scholar
[62] Kulkarni GS, Reddy K, Zhong Z, Fan X. Graphene nanoelectronic heterodyne sensor for rapid and sensitive vapour detection. Nat Commun. 2014;5(1):1–7.10.1038/ncomms5376Suche in Google Scholar PubMed
[63] Alahi ME, Nag A, Mukhopadhyay SC, Burkitt L. A temperature-compensated graphene sensor for nitrate monitoring in real-time application. Sens Actuator A Phys. 2018;269:79–90.10.1016/j.sna.2017.11.022Suche in Google Scholar
[64] Ratinac KR, Yang W, Ringer SP, Braet F. Toward ubiquitous environmental gas sensors-capitalizing on the promise of graphene. Env Sci Technol. 2010;44(4):1167–76.10.1021/es902659dSuche in Google Scholar PubMed
[65] Segal M. Selling graphene by the ton. Nat Nanotechnol. 2009;4(10):612–4.10.1038/nnano.2009.279Suche in Google Scholar PubMed
[66] Mohd Nurazzi N, Muhammad Asyraf MR, Khalina A, Abdullah N, Sabaruddin FA, Kamarudin SH, et al. Fabrication, functionalization, and application of carbon nanotube-reinforced polymer composite: an overview. Polymers. 2021;13(7):1047.10.3390/polym13071047Suche in Google Scholar PubMed PubMed Central
[67] Lu G, Yu K, Ocola LE, Chen J. Ultrafast room temperature NH3 sensing with positively gated reduced graphene oxide field-effect transistors. Chem Commun. 2011;47(27):7761–3.10.1039/c1cc12658jSuche in Google Scholar PubMed
[68] Gutés A, Hsia B, Sussman A, Mickelson W, Zettl A, Carraro C, et al. Graphene decoration with metal nanoparticles: towards easy integration for sensing applications. Nanoscale. 2012;4(2):438–40.10.1039/C1NR11537ESuche in Google Scholar
[69] Niu F, Liu JM, Tao LM, Wang W, Song WG. Nitrogen and silica co-doped graphene nanosheets for NO2 gas sensing. J Mater Chem A. 2013;1(20):6130–3.10.1039/c3ta11070bSuche in Google Scholar
[70] Russo PA, Donato N, Leonardi SG, Baek S, Conte DE, Neri G, et al. Room‐temperature hydrogen sensing with heteronanostructures based on reduced graphene oxide and tin oxide. Angew Chem Int Ed. 2012;51(44):11053–57.10.1002/anie.201204373Suche in Google Scholar PubMed
[71] Paul RK, Badhulika S, Saucedo NM, Mulchandani A. Graphene nanomesh as highly sensitive chemiresistor gas sensor. Anal Chem. 2012;84(19):8171–8.10.1021/ac3012895Suche in Google Scholar PubMed PubMed Central
[72] Tiwari SK, Sahoo S, Wang N, Huczko A. Graphene research and their outputs: status and prospect. J Sci Adv Mater Dev. 2020;5(1):10–29.10.1016/j.jsamd.2020.01.006Suche in Google Scholar
[73] Mao S, Yu K, Cui S, Bo Z, Lu G, Chen J. A new reducing agent to prepare single-layer, high-quality reduced graphene oxide for device applications. Nanoscale. 2011;3(7):2849–53.10.1039/c1nr10270bSuche in Google Scholar PubMed
[74] Yang W, Mao S, Yang J, Shang T, Song H, Mabon J, et al. Large-deformation and high-strength amorphous porous carbon nanospheres. Sci Rep. 2016;6(1):1–9.10.1038/srep24187Suche in Google Scholar PubMed PubMed Central
[75] Alemour B, Yaacob MH, Lim HN, Hassan MR. Review of electrical properties of graphene conductive composites. Int J Nanoelectron Mater. 2018;11(4):371–98.Suche in Google Scholar
[76] AZoM. Graphite (C) – Classifications, properties & applications. United Kingdom; 2002; Available from: https://www.azom.comSuche in Google Scholar
[77] Castañeda LF, Walsh FC, Nava JL, de León CP. Graphite felt as a versatile electrode material: properties, reaction environment, performance and applications. Electrochim Acta. 2017;258:1115–39.10.1016/j.electacta.2017.11.165Suche in Google Scholar
[78] Spahr ME, Zürcher S, Rodlert-bacilieri M, Mornaghini F, Gruenberger TM, Imertech SAS. High-conductive carbon black with low viscosity. United States patent US 10870761; 2020 Dec 22.Suche in Google Scholar
[79] Lu W, He T, Xu B, He X, Adidharma H, Radosz M, et al. Progress in catalytic synthesis of advanced carbon nanofibers. J Mater Chem A. 2017;5(27):13863–81.10.1039/C7TA02007DSuche in Google Scholar
[80] David B, Patrick P, Andrew P. Carbon nanofiber applications & properties. Germany; 2020; Available from: https://www.sigmaaldrich.comSuche in Google Scholar
[81] Kavitha M, Kalpana AM. Carbon nanotubes: properties and applications-A brief review. i-manager’s J ElectrEng. 2017;7(3):1–6.10.26634/jele.7.3.13558Suche in Google Scholar
[82] Spinelle L, Gerboles M, Kok G, Persijn S, Sauerwald T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors. 2017;17(7):1–30.10.3390/s17071520Suche in Google Scholar PubMed PubMed Central
[83] Norizan MN, Zulaikha NS, Norhana AB, Syakir MI, Norli A. Carbon nanotubes-based sensor for ammonia gas detection–an overview. Polimery. 2021;66(3):175–86.10.14314/polimery.2021.3.3Suche in Google Scholar
[84] Giddey SP, Badwal SP, Kulkarni A. Review of electrochemical ammonia production technologies and materials. Int J Hydrog Energy. 2013;38(34):14576–94.10.1016/j.ijhydene.2013.09.054Suche in Google Scholar
[85] Miller DR, Akbar SA, Morris PA. Nanoscale metal oxide-based heterojunctions for gas sensing: a review. Sens Actuators B Chem. 2014;204:250–72.10.1016/j.snb.2014.07.074Suche in Google Scholar
[86] Ahuja T, Kumar D. Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sens Actuators B Chem. 2009;136(1):275–86.10.1016/j.snb.2008.09.014Suche in Google Scholar
[87] Baharuddin AA, Ang BC, Haseeb AS, Wong YC, Wong YH. Advances in chemiresistive sensors for acetone gas detection. Mater Sci Semicond Process. 2019;103:1–19.10.1016/j.mssp.2019.104616Suche in Google Scholar
[88] Hou L, Zhang C, Li L, Du C, Li X, Kang XF, et al. CO gas sensors based on p-type CuO nanotubes and CuO nanocubes: morphology and surface structure effects on the sensing performance. Talanta. 2018;188:41–9.10.1016/j.talanta.2018.05.059Suche in Google Scholar PubMed
[89] Jaisutti R, Lee M, Kim J, Choi S, Ha TJ, Kim J, et al. Ultrasensitive room-temperature operable gas sensors using p-type Na: ZnO nanoflowers for diabetes detection. ACS Appl Mater Interfaces. 2017;9(10):8796–804.10.1021/acsami.7b00673Suche in Google Scholar PubMed
[90] Tian W, Liu X, Yu W. Research progress of gas sensor based on graphene and its derivatives: a review. Appl Sci. 2018;8(7):1–21.10.3390/app8071118Suche in Google Scholar
[91] Jawaid M, Ahmad A, Lokhat D. Graphene-based nanotechnologies for energy and environmental applications. Netherlands: Elsevier; 2019.10.1016/B978-0-12-815811-1.00002-8Suche in Google Scholar
[92] Jeevitha G, Abhinayaa R, Mangalaraj D, Ponpandian N, Meena P, Mounasamy V, et al. Porous reduced graphene oxide (rGO)/WO3 nanocomposites for the enhanced detection of NH3 at room temperature. Nanoscale Adv. 2019;1(5):1799–811.10.1039/C9NA00048HSuche in Google Scholar
[93] Joshi A, Gangal SA, Gupta SK. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens Actuators B Chem. 2011;156(2):938–42.10.1016/j.snb.2011.03.009Suche in Google Scholar
[94] Leenaerts O, Partoens B, Peeters FM. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: a first-principles study. Phys Rev B. 2008;77(12):1–6.10.1103/PhysRevB.77.125416Suche in Google Scholar
[95] Tang X, Raskin JP, Kryvutsa N, Hermans S, Slobodian O, Nazarov AN, et al. An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sens Actuators B Chem. 2020;305:1–10.10.1016/j.snb.2019.127423Suche in Google Scholar
[96] Yavari F, Chen Z, Thomas AV, Ren W, Cheng HM, Koratkar N. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network. Sci Rep. 2011;1(1):1–5.10.1038/srep00166Suche in Google Scholar PubMed PubMed Central
[97] Gerasimov G, Pogosbekian M. Graphene-based gas sensors. in Advanced environmental analysis, United Kingdom; 2016. p. 133–52.10.1039/9781782629139-00133Suche in Google Scholar
[98] Capone S, Forleo A, Francioso L, Rella R, Siciliano P, Spadavecchia J, et al. Solid state gas sensors: state of the art and future activities. J Optoelectron Adv Mater. 2003;5(5):1335–48.10.1002/chin.200429283Suche in Google Scholar
[99] Bak SY, Lee J, Kim Y, Lee SH, Woo K, Lee S, et al. Sensitivity improvement of urchin-like ZnO nanostructures using 2wo-dimensional electron gas in MgZnO/ZnO. Sensors. 2019;19(23):5195.10.3390/s19235195Suche in Google Scholar PubMed PubMed Central
[100] Tyagi D, Wang H, Huang W, Hu L, Tang Y, Guo Z, et al. Recent advances in two-dimensional-material-based sensing technology toward health and environmental monitoring applications. Nanoscale. 2020;12(6):3535–59.10.1039/C9NR10178KSuche in Google Scholar
[101] Chu BH, Lo CF, Nicolosi J, Chang CY, Chen V, Strupinski W, et al. Hydrogen detection using platinum coated graphene grown on SiC. Sens Actuators B Chem. 2011;157(2):500–3.10.1016/j.snb.2011.05.007Suche in Google Scholar
[102] Chen CW, Hung SC, Yang MD, Yeh CW, Wu CH, Chi GC, et al. Oxygen sensors made by monolayer graphene under room temperature. Appl Phys Lett. 2011;99(24):1–3.10.1063/1.3668105Suche in Google Scholar
[103] Zhang S, Zhou J, Wang Q, Chen X, Kawazoe Y, Jena P. Penta-graphene: a new carbon allotrope. Proceedings of the National Academy of Sciences; 2015.10.1073/pnas.1416591112Suche in Google Scholar PubMed PubMed Central
[104] Cheng MQ, Chen Q, Yang K, Huang WQ, Hu WY, Huang GF. Penta-graphene as a potential gas sensor for NOx detection. Nanoscale Res Lett. 2019;14(1):1–8.10.1186/s11671-019-3142-4Suche in Google Scholar PubMed PubMed Central
[105] Lee G, Yang G, Cho A, Han JW, Kim J. Defect-engineered graphene chemical sensors with ultrahigh sensitivity. Phys Chem Chem Phys. 2016;18(21):14198–204.10.1039/C5CP04422GSuche in Google Scholar
[106] Kumar S, Kaushik S, Pratap R, Raghavan S. Graphene on paper: a simple, low-cost chemical sensing platform. ACS Appl Mater Interfaces. 2015;7(4):2189–94.10.1021/am5084122Suche in Google Scholar PubMed
[107] Seekaew Y, Phokharatkul D, Wisitsoraat A, Wongchoosuk C. Highly sensitive and selective room-temperature NO2 gas sensor based on bilayer transferred chemical vapor deposited graphene. Appl Surf Sci. 2017;404:357–63.10.1016/j.apsusc.2017.01.286Suche in Google Scholar
[108] Jeong SY, Jeong S, Lee SW, Kim ST, Kim D, Jeong HJ, et al. Enhanced response and sensitivity of self-corrugated graphene sensors with anisotropic charge distribution. Sci Rep. 2015;5(1):1–9.10.1038/srep11216Suche in Google Scholar
[109] Ruffino F, Meli G, Grimaldi MG. Nanoscale electrical characteristics of metal (Au, Pd)–graphene–metal (Cu) contacts. Solid State Commun. 2016;225:1–6.10.1016/j.ssc.2015.10.010Suche in Google Scholar
[110] Khan ZU, Kausar A, Ullah H, Badshah A, Khan WU. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: properties and fabrication techniques. J Plast Film Sheeting. 2016;32(4):336–79.10.1177/8756087915614612Suche in Google Scholar
[111] Choi YR, Yoon YG, Choi KS, Kang JH, Shim YS, Kim YH, et al. Role of oxygen functional groups in graphene oxide for reversible room-temperature NO2 sensing. Carbon. 2015;91:178–87.10.1016/j.carbon.2015.04.082Suche in Google Scholar
[112] Jang HW, Shim YS, Kim YH. Heaterless operation of chemoresistive gas sensors for further functional convergence. Smart sensors for health and environment monitoring. Germany: Springer; 2015. p. 189–21210.1007/978-94-017-9981-2_8Suche in Google Scholar
[113] Peng Y, Li J. Ammonia adsorption on graphene and graphene oxide: a first-principles study. Front Env Sci Eng. 2013;7(3):403–11.10.1007/s11783-013-0491-6Suche in Google Scholar
[114] Varghese SS, Lonkar S, Singh KK, Swaminathan S, Abdala A. Recent advances in graphene based gas sensors. Sens Actuators B Chem. 2015;218:160–83.10.1016/j.snb.2015.04.062Suche in Google Scholar
[115] Mattson EC, Pande K, Unger M, Cui S, Lu G, Gajdardziska-Josifovska M, et al. Exploring adsorption and reactivity of NH3 on reduced graphene oxide. J Phys Chem C. 2013;117(20):10698–707.10.1021/jp3122853Suche in Google Scholar
[116] Alam MK, Rahman MM, Rahman MM, Kim D, Asiri AM, Khan FA. In-situ synthesis of gold nanocrystals anchored graphene oxide and its application in biosensor and chemical sensor. J Electroanal Chem. 2019;835:329–37.10.1016/j.jelechem.2019.01.023Suche in Google Scholar
[117] Yu C, Wu Y, Liu X, Fu F, Gong Y, Rao YJ, et al. Miniature fiber-optic NH3 gas sensor based on Pt nanoparticle-incorporated graphene oxide. Sens Actuators B Chem. 2017;244:107–13.10.1016/j.snb.2016.12.126Suche in Google Scholar
[118] Hunter GW, Akbar S, Bhansali S, Daniele M, Erb PD, Johnson K, et al. Editors’ choice – critical review – a critical review of solid state gas sensors. J Electrochem Soc. 2020;167(3):1–30.10.1149/1945-7111/ab729cSuche in Google Scholar
[119] Park MS, Kim KH, Kim MJ, Lee YS. NH3 gas sensing properties of a gas sensor based on fluorinated graphene oxide. Colloids Surf A Physicochem Eng Asp. 2016;490:104–9.10.1016/j.colsurfa.2015.11.028Suche in Google Scholar
[120] Prezioso S, Perrozzi F, Giancaterini L, Cantalini C, Treossi E, Palermo V, et al. Graphene oxide as a practical solution to high sensitivity gas sensing. J Phys Chem C. 2013;117(20):10683–90.10.1021/jp3085759Suche in Google Scholar
[121] Wang J, Singh B, Park JH, Rathi S, Lee IY, Maeng S, et al. Dielectrophoresis of graphene oxide nanostructures for hydrogen gas sensor at room temperature. Sens Actuators B Chem. 2014;194:296–302.10.1016/j.snb.2013.12.009Suche in Google Scholar
[122] Some S, Xu Y, Kim Y, Yoon Y, Qin H, Kulkarni A, et al. Highly sensitive and selective gas sensor using hydrophilic and hydrophobic graphenes. Sci Rep. 2013;3:1868.10.1038/srep01868Suche in Google Scholar PubMed PubMed Central
[123] Duy LT, Kim DJ, Trung TQ, Dang VQ, Kim BY, Moon HK, et al. High performance three‐dimensional chemical sensor platform using reduced graphene oxide formed on high aspect‐ratio micro‐pillars. Adv Funct Mater. 2015;25(6):883–90.10.1002/adfm.201401992Suche in Google Scholar
[124] Veluswamy P, Sathiyamoorthy S, Santhoshkumar P, Karunakaran G, Lee CW, Kuznetsov D, Kadarkaraithangam J, et al. Sono-synthesis approach of reduced graphene oxide for ammonia vapour detection at room temperature. Ultrason Sonochem. 2018;48:555–66.10.1016/j.ultsonch.2018.07.012Suche in Google Scholar PubMed
[125] Mao S, Cui S, Lu G, Yu K, Wen Z, Chen J. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J Mater Chem. 2012;22(22):11009–13.10.1039/c2jm30378gSuche in Google Scholar
[126] Panda D, Nandi A, Datta SK, Saha H, Majumdar S. Selective detection of carbon monoxide (CO) gas by reduced graphene oxide (rGO) at room temperature. RSC Adv. 2016;6(53):47337–48.10.1039/C6RA06058GSuche in Google Scholar
[127] Lee K, Yoo YK, Chae MS, Hwang KS, Lee J, Kim H, et al. Highly selective reduced graphene oxide (rGO) sensor based on a peptide aptamer receptor for detecting explosives. Sci Rep. 2019;9(1):1–9.10.1038/s41598-019-45936-zSuche in Google Scholar PubMed PubMed Central
[128] Zhang LS, Wang WD, Liang XQ, Chu WS, Song WG, Wang W, et al. Characterization of partially reduced graphene oxide as room temperature sensor for H2. Nanoscale. 2011;3(6):2458–60.10.1039/c1nr10187kSuche in Google Scholar PubMed
[129] Lipatov A, Varezhnikov A, Wilson P, Sysoev V, Kolmakov A, Sinitskii A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale. 2013;5(12):5426–34.10.1039/c3nr00747bSuche in Google Scholar PubMed
[130] Robinson JT, Perkins FK, Snow ES, Wei Z, Sheehan PE. Reduced graphene oxide molecular sensors. Nano Lett. 2008;8(10):3137–40.10.1021/nl8013007Suche in Google Scholar PubMed
[131] Li X, Zhou X, Guo H, Wang C, Liu J, Sun P, et al. Design of Au@ ZnO yolk–shell nanospheres with enhanced gas sensing properties. ACS Appl Mater Interfaces. 2014;6(21):18661–7.10.1021/am5057322Suche in Google Scholar PubMed
[132] Wang L, Lou Z, Deng J, Zhang R, Zhang T. Ethanol gas detection using a yolk–shell (core–shell) α-Fe2O3 nanospheres as sensing material. ACS Appl Mater Interfaces. 2015;7(23):13098–104.10.1021/acsami.5b03978Suche in Google Scholar PubMed
[133] Yang X, Zhang S, Zhao L, Sun P, Wang T, Liu F, et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens Actuators B Chem. 2019;281:415–23.10.1016/j.snb.2018.10.138Suche in Google Scholar
[134] Ma ZH, Yu RT, Song JM. Facile synthesis of Pr-doped In2O3 nanoparticles and their high gas sensing performance for ethanol. Sens Actuators B Chem. 2020;305:127377.10.1016/j.snb.2019.127377Suche in Google Scholar
[135] Hu L, Hu P, Chen Y, Lin Z, Qiu C. Synthesis and gas-sensing property of highly self-assembled tungsten oxide nanosheets. Front Chem. 2018;6:452.10.3389/fchem.2018.00452Suche in Google Scholar PubMed PubMed Central
[136] Sui L, Yu T, Zhao D, Cheng X, Zhang X, Wang P, et al. In situ deposited hierarchical CuO/NiO nanowall arrays film sensor with enhanced gas sensing performance to H2S. J Hazard Mater. 2020;385:121570.10.1016/j.jhazmat.2019.121570Suche in Google Scholar PubMed
[137] Feng C, Kou X, Chen B, Qian G, Sun Y, Lu G. One-pot synthesis of In doped NiO nanofibers and their gas sensing properties. Sens Actuators B Chem. 2017;253:584–91.10.1016/j.snb.2017.06.115Suche in Google Scholar
[138] Wang T, Liu B, Li Q, Wang S. Controllable construction of Cr2O3–ZnO hierarchical heterostructures and their formaldehyde gas sensing properties. Mater Lett. 2018;221:260–3.10.1016/j.matlet.2018.03.073Suche in Google Scholar
[139] Cao J, Wang S, Zhang H, Zhang T. Facile construction of Co3O4 porous microspheres with enhanced acetone gas sensing performances. Mater Sci Semicond Process. 2019;101:10–5.10.1016/j.mssp.2019.05.014Suche in Google Scholar
[140] Kim HJ, Lee JH. Highly sensitive and selective gas sensors using p-type oxide semiconductors: overview. Sens Actuators B Chem. 2014;192:607–27.10.1016/j.snb.2013.11.005Suche in Google Scholar
[141] Latif U, Dickert FL. Graphene hybrid materials in gas sensing applications. Sensors. 2015;15(12):30504–24.10.3390/s151229814Suche in Google Scholar
[142] Yi J, Lee JM, Park WI. Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive flexible gas sensors. Sens Actuators B Chem. 2011;155(1):264–9.10.1016/j.snb.2010.12.033Suche in Google Scholar
[143] Singh G, Choudhary A, Haranath D, Joshi AG, Singh N, Singh S, et al. ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon. 2012;50(2):385–94.10.1016/j.carbon.2011.08.050Suche in Google Scholar
[144] Cuong TV, Pham VH, Chung JS, Shin EW, Yoo DH, Hahn SH, et al. Solution-processed ZnO-chemically converted graphene gas sensor. Mater Lett. 2010;64(22):2479–82.10.1016/j.matlet.2010.08.027Suche in Google Scholar
[145] Deng S, Tjoa V, Fan HM, Tan HR, Sayle DC, Olivo M, et al. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J Am Chem Soc. 2012;134(10):4905–17.10.1021/ja211683mSuche in Google Scholar
[146] Yamaura H, Moriya K, Miura N, Yamazoe N. Mechanism of sensitivity promotion in CO sensor using indium oxide and cobalt oxide. Sens Actuators B Chem. 2000;65(1–3):39–41. Sens Actuators B Chem. 2001;77(1–2):330–334.10.1016/S0925-4005(99)00456-6Suche in Google Scholar
[147] Choi US, Sakai G, Shimanoe K, Yamazoe N. Sensing properties of SnO2–Co3O4 composites to CO and H2. Sens Actuators B Chem. 2004;98(2–3):166–73.10.1016/j.snb.2003.09.033Suche in Google Scholar
[148] Cao AM, Hu JS, Liang HP, Song WG, Wan LJ, He XL, et al. Hierarchically structured cobalt oxide (Co3O4): the morphology control and its potential in sensors. J Phys Chem B. 2006;110(32):15858–63.10.1021/jp0632438Suche in Google Scholar PubMed
[149] Chen N, Li X, Wang X, Yu J, Wang J, Tang Z, et al. Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens Actuators B Chem. 2013;188:902–8.10.1016/j.snb.2013.08.004Suche in Google Scholar
[150] Seo MH, Yuasa M, Kida T, Huh JS, Shimanoe K, Yamazoe N. Gas sensing characteristics and porosity control of nanostructured films composed of TiO2 nanotubes. Sens Actuators B Chem. 2009;137(2):513–20.10.1016/j.snb.2009.01.057Suche in Google Scholar
[151] Liang Y, Li Y, Wang H, Zhou J, Wang J, Regier T, et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater. 2011;10(10):780–6.10.1038/nmat3087Suche in Google Scholar PubMed
[152] Zhang B, Cheng M, Liu G, Gao Y, Zhao L, Li S, et al. Room temperature NO2 gas sensor based on porous Co3O4 slices/reduced graphene oxide hybrid. Sens Actuators B Chem. 2018;263:387–99.10.1016/j.snb.2018.02.117Suche in Google Scholar
[153] Pan J, Liu W, Quan L, Han N, Bai S, Luo R, et al. Cu2O and rGO hybridizing for enhancement of low-concentration NO2 sensing at room temperature. Ind Eng Chem Res. 2018;57(31):10086–94.10.1021/acs.iecr.8b01430Suche in Google Scholar
[154] Lassesson A, Schulze M, Van Lith J, Brown SA. Tin oxide nanocluster hydrogen and ammonia sensors. Nanotechnology. 2007;19(1):015502.10.1088/0957-4484/19/01/015502Suche in Google Scholar PubMed
[155] Chen Z, Pan D, Zhao B, Ding G, Jiao Z, Wu M, et al. Insight on fractal assessment strategies for tin dioxide thin films. ACS Nano. 2010;4(2):1202–8.10.1021/nn901635fSuche in Google Scholar PubMed
[156] Gyger F, Hübner M, Feldmann C, Barsan N, Weimar U. Nanoscale SnO2 hollow spheres and their application as a gas-sensing material. Chem Mater. 2010;22(16):4821–7.10.1021/cm1011235Suche in Google Scholar
[157] Fan G, Wang Y, Hu M, Luo Z, Li G. Synthesis of flowerlike nano-SnO2 and a study of its gas sensing response. Meas Sci Technol. 2011;22(4):045203.10.1088/0957-0233/22/4/045203Suche in Google Scholar
[158] Gurlo A. Nanosensors: towards morphological contol of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale. 2011;3(1):154–65.10.1039/C0NR00560FSuche in Google Scholar PubMed
[159] Neri G, Leonardi SG, Latino M, Donato N, Baek S, Conte DE, et al. Sensing behavior of SnO2/reduced graphene oxide nanocomposites toward NO2. Sens Actuators B Chem. 2013;179:61–8.10.1016/j.snb.2012.10.031Suche in Google Scholar
[160] Zhang H, Feng J, Fei T, Liu S, Zhang T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens Actuators B Chem. 2014;190:472–8.10.1016/j.snb.2013.08.067Suche in Google Scholar
[161] Cui S, Wen Z, Mattson EC, Mao S, Chang J, Weinert M, et al. Indium-doped SnO2 nanoparticle–graphene nanohybrids: simple one-pot synthesis and their selective detection of NO2. J Mater Chem A. 2013;1(14):4462–7.10.1039/c3ta01673kSuche in Google Scholar
[162] Su J, Guo L, Bao N, Grimes CA. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011;11(5):1928–33.10.1021/nl2000743Suche in Google Scholar PubMed
[163] Rossinyol E, Prim A, Pellicer E, Arbiol J, Hernández‐Ramírez F, Peiro F, et al. Synthesis and characterization of chromium‐doped mesoporous tungsten oxide for gas sensing applications. Adv Funct Mater. 2007;17(11):1801–6.10.1002/adfm.200600722Suche in Google Scholar
[164] Hung CM, Van Duy N, Van Quang V, Van Toan N, Van Hieu N, Hoa ND. Facile synthesis of ultrafine rGO/WO3 nanowire nanocomposites for highly sensitive toxic NH3 gas sensors. Mater Res Bull. 2020;125:110810.10.1016/j.materresbull.2020.110810Suche in Google Scholar
[165] Jiang Z, Chen W, Jin L, Cui F, Song Z, Zhu C. High performance acetylene sensor with heterostructure based on WO3 nanolamellae/reduced graphene oxide (rGO) nanosheets operating at low temperature. Nanomater. 2018;8(11):909.10.3390/nano8110909Suche in Google Scholar PubMed PubMed Central
[166] Su PG, Peng SL. Fabrication and NO2 gas-sensing properties of reduced graphene oxide/WO3 nanocomposite films. Talanta. 2015;132:398–405.10.1016/j.talanta.2014.09.034Suche in Google Scholar PubMed
[167] Punetha D, Pandey SK. Sensitivity enhancement of ammonia gas sensor based on hydrothermally synthesized rGO/WO3 nanocomposites. IEEE Sens J. 2019;20(4):1738–45.10.1109/JSEN.2019.2950781Suche in Google Scholar
[168] He JQ, Yin J, Liu D, Zhang LX, Cai FS, Bie LJ. Enhanced acetone gas-sensing performance of La2O3-doped flowerlike ZnO structure composed of nanorods. Sens Actuators B Chem. 2013;182:170–5.10.1016/j.snb.2013.02.085Suche in Google Scholar
[169] Na CW, Park SY, Lee JH. Punched ZnO nanobelt networks for highly sensitive gas sensors. Sens Actuators B Chem. 2012;174:495–9.10.1016/j.snb.2012.07.094Suche in Google Scholar
[170] Das S, Roy S, Bhattacharya TS, Sarkar CK. Efficient room temperature hydrogen gas sensor using ZnO nanoparticles-reduced graphene oxide nanohybrid. IEEE Sens J. 2020;21(2):1264–72.10.1109/JSEN.2020.3020755Suche in Google Scholar
[171] Liu J, Li S, Zhang B, Xiao Y, Gao Y, Yang Q, et al. Ultrasensitive and low detection limit of nitrogen dioxide gas sensor based on flower-like ZnO hierarchical nanostructure modified by reduced graphene oxide. Sens Actuators B Chem. 2017;249:715–24.10.1016/j.snb.2017.04.190Suche in Google Scholar
[172] Cao P, Cai Y, Pawar D, Navale ST, Rao CN, Han S, et al. Down to ppb level NO2 detection by ZnO/rGO heterojunction based chemiresistive sensors. Chem Eng J. 2020;401:125491.10.1016/j.cej.2020.125491Suche in Google Scholar
[173] Tang Z, Deng X, Zhang Y, Guo X, Yang J, Zhu C, et al. MoO3 nanoflakes coupled reduced graphene oxide with enhanced ethanol sensing performance and mechanism. Sens Actuators B Chem. 2019;297:126730.10.1016/j.snb.2019.126730Suche in Google Scholar
[174] Bai S, Chen C, Luo R, Chen A, Li D. Synthesis of MoO3/reduced graphene oxide hybrids and mechanism of enhancing H2S sensing performances. Sens Actuators B Chem. 2015;216:113–20.10.1016/j.snb.2015.04.036Suche in Google Scholar
[175] Ye Z, Tai H, Xie T, Yuan Z, Liu C, Jiang Y. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide. Sens Actuators B Chem. 2016;223:149–56.10.1016/j.snb.2015.09.102Suche in Google Scholar
[176] Li X, Zhao Y, Wang X, Wang J, Gaskov AM, Akbar SA. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens Actuators B Chem. 2016;230:330–6.10.1016/j.snb.2016.02.069Suche in Google Scholar
[177] Ye Z, Tai H, Guo R, Yuan Z, Liu C, Su Y, et al. Excellent ammonia sensing performance of gas sensor based on graphene/titanium dioxide hybrid with improved morphology. Appl Surf Sci. 2017;419:84–90.10.1016/j.apsusc.2017.03.251Suche in Google Scholar
[178] Sadasivuni KK, Ponnamma D, Kim J, Thomas S. Graphene-based polymer nanocomposites in electronics. Germany: Springer; 2015.10.1007/978-3-319-13875-6Suche in Google Scholar
[179] Kasap S, Capper P. Molecular electronics. In Springer handbook of electronic and photonic materials. Germany: Springer; 2017. p. 1257–79.10.1007/978-3-319-48933-9Suche in Google Scholar
[180] Khan AA, Khan A, Rahman MM, Asiri AM, Oves M. Sensor development of 1, 2 dichlorobenzene based on polypyrole/Cu-doped ZnO (PPY/CZO) nanocomposite embedded silver electrode and their antimicrobial studies. Int J Biol Macromol. 2017;98:256–67.10.1016/j.ijbiomac.2017.02.005Suche in Google Scholar PubMed
[181] Rahman MM, Hussein MA, Alamry KA, Al-Shehry FM, Asiri AM. Polyaniline/graphene/carbon nanotubes nanocomposites for sensing environmentally hazardous 4-aminophenol. Nano-Struct Nano-Objects. 2018;15:63–74.10.1016/j.nanoso.2017.08.006Suche in Google Scholar
[182] Husain A, Ahmad S, Mohammad F. Synthesis, characterisation and ethanol sensing application of polythiophene/graphene nanocomposite. Mater Chem Phys. 2020;239:122324.10.1016/j.matchemphys.2019.122324Suche in Google Scholar
[183] Beduk T, Bihar E, Surya SG, Castillo AN, Inal S, Salama KN. A paper-based inkjet-printed PEDOT: PSS/ZnO sol–gel hydrazine sensor. Sens Actuators B Chem. 2020;306:127539.10.1016/j.snb.2019.127539Suche in Google Scholar
[184] Shah AH. Applications of carbon nanotubes and their polymer nanocomposites for gas sensors. In Carbon nanotubes-current progress of their polymer composites. IntechOpen; 2016;459–94.10.5772/63058Suche in Google Scholar
[185] Mora A, Han F, Lubineau GL. Estimating and understanding the efficiency of nanoparticles in enhancing the conductivity of carbon nanotube/polymer composites. Results Phys. 2018;10:81–90.10.1016/j.rinp.2018.05.019Suche in Google Scholar
[186] Andre RS, Shimizu FM, Miyazaki CM, Riul Jr A, Manzani D, Ribeiro SJ, et al. Hybrid layer-by-layer (LbL) films of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sens Actuators B Chem. 2017;238:795–801.10.1016/j.snb.2016.07.099Suche in Google Scholar
[187] Huang X, Hu N, Gao R, Yu Y, Wang Y, Yang Z, et al. Reduced graphene oxide–polyaniline hybrid: preparation, characterization and its applications for ammonia gas sensing. J Mater Chem. 2012;22(42):22488–95.10.1039/c2jm34340aSuche in Google Scholar
[188] Li S, Wang T, Yang Z, He J, Wang J, Zhao L, et al. Room temperature high performance NH3 sensor based on GO-rambutan-like polyaniline hollow nanosphere hybrid assembled to flexible PET substrate. Sens Actuators B Chem. 2018;273:726–34.10.1016/j.snb.2018.06.072Suche in Google Scholar
[189] Zhang D, Wu Z, Zong X. Flexible and highly sensitive H2S gas sensor based on in situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens Actuators B Chem. 2019;289:32–41.10.1016/j.snb.2019.03.055Suche in Google Scholar
[190] Xie T, Xie G, Zhou Y, Huang J, Wu M, Jiang Y, et al. Thin film transistors gas sensors based on reduced graphene oxide poly (3-hexylthiophene) bilayer film for nitrogen dioxide detection. Chem Phys Lett. 2014;614:275–81.10.1016/j.cplett.2014.09.028Suche in Google Scholar
[191] Ye Z, Jiang Y, Tai H, Yuan Z. The investigation of reduced graphene oxide/P3HT composite films for ammonia detection. Integr Ferroelectr. 2014;154(1):73–81.10.1080/10584587.2014.904148Suche in Google Scholar
[192] Yang Y, Yang X, Yang W, Li S, Xu J, Jiang Y. Porous conducting polymer and reduced graphene oxide nanocomposites for room temperature gas detection. RSC Adv. 2014;4(80):42546–53.10.1039/C4RA06560CSuche in Google Scholar
[193] Pasha A, Khasim S, Khan FA, Dhananjaya N. Fabrication of gas sensor device using poly (3, 4-ethylenedioxythiophene)-poly (styrenesulfonate)-doped reduced graphene oxide organic thin films for detection of ammonia gas at room temperature. Iran Polym J. 2019;28(3):183–92.10.1007/s13726-019-00689-4Suche in Google Scholar
[194] Qin Y, Zhang B, Zhang Z. Combination of PPy with three-dimensional rGO to construct bioinspired nanocomposite for NH3-sensing enhancement. Org Electron. 2019;70:240–5.10.1016/j.orgel.2019.04.023Suche in Google Scholar
[195] Yu X, Zhang W, Zhang P, Su Z. Fabrication technologies and sensing applications of graphene-based composite films: advances and challenges. Biosens Bioelectron. 2017;89:72–84.10.1016/j.bios.2016.01.081Suche in Google Scholar PubMed
[196] Zhou L, Shen F, Tian X, Wang D, Zhang T, Chen W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale. 2013;5(4):1564–9.10.1039/c2nr33164kSuche in Google Scholar PubMed
[197] Hong J, Lee S, Seo J, Pyo S, Kim J, Lee T. A highly sensitive hydrogen sensor with gas selectivity using a PMMA membrane-coated Pd nanoparticle/single-layer graphene hybrid. ACS Appl Mater Interfaces. 2015;7(6):3554–61.10.1021/am5073645Suche in Google Scholar PubMed
[198] Al-Mashat L, Shin K, Kalantar-Zadeh K, Plessis JD, Han SH, Kojima RW, et al. Graphene/polyaniline nanocomposite for hydrogen sensing. J Phys Chem C. 2010;114(39):16168–73.10.1021/jp103134uSuche in Google Scholar
[199] Parmar M, Balamurugan C, Lee DW. PANI and graphene/PANI nanocomposite films – Comparative toluene gas sensing behavior. Sensors. 2013;13(12):16611–24.10.3390/s131216611Suche in Google Scholar PubMed PubMed Central
[200] Xiang C, Jiang D, Zou Y, Chu H, Qiu S, Zhang H, et al. Ammonia sensor based on polypyrrole–graphene nanocomposite decorated with titania nanoparticles. Ceram Int. 2015;41(5):6432–8.10.1016/j.ceramint.2015.01.081Suche in Google Scholar
[201] Seekaew Y, Lokavee S, Phokharatkul D, Wisitsoraat A, Kerdcharoen T, Wongchoosuk C. Low-cost and flexible printed graphene–PEDOT: PSS gas sensor for ammonia detection. Org Electron. 2014;15(11):2971–81.10.1016/j.orgel.2014.08.044Suche in Google Scholar
[202] Dehsari HS, Gavgani JN, Hasani A, Mahyari M, Shalamzari EK, Salehi A, et al. Copper(ii) phthalocyanine supported on a three-dimensional nitrogen-doped graphene/PEDOT-PSS nanocomposite as a highly selective and sensitive sensor for ammonia detection at room temperature. RSC Adv. 2015;5(97):79729–37.10.1039/C5RA13976GSuche in Google Scholar
[203] Gavgani JN, Hasani A, Nouri M, Mahyari M, Salehi A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens Actuators B Chem. 2016;229:239–48.10.1016/j.snb.2016.01.086Suche in Google Scholar
[204] Hakimi M, Salehi A, Boroumand FA, Mosleh N. Fabrication of a room temperature ammonia gas sensor based on polyaniline with N-doped graphene quantum dots. IEEE Sens J. 2018;18(6):2245–52.10.1109/JSEN.2018.2797118Suche in Google Scholar
[205] An X, Jimmy CY, Wang Y, Hu Y, Yu X, Zhang G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J Mater Chem. 2012;22(17):8525–31.10.1039/c2jm16709cSuche in Google Scholar
[206] Esfandiar A, Irajizad A, Akhavan O, Ghasemi S, Gholami MR. Pd–WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int J Hydrog Energy. 2014;39(15):8169–79.10.1016/j.ijhydene.2014.03.117Suche in Google Scholar
[207] Wu Z, Chen X, Zhu S, Zhou Z, Yao Y, Quan W, et al. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens Actuators B Chem. 2013;178:485–93.10.1016/j.snb.2013.01.014Suche in Google Scholar
[208] Tian J, Yang G, Jiang D, Su F, Zhang Z. A hybrid material consisting of bulk-reduced TiO2, graphene oxide and polyaniline for resistance based sensing of gaseous ammonia at room temperature. Microchim Acta. 2016;183(11):2871–8.10.1007/s00604-016-1912-6Suche in Google Scholar
[209] Lu G, Ocola LE, Chen J. Gas detection using low-temperature reduced graphene oxide sheets. Appl Phys Lett. 2009;94(8):083111.10.1063/1.3086896Suche in Google Scholar
[210] Fowler JD, Allen MJ, Tung VC, Yang Y, Kaner RB, Weiller BH. Practical chemical sensors from chemically derived graphene. ACS Nano. 2009;3(2):301–6.10.1021/nn800593mSuche in Google Scholar PubMed
[211] Liu X, Cui J, Sun J, Zhang X. 3D graphene aerogel-supported SnO2 nanoparticles for efficient detection of NO2. RSC Adv. 2014;4(43):22601–5.10.1039/c4ra02453bSuche in Google Scholar
[212] Xiao Y, Yang Q, Wang Z, Zhang R, Gao Y, Sun P, et al. Improvement of NO2 gas sensing performance based on discoid tin oxide modified by reduced graphene oxide. Sens Actuators B. 2016;227:419–26.10.1016/j.snb.2015.11.051Suche in Google Scholar
[213] Srivastava S, Jain K, Singh VN, Singh S, Vijayan N, Dilawar N, et al. Faster response of NO2 sensing in graphene–WO3 nanocomposites. Nanotechnology. 2012;23(20):205501.10.1088/0957-4484/23/20/205501Suche in Google Scholar PubMed
[214] Jie X, Zeng D, Zhang J, Xu K, Wu J, Zhu B, et al. Graphene-wrapped WO3 nanospheres with room-temperature NO2 sensing induced by interface charge transfer. Sens Actuators B Chem. 2015;220:201–9.10.1016/j.snb.2015.05.047Suche in Google Scholar
[215] Liu X, Li J, Sun J, Zhang X. 3D Fe3O4 nanoparticle/graphene aerogel for NO2 sensing at room temperature. RSC Adv. 2015;5(90):73699–704.10.1039/C5RA14857JSuche in Google Scholar
[216] Liu X, Sun J, Zhang X. Novel 3D graphene aerogel–ZnO composites as efficient detection for NO2 at room temperature. Sens Actuators B Chem. 2015;211:220–6.10.1016/j.snb.2015.01.083Suche in Google Scholar
[217] Abideen ZU, Katoch A, Kim JH, Kwon YJ, Kim HW, Kim SS. Excellent gas detection of ZnO nanofibers by loading with reduced graphene oxide nanosheets. Sens Actuators B Chem. 2015;221:1499–507.10.1016/j.snb.2015.07.120Suche in Google Scholar
[218] Huang XL, Hu NT, Wang YY, Zhang YF. Ammonia gas sensor based on aniline reduced graphene oxide. In Advanced materials research. Trans Tech Publications Ltd; 2013;669:79–84.10.4028/www.scientific.net/AMR.669.79Suche in Google Scholar
[219] Bai S, Zhao Y, Sun J, Tian Y, Luo R, Li D, et al. Ultrasensitive room temperature NH3 sensor based on a graphene–polyaniline hybrid loaded on PET thin film. Chem Comm. 2015;51(35):7524–7.10.1039/C5CC01241DSuche in Google Scholar PubMed
[220] Ye Z, Jiang Y, Tai H, Guo N, Xie G, Yuan Z. The investigation of reduced graphene oxide@ SnO2–polyaniline composite thin films for ammonia detection at room temperature. J Mater Sci Mater Electron. 2015;26(2):833–41.10.1007/s10854-014-2472-3Suche in Google Scholar
[221] Lin Q, Li Y, Yang M. Tin oxide/graphene composite fabricated via a hydrothermal method for gas sensors working at room temperature. Sens Actuators B Chem. 2012;173:139–47.10.1016/j.snb.2012.06.055Suche in Google Scholar
[222] Zhou Y, Jiang Y, Xie G, Wu M, Tai H. Gas sensors for CO2 detection based on RGO–PEI films at room temperature. Chin Sci Bull. 2014;59(17):1999–2005.10.1007/s11434-014-0253-2Suche in Google Scholar
[223] Naikoo RA, Tomar R. Fabrication of a novel Zeolite-X/Reduced graphene oxide/Polypyrrole nanocomposite and its role in sensitive detection of CO. Mater Chem Phys. 2018;211:225–33.10.1016/j.matchemphys.2018.02.021Suche in Google Scholar
[224] Mishra SK, Tripathi SN, Choudhary V, Gupta BD. Surface plasmon resonance-based fiber optic methane gas sensor utilizing graphene-carbon nanotubes-poly(methyl methacrylate) hybrid nanocomposite. Plasmonics. 2015;10(5):1147–57.10.1007/s11468-015-9914-5Suche in Google Scholar
© 2021 Norizan M. Nurazzi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

![Figure 1
Two layers of graphene (right) that show a superconducting properties when it twisted at angle 1.1°. Adapted from ref. [21,22].](/document/doi/10.1515/ntrev-2021-0030/asset/graphic/j_ntrev-2021-0030_fig_001.jpg)
