Abstract
C16H16N2O2, orthorhombic, Pbca (no. 61), a = 12.5980(5) Å, b = 12.8535(4) Å, c = 17.5963(7) Å, V = 2849.34(18) Å3, Z = 8, Rgt(F) = 0.0464, wRref(F2) = 0.1396, T = 298 K.
1 Source of materials
The synthesis of (Z)-2-hydroxy–N′-(1-(o-tolyl)ethylidene)benzohydrazide single crystals was performed by reacting 1 mmol of salicyl hydrazide with 1 mmol of 2′-methylacetophenone in 20 mL of ethanol as the solvent. To catalyze the reaction, 3 drops of acetic acid were added to the mixture. The reaction was carried out under reflux at 50 °C for 3 h, during which the condensation reaction occurred, yielding the crude product. Upon completion, the crude product (0.05 g) was filtered and then dissolved in a small amount of hot ethanol to facilitate crystallization. The resulting solution was allowed to cool slowly to room temperature, and the container was left open to allow gradual evaporation of the solvent. Over time, single crystals of the title compound formed (Table 1).
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.30 × 0.10 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.68 mm−1 |
| Diffractometer, scan mode: | Bruker D8, ω scans |
| θmax, completeness: | 68.3°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 29030, 2608, 0.087 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2,136 |
| N(param)refined: | 227 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
2 Experimental details
Single-crystal X-ray diffraction (XRD) analysis was performed using a Bruker D8 Venture diffractometer with CuKα radiation at room temperature. 1 The initial structure solution was obtained using ShelXT, 2 followed by refinement using ShelXL 3 within the Olex2 software 4 environment. Hydrogen atoms were positioned in geometrically idealized positions and were refined as riding atoms.
3 Comment
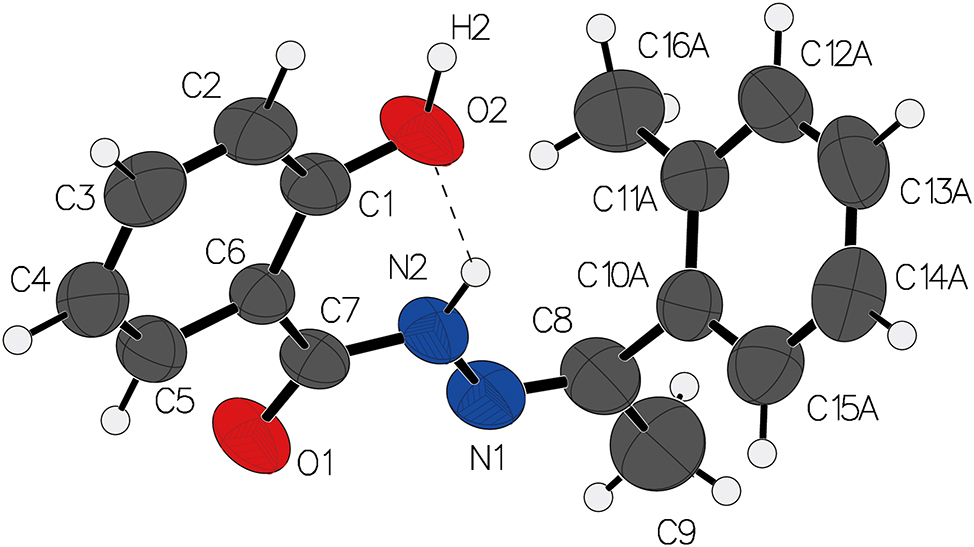
Benzohydrazide derivatives, particularly those with functional substituents such as hydroxyl or aromatic groups, exhibit intriguing properties, including enhanced solubility, structural versatility, and potential biological activity. 5 The study of benzohydrazide single crystals has significant implications in the fields of materials science, drug development, and coordination chemistry. 6 , 7 , 8 , 9 , 10 Additionally, the synthesis of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide single crystals in this article provides valuable insights into the stability and reactivity of this class of compounds, paving the way for further exploration in advanced material design and therapeutic applications.
The central hydrazone group (C=N–N) of the title compound adopts a Z-configuration The C=N bond length is found to be 1.274 Å, consistent with the expected double bond character, while the N–N bond is slightly longer, reflecting partial single bond character. The C=O bond of the hydrazide group is 1.228 Å, further emphasizing the electron-withdrawing nature of the carbonyl group in the hydrazide moiety. 11 , 12 , 13 , 14 , 15 , 16 The hydroxyl group at position 2 of the benzene ring plays a crucial role in the molecular conformation, forming an intramolecular hydrogen bond with the hydrazide nitrogen (N–H⋯O) at a distance of 2.004 Å.
The ethylidene group attached to the hydrazone nitrogen, derived from o-tolyl, displays a slightly distorted conformation relative to the benzene ring, with a dihedral angle of 55° between the C–C bond of the ethylidene and the plane of the aromatic ring. The distance between the centers of the benzene rings in the crystal is 3.85 Å, suggesting weak π–π interactions that contribute to the overall packing efficiency. 17 , 18 , 19
In the crystal lattice, molecules are arranged in layers, with the primary intermolecular forces being hydrogen bonds (O2–H2⋯O1) between the hydroxyl group of one molecule and the hydrazide oxygen atom of a neighboring molecule.
Acknowledgments
This work was financially supported by the 2024 Key Scientific Research Program Projects of the Shaanxi Provincial Department of Education (Key Laboratory Projects, 24JS004), the 2023 research and development project of the Xianyang Science and Technology Bureau (L2023-ZDYF-SF-030), Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city (2021QXNL-PT-0008), School-level Scientific and Technological Innovation Team for Design, Synthesis and Structural Modification of Drug Molecules (2024KCTD04).
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr., Section A: Found. Adv. 2015, 71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr., Section C: Struct. Chem. 2015, 71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Korkmaz, I. N.; Türkeş, C.; Demir, Y.; Öztekin, A.; Özdemir, H.; Beydemir, Ş. Biological Evaluation and In Silico Study of Benzohydrazide Derivatives as Paraoxonase 1 Inhibitors. J. Biochem. Mol. Toxicol. 2022, 36, e23180; https://doi.org/10.1002/jbt.23180.Search in Google Scholar PubMed
6. Bai, Z.-C.; Li, H.; Liu, Y.; Jing, Z.-L. N′-(2,4-dichlorobenzylidene)-2-methoxybenzohydrazide. Acta Crystallogr. Section E 2006, 62, o2295–o2296, https://doi.org/10.1107/s1600536806017053.Search in Google Scholar
7. Fun, H.-K.; Horkaew, J.; Chantrapromma, S. (E)-4-hydroxy-N′-(3-hydroxy-4-methoxybenzylidene)benzohydrazide. Acta Crystallogr. Section E 2011, 67, o2644–o2645, https://doi.org/10.1107/s1600536811036579.Search in Google Scholar PubMed PubMed Central
8. Yang, J.-G.; Pan, F.-Y. 2′-(2-fluorobenzylidene)-2-hydroxybenzohydrazide. Acta Crystallogr. Section E 2004, 60, o2009–o2010, https://doi.org/10.1107/s1600536804024614.Search in Google Scholar
9. Bai, B.; Zhang, M.; Ji, N.; Wei, J.; Wang, H.; Li, M. E–Z Isomerization of the –C=N– Bond in Anthracene-Based Acylhydrazone Derivatives under Visible Light. Chem. Commun. 2017, 53, 2693–2696; https://doi.org/10.1039/c6cc08403f.Search in Google Scholar PubMed
10. Yu, H.; Ren, W.; Lu, H.; Liang, Y.; Wang, Q. Synthesis and Piezochromic Luminescence Study of a Coumarin Hydrozone Compound. Chem. Commun. 2016, 52, 7387–7389; https://doi.org/10.1039/c6cc02937j.Search in Google Scholar PubMed
11. Alifu, Z.; Nizhamu, M.; Ablajan, K. Efficient Synthesis of N′-benzylidene-2-hydroxymethylbenzohydrazides from the One-Pot Reaction of Phthalide, Hydrazine and Aldehydes. Res. Chem. Intermed. 2019, 45, 4779–4788, https://doi.org/10.1007/s11164-019-03863-8.Search in Google Scholar
12. Ferraresi-Curotto, V.; Echeverría, G. A.; Piro, O. E.; Pis-Diez, R.; González-Baró, A. C. Structural, Spectroscopic and DFT Study of 4-methoxybenzohydrazide Schiff Bases. A New Series of Polyfunctional Ligands. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 137, 692–700; https://doi.org/10.1016/j.saa.2014.08.095.Search in Google Scholar PubMed
13. Al-Wahaibi, L. H.; Blacque, O.; Tiekink, E. R. T.; El-Emam, A. A. Crystal Structure of 4-Chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3. Z. Kristallogr. - New Cryst. Struct. 2023, 238, 631–633, https://doi.org/10.1515/ncrs-2023-0125.Search in Google Scholar
14. Qiao, A.-L.; Guo, Z.-F. The Crystal Structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O. Z. Kristallogr. - New Cryst. Struct. 2023, 238, 639–640, https://doi.org/10.1515/ncrs-2023-0127.Search in Google Scholar
15. Wen, J.; Liu, L.; Tang, W.; Liu, B. The Crystal Structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2. Z. Kristallogra. - New Cryst. Struct. 2023, 238, 1205–1207, https://doi.org/10.1515/ncrs-2023-0404.Search in Google Scholar
16. Zhu, N.; Zhao, J.; Zhang, L. Crystal Structure of E-2-Chloro-N′-(1-(5-Chloro-2-hydroxyphenyl)propylidene)benzohydrazide, C16H14Cl2N2O2. Z. Kristallogr. - New Cryst. Struct. 2022, 237, 1065–1066, https://doi.org/10.1515/ncrs-2022-0413.Search in Google Scholar
17. Xue, L.-W.; Han, Y.-J.; Zhao, G.-Q.; Feng, Y.-X. Synthesis, Characterization and Crystal Structures of N′-(5-bromo-2-hydroxybenzylidene)-2-fluorobenzohydrazide in Unsolvated and Monohydrate Forms. J. Chem. Crystallogr. 2011, 41, 1599–1603, https://doi.org/10.1007/s10870-011-0146-z.Search in Google Scholar
18. Zhu, H.-Y. Synthesis, Characterization, and Crystal Structures of 3-bromo-N′-(2-methoxybenzylidene)benzohydrazide and N′-(2-methoxybenzylidene)-3,4-methylenedioxybenzohydrazide. J. Chem. Crystallogr. 2011, 41, 1785–1789, https://doi.org/10.1007/s10870-011-9982-0.Search in Google Scholar
19. Mishra, M.; Tiwari, K.; Mourya, P.; Singh, M. M.; Singh, V. P. Synthesis, Characterization and Corrosion Inhibition Property of Nickel(II) and Copper(II) Complexes with Some Acylhydrazine Schiff Bases. Polyhedron 2015, 89, 29; https://doi.org/10.1016/j.poly.2015.01.003.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O


