Abstract
C21H16ClFeNO, monoclinic, P21/c (no. 14), a = 14.6936(11) Å, b = 9.3784(6) Å, c = 12.7854(8) Å, β = 105.183(2)°, V = 1700.4(2) Å3, Z = 4, R gt(F) = 0.0584 wR ref(F 2) = 0.1719, T = 170 K.
1 Source of materials
To a solution of 1–ferrocenyl-3-(4-chlorophenyl)-2-propen-1-one (3.50 g, 10 mmol) and tosylmethyl isocyanide (2.15 g, 11 mmol) in N,N-dimethylformamide (25 mL) was added potassium tert-butoxide (2.24 g, 20 mmol). The mixture was stirred at room temperature for 12 h, until the TLC indicated the reaction was completed. The mixture was diluted with brine, and then extracted with ethyl acetate (3 × 30 mL). The organic phase was washed with brine (30 mL), dried with anhydrous sodium sulphate, and then concentrated under pressure. The title compound was separated by silica-gel column chromatography with ethyl acetate-petroleum ether (25 %) gradient solvent system. The target product was obtained as a white solid. Yield: 68.5 %. For crystal growth, the product was dissolved in a minimal amount of hot ethanol and slowly cooled to room temperature (Tables 1 and 2).
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.11 × 0.09 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.05 mm−1 |
| Diffractometer, scan mode: | Bruker D8 VENTURE, φ and ω scans |
| θ max, completeness: | 26.6°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 12549, 3530, 0.068 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2,456 |
| N(param)refined: | 226 |
| Programs: | Bruker, 1 SHELX 2 , 3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | U iso*/U eq | |
|---|---|---|---|---|
| Fe1 | 0.12689 (4) | 0.40872 (7) | 0.37890 (5) | 0.0275 (2) |
| Cl1 | 0.77898 (10) | 0.45575 (17) | 0.95474 (11) | 0.0551 (4) |
| O1 | 0.3348 (2) | 0.3562 (4) | 0.6660 (2) | 0.0292 (7) |
| N1 | 0.4420 (2) | 0.1944 (4) | 0.3871 (3) | 0.0288 (9) |
| H1 | 0.443510 | 0.153855 | 0.325523 | 0.035* |
| C1 | 0.0440 (4) | 0.2318 (6) | 0.3689 (5) | 0.0493 (15) |
| H1A | 0.026590 | 0.186904 | 0.427362 | 0.059* |
| C2 | 0.1244 (3) | 0.1991 (5) | 0.3319 (4) | 0.0372 (11) |
| H2 | 0.170527 | 0.128743 | 0.361498 | 0.045* |
| C3 | 0.1241 (3) | 0.2890 (5) | 0.2436 (4) | 0.0371 (11) |
| H3 | 0.169694 | 0.289950 | 0.202884 | 0.045* |
| C4 | 0.0438 (4) | 0.3776 (6) | 0.2263 (4) | 0.0480 (15) |
| H4 | 0.026161 | 0.448650 | 0.171817 | 0.058* |
| C5 | −0.0059 (3) | 0.3429 (7) | 0.3036 (5) | 0.0555 (17) |
| H5 | −0.062492 | 0.386282 | 0.310524 | 0.067* |
| C6 | 0.1058 (3) | 0.5863 (5) | 0.4622 (4) | 0.0297 (10) |
| H6 | 0.046317 | 0.624788 | 0.463732 | 0.036* |
| C7 | 0.1580 (3) | 0.4837 (5) | 0.5352 (3) | 0.0279 (10) |
| H7 | 0.139987 | 0.441860 | 0.594392 | 0.034* |
| C8 | 0.2431 (3) | 0.4540 (5) | 0.5036 (3) | 0.0227 (9) |
| C9 | 0.2417 (3) | 0.5418 (5) | 0.4113 (3) | 0.0264 (9) |
| H9 | 0.289314 | 0.545450 | 0.373403 | 0.032* |
| C10 | 0.1577 (3) | 0.6215 (5) | 0.3865 (4) | 0.0324 (11) |
| H10 | 0.138905 | 0.687842 | 0.328685 | 0.039* |
| C11 | 0.3238 (3) | 0.3695 (5) | 0.5673 (3) | 0.0242 (9) |
| C12 | 0.3902 (3) | 0.3077 (5) | 0.5121 (3) | 0.0232 (9) |
| C13 | 0.3666 (3) | 0.2609 (5) | 0.4068 (3) | 0.0274 (10) |
| H13 | 0.307077 | 0.273153 | 0.356037 | 0.033* |
| C14 | 0.5156 (3) | 0.2001 (5) | 0.4781 (3) | 0.0280 (10) |
| H14 | 0.576816 | 0.162377 | 0.484632 | 0.034* |
| C15 | 0.4868 (3) | 0.2692 (5) | 0.5584 (3) | 0.0231 (9) |
| C16 | 0.5523 (3) | 0.3104 (5) | 0.6626 (3) | 0.0224 (9) |
| C17 | 0.5507 (3) | 0.4485 (5) | 0.7038 (3) | 0.0262 (9) |
| H17 | 0.502167 | 0.512696 | 0.669080 | 0.031* |
| C18 | 0.6193 (3) | 0.4925 (5) | 0.7946 (3) | 0.0289 (10) |
| H18 | 0.617676 | 0.586067 | 0.822470 | 0.035* |
| C19 | 0.6896 (3) | 0.3990 (5) | 0.8438 (4) | 0.0325 (11) |
| C20 | 0.6919 (3) | 0.2610 (5) | 0.8077 (4) | 0.0324 (11) |
| H20 | 0.740068 | 0.197122 | 0.843962 | 0.039* |
| C21 | 0.6222 (3) | 0.2166 (5) | 0.7170 (4) | 0.0300 (10) |
| H21 | 0.622439 | 0.121277 | 0.691958 | 0.036* |
2 Experimental details
The crystal structure was solved via Direct Methods using the SHELXT program 2 and refined through full-matrix least squares on F 2 using SHELXL. 3 Non-hydrogen atoms were refined anisotropically, while hydrogen atoms were placed in geometrically idealized positions and refined using a riding model.
3 Comment
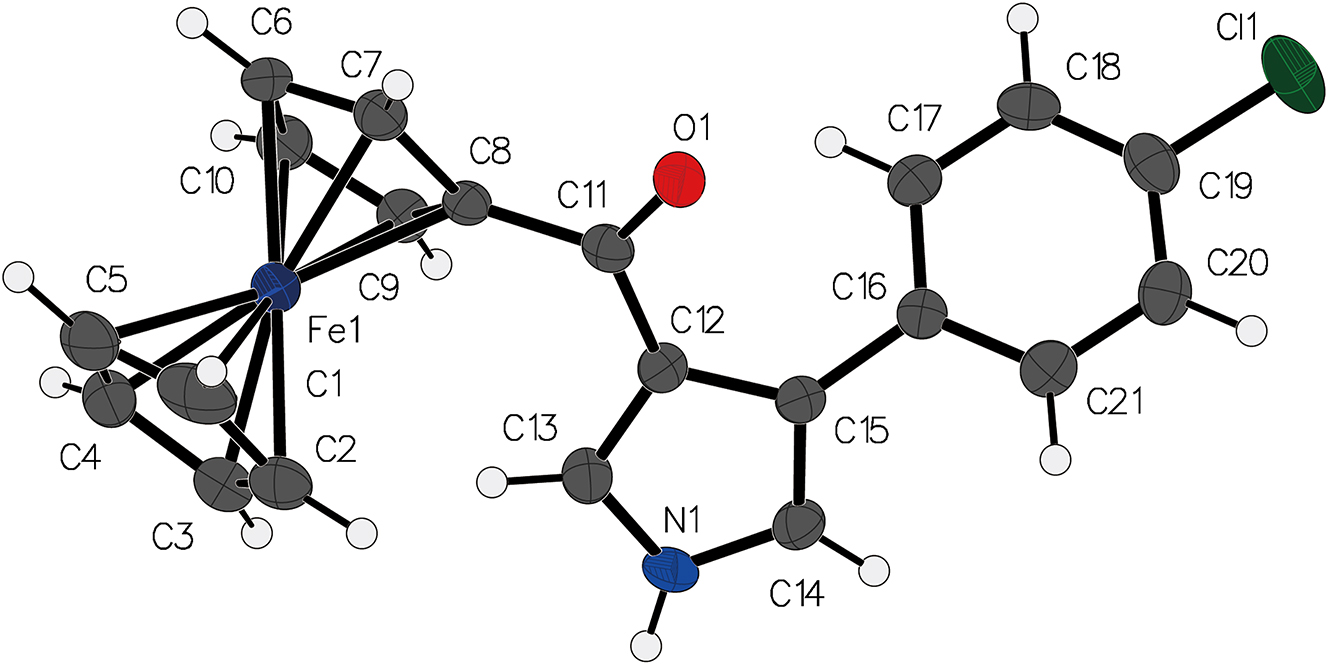
Ferrocene, known for its robust and well-defined sandwich structure, serves as an ideal scaffold for incorporating diverse functional moieties, 4 The single-crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, with the incorporation of a pyrrole-3-carbonyl group, along with a 4-chlorophenyl substituent, introduces additional versatility to the molecule, potentially enhancing its applications in fields. 5 , 6 , 7 , 8 , 9 , 10
In the crystal of the title compound, the iron center of ferrocene is coordinated to two cyclopentadienyl rings, which adopt a parallel alignment, a characteristic feature of the ferrocene structure. 11 , 12 , 13 , 14 The functionalized pyrrole-3-carbonyl group is attached to the ferrocene scaffold through the carbonyl carbon atom (C11), which interacts with the C12 atom and connects the two ring systems. The bond length of C11–C8 is 1.480(6) Å, and the bond length of C11–C12 is 1.465(7) Å. The 4-chlorophenyl group, represented by atoms C16 to C21 in the structure, is positioned at the para position relative to the pyrrole ring, as indicated by the spatial arrangement in the crystal. The chlorine atom (Cl1) is attached to carbon atom C19, and the bond length of Cl1–C19 is 1.745(5) Å.
The dihedral angle between the two cyclopentadienyl rings is measured to be 5.2°, reflecting a nearly parallel alignment, which is in agreement with the structural features observed in other ferrocenyl compounds. 15 , 16 , 17 , 18 Furthermore, the dihedral angle between the chlorophenyl and pyrrole rings is determined to be 42.3°, which is slightly smaller than the 51° angle reported for the analogous compound 4-(2-chlorophenyl)-1H-pyrrol-3-yl (ferrocenyl)methanone. 19
Acknowledgments
This work was financially supported by the projects of Social Development in Shaanxi Province Science and Technology Department (2023–YBSF-036), the 2023 research and development project of the Xianyang Science and Technology Bureau (L2023–ZDYF–SF-030), Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city (2021QXNL–PT-0008), School-level Scientific and Technological Innovation Team for Design, Synthesis and Structural Modification of Drug Molecules (2024KCTD04).
References
1. Bruker. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Zábranský, M.; Císařová, I.; Štěpnička, P. Ferrocenylmethylation Reactions with a Phosphinoferrocene Betaine. Dalton Trans. 2015, 44, 14494–14506; https://doi.org/10.1039/c5dt01877c.Search in Google Scholar PubMed
5. Deck, P. A.; Kroll, C. E.; Gary Hollis, W.; Fronczek, F. R. Conformational Control of Intramolecular Arene Stacking in Ferrocene Complexes Bearing Tert-Butyl and Pentafluorophenyl Substituents. J. Organomet. Chem. 2001, 637–639, 107–115; https://doi.org/10.1016/s0022-328x(01)00878-6.Search in Google Scholar
6. Wang, X.-L.; Li, H.-M. Crystal Structure of 4-(1H-Imidazol-1-yl)-6-pyrimidinylferrocene, C17H14FeN4. Z. Kristallogr. New Cryst. Struct. 2016, 231, 141–143; https://doi.org/10.1515/ncrs-2015-0062.Search in Google Scholar
7. Chamkin, A. A.; Krivykh, V. V.; Nikitin, O. M.; Kreindlin, A. Z.; Shteltser, N. A.; Dolgushin, F. M.; Artyushin, O. I.; Ikonnikov, N. S.; Borisov, Y. A.; Belousov, Y. A.; Ustynyuk, N. A. Direct Phosphination of Ferrocenium Ion with Tertiary Phosphines by the Mechanism of Oxidative Nucleophilic Substitution. Eur. J. Inorg. Chem. 2018, 2018, 4494–4504; https://doi.org/10.1002/ejic.201800961.Search in Google Scholar
8. Shameem, M. A.; Esfandiarfard, K.; Öberg, E.; Ott, S.; Orthaber, A. Direct, Sequential, and Stereoselective Alkynylation of C,C-Dibromophosphaalkenes. Chem. – Eur. J. 2016, 22, 10614–10619; https://doi.org/10.1002/chem.201601955.Search in Google Scholar PubMed
9. Champaka, G.; Senthilkumar, K.; David, E.; Shanmugan, S.; Palanisami, N. Monomeric Zinc Ferrocene Carboxylate [Zn(FcCOO)(3,5-dmp)2Cl] Derived from 3,5-dimethylpyrazole: Structural, Optical, Electrochemical and Antimicrobial Studies. J. Mol. Struct. 2021, 1228, 129749; https://doi.org/10.1016/j.molstruc.2020.129749.Search in Google Scholar
10. Liu, X. F.; Li, R. F.; Fu, X.; Shen, H.; wen, M.; Feng, X. Syntheses, Characterizations, and Reactivity of Two Cu(I)–Amido Complexes: Proposed Intermediate in Cu(I)–Catalyzed Goldberg Reaction. Russ. J. Coord. Chem. 2018, 44, 353–358; https://doi.org/10.1134/s1070328418050044.Search in Google Scholar
11. Peng, Y.-D.; Zhou, L.-S.; Chen, L.-L.; Ma, L.; Zhao, Y.; Zhang, W.-W.; Zuo, J.-L. Ferrocene-isocoumarin Conjugated Molecules: Synthesis, Structural Characterization, Electronic Properties, and DFT–TDDFT Computational Study. Dalton Trans. 2015, 44, 14465–14474; https://doi.org/10.1039/c5dt02169c.Search in Google Scholar PubMed
12. Chen, Z.-F.; Zou, H.-L.; Liang, H.; Yuan, R.-X.; Zhang, Y. Ferrocenecarbaldehyde Isonicotinyl Hydrazide. Appl. Organomet. Chem. 2004, 18, 438–439; https://doi.org/10.1002/aoc.691.Search in Google Scholar
13. Panaka, S.; Trivedi, R.; Jaipal, K.; Giribabu, L.; Sujitha, P.; Kumar, C. G.; Sridhar, B. Ferrocenyl Chalcogeno (Sugar) Triazole Conjugates: Synthesis, Characterization and Anticancer Properties. J. Organomet. Chem. 2016, 813, 125–130; https://doi.org/10.1016/j.jorganchem.2016.04.011.Search in Google Scholar
14. Shafir, A.; Fiedler, D.; Arnold, J. Formation of 1 : 1 Complexes of Ferrocene-Containing Salen Ligands with Mg, Ti and Zr. J. Chem. Soc., Dalton Trans. 2002, 555–560; https://doi.org/10.1039/b107066p.Search in Google Scholar
15. Kang, T.; Kim, N.; Cheng, P. T.; Zhang, H.; Foo, K.; Engle, K. M. Nickel-Catalyzed 1,2-Carboamination of Alkenyl Alcohols. J. Am. Chem. Soc. 2021, 143, 13962–13970; https://doi.org/10.1021/jacs.1c07112.Search in Google Scholar PubMed
16. Höcher, T.; Blaurock, S.; Hey–Hawkins, E. Novel Ferrocene Derivatives with PH–Functionalized Phosphanylalkylcyclopentadienyl Ligands: Syntheses and Molecular Structures of rac-[Fe{(η5–C5H4)CMe2PHR}2] (R = Ph, Mes) and Rac-[Fe{(η5–C5H4)CMe2PHR(Cp*TaCl4)}2]. Eur. J. Inorg. Chem. 2002, 2002, 1174–1180; https://doi.org/10.1002/1099-0682(200207)2002:7<1883::aid-ejic1883>3.0.co;2-1.10.1002/1099-0682(200205)2002:5<1174::AID-EJIC1174>3.0.CO;2-ASearch in Google Scholar
17. Erb, W.; Wen, M.; Pierre Hurvois, J.; Mongin, F.; Halauko, Y. S.; Ivashkevich, O. A.; Matulis, V. E.; Roisnel, T. O-Isopropylferrocenesulfonate: Synthesis of Polysubstituted Derivatives and Electrochemical Study. Eur. J. Inorg. Chem. 2021, 2021, 3165–3176; https://doi.org/10.1002/ejic.202100448.Search in Google Scholar
18. Ramírez-Gómez, A.; Gutiérrez-Hernández, A. I.; Alvarado-Castillo, M. A.; Toscano, R. A.; Ortega-Alfaro, M. C.; López-Cortés, J. G. Selenoamides as Powerful Scaffold to Build Imidazo[1,5-a]pyridines Using a Grinding Protocol. J. Organomet. Chem. 2020, 919, 121315; https://doi.org/10.1016/j.jorganchem.2020.121315.Search in Google Scholar
19. Zhang, C.; Zhu, Z.; Tang, W.; Xu, X.; Liu, B. Crystal Structure of (4-(2-Chlorophenyl)-1H-Pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO. Z. Kristallogr. New Cryst. Struct. 2024, 239, 375–377; https://doi.org/10.1515/ncrs-2024-0007.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

