Abstract
C20H18F2N2O, monoclinic, P21/n (no. 14), a = 10.7795(8) Å, b = 12.7526(7) Å, c = 13.4631(10) Å, β = 111.105(8)°, V = 1726.6(2) Å3, Z = 4, Rgt(F) = 0.0737 wR ref (F2) = 0.1862, T = 293 K.
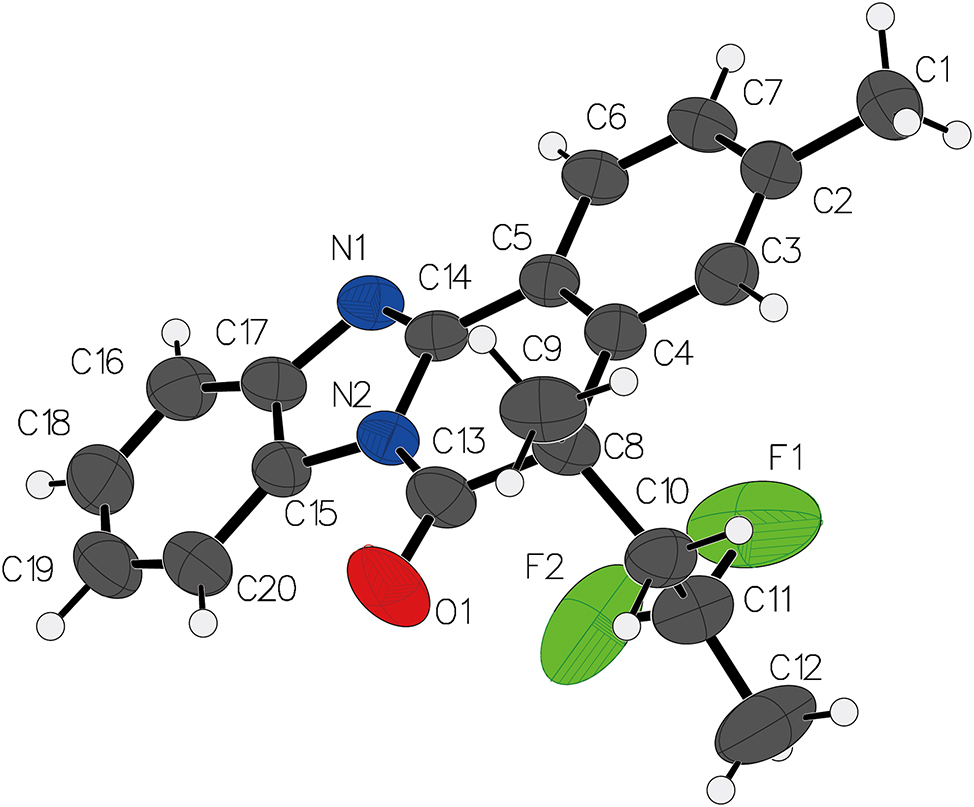
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | colourless plate |
| Size: | 0.21 × 0.15 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Rigaku, φ and ω scans |
| θmax, completeness: | 29.2°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 19689, 4225, 0.046 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2,300 |
| N(param)refined: | 229 |
| Programs: | Rigaku, 1 SHELX, 2 , 3 Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| F1 | 0.8538 (3) | 0.5073 (3) | 0.4103 (2) | 0.1526 (13) |
| F2 | 0.6637 (3) | 0.47467 (19) | 0.40747 (19) | 0.1385 (12) |
| O1 | 0.4223 (2) | 0.59949 (18) | 0.2647 (2) | 0.0874 (8) |
| N1 | 0.5302 (2) | 0.34805 (16) | 0.07715 (16) | 0.0433 (5) |
| N2 | 0.48549 (19) | 0.47779 (16) | 0.17250 (16) | 0.0408 (5) |
| C1 | 1.0462 (3) | 0.6615 (3) | 0.1559 (3) | 0.0680 (9) |
| H1A | 1.122723 | 0.634990 | 0.212416 | 0.102* |
| H1B | 1.034022 | 0.734216 | 0.168542 | 0.102* |
| H1C | 1.059254 | 0.654320 | 0.089351 | 0.102* |
| C2 | 0.9243 (3) | 0.6002 (2) | 0.1518 (2) | 0.0478 (7) |
| C3 | 0.8402 (3) | 0.6374 (2) | 0.2007 (2) | 0.0480 (7) |
| H3 | 0.862013 | 0.699483 | 0.239444 | 0.058* |
| C4 | 0.7240 (2) | 0.58549 (19) | 0.1941 (2) | 0.0415 (6) |
| C5 | 0.6921 (2) | 0.49260 (18) | 0.13524 (19) | 0.0371 (6) |
| C6 | 0.7771 (2) | 0.4543 (2) | 0.0862 (2) | 0.0431 (6) |
| H6 | 0.756346 | 0.392336 | 0.047263 | 0.052* |
| C7 | 0.8905 (3) | 0.5074 (2) | 0.0951 (2) | 0.0489 (7) |
| H7 | 0.946381 | 0.480617 | 0.062293 | 0.059* |
| C8 | 0.6322 (3) | 0.6320 (2) | 0.2466 (2) | 0.0447 (6) |
| C9 | 0.5858 (3) | 0.7412 (2) | 0.1969 (3) | 0.0626 (9) |
| H9A | 0.661671 | 0.785936 | 0.210115 | 0.094* |
| H9B | 0.526910 | 0.771410 | 0.228155 | 0.094* |
| H9C | 0.539999 | 0.733898 | 0.121472 | 0.094* |
| C10 | 0.7026 (3) | 0.6475 (2) | 0.3680 (2) | 0.0537 (7) |
| H10A | 0.777002 | 0.694756 | 0.379163 | 0.064* |
| H10B | 0.640900 | 0.682559 | 0.394507 | 0.064* |
| C11 | 0.7540 (4) | 0.5517 (3) | 0.4351 (3) | 0.0701 (9) |
| C12 | 0.8018 (5) | 0.5663 (4) | 0.5514 (3) | 0.1075 (15) |
| H12A | 0.730731 | 0.592566 | 0.571384 | 0.161* |
| H12B | 0.874097 | 0.615470 | 0.572469 | 0.161* |
| H12C | 0.831870 | 0.500290 | 0.585997 | 0.161* |
| C13 | 0.5054 (3) | 0.5700 (2) | 0.2303 (2) | 0.0515 (7) |
| C14 | 0.5721 (2) | 0.43685 (18) | 0.12580 (19) | 0.0370 (6) |
| C15 | 0.3805 (2) | 0.40557 (19) | 0.15053 (19) | 0.0412 (6) |
| C16 | 0.4097 (3) | 0.3267 (2) | 0.0917 (2) | 0.0439 (6) |
| C17 | 0.3253 (3) | 0.2417 (2) | 0.0561 (2) | 0.0565 (8) |
| H17 | 0.342928 | 0.189312 | 0.015026 | 0.068* |
| C18 | 0.2141 (3) | 0.2377 (3) | 0.0840 (3) | 0.0625 (8) |
| H18 | 0.156308 | 0.180970 | 0.062184 | 0.075* |
| C19 | 0.1871 (3) | 0.3167 (3) | 0.1439 (3) | 0.0631 (9) |
| H19 | 0.111314 | 0.311455 | 0.161304 | 0.076* |
| C20 | 0.2686 (3) | 0.4029 (2) | 0.1787 (2) | 0.0544 (7) |
| H20 | 0.249782 | 0.455933 | 0.218544 | 0.065* |
1 Source of materials
The target compound was synthesized via an electrochemical method under the following typical conditions: A 20 mL test tube equipped with a stirring bar was charged with 2-methyl-1-(2-phenyl-1H-benzo[d]imidazol-1-yl)prop-2-en-1-one (0.2 mmol), sodium difluoromethanesulfinate (MeCF2 SO2 Na, 0.6 mmol), lithium perchlorate (LiClO4, 0.3 M), acetonitrile (MeCN, 4.5 mL), and water (H2O, 1.5 mL). A carbon plate (10 mm × 10 mm × 3 mm) and a platinum plate (10 mm × 10 mm × 0.2 mm) were employed as the anode and cathode, respectively. The electrolysis was performed at a constant voltage of 2.1 V at room temperature for 3 h. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (EtOAc, 10 mL × 3). The combined organic layers were dried over anhydrous sodium sulfate (Na2 SO4) and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography.
2 Experimental details
Hydrogen atoms were positioned in geometrically idealized locations and refined using a riding model. The crystal structure was solved using ShelXT 2 and refined with ShelXL, 3 implemented within the Olex2 software suite 4 .
3 Comment
Benzo[4,5]imidazo[2,1-a]isoquinoline derivatives have garnered significant attention in recent years due to their diverse biological activities and potential applications in medicinal chemistry. 5 , 6 , 7 , 8 , 9 , 10 , 11 The present study focuses on the synthesis and structural elucidation of 5-(2,2-difluoropropyl) -5-methylbenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one.
The central benzo[4,5]imidazo[2,1-a]isoquinoline core exhibits a nearly planar conformation, with the maximum deviation from the mean plane being observed at C8 by 0.93 Å. This planarity is consistent with the conjugated aromatic system, facilitating π-π stacking interactions in the crystal. 12 , 13 , 14 , 15 , 16 , 17 The substituents at the 5-position include a 2,2-difluoropropyl group and a methyl group. The difluoropropyl group adopts an extended conformation, with the C–C–F bond angles ranging from 108.0° to 110.9°, while the C–F bond lengths are measured as 1.338(5) Å and 1.359(6) Å. The presence of the two electronegative fluorine atoms introduces notable steric and electronic effects, which influence the overall molecular packing.
The molecular packing is stabilized by a combination of π-π stacking and weak intermolecular interactions. Significant π-π stacking occurs between adjacent aromatic rings, with a centroid-to-centroid distance of 3.2 Å. Additionally, weak C–H…F interactions are observed, contributing to the stability of the three-dimensional crystal lattice.
In summary, the structural elucidation of 5-(2,2-difluoropropyl)-5-methylbenzo [4,5]imidazo[2,1-a]isoquinolin-6(5H)-one highlights the planar aromatic framework, the spatial arrangement of the fluorinated propyl group, and the key intermolecular interactions that govern its crystal packing.
Acknowledgments
This work was financially supported by the projects of Natural Science Foundation of Shannxi Province (2024JC–YBMS-733, 2022JM-561), the 2023 research and development project of the Xianyang Science and Technology Bureau (L2023–ZDYF–SF-030), Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city (2021QXNL–PT-0008), Doctoral research fund project of Xianyang Vocational and Technical College (2021BK01) and the scientific research fund project of Xianyang Vocational and Technical College (2020KJB02).
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. Shelxt–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A: Found. Adv. 2015, 71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Kraus, G. A.; Gupta, V.; Kohut, M.; Singh, N. A Direct Synthesis of 5, 6-Dihydroindolo[2,1-a]isoquinolines that Exhibit Immunosuppressive Activity. Bioorg. Med. Chem. Lett. 2009, 19, 5539–5542; https://doi.org/10.1016/j.bmcl.2009.08.057.Search in Google Scholar PubMed
6. Low, J. N.; Insuasty, B.; Torres, H.; Cobo, J. 6-(4–Fluorophenyl)-5, 6-dihydrobenzimidazo[1,2-c]quinazoline. Acta Crystallogr. Sect. E 2003, 59, o1801–o1803; https://doi.org/10.1107/s1600536803023663.Search in Google Scholar
7. Nandi, S.; Samanta, S.; Jana, S.; Ray, J. K. Synthesis of Substituted Benzimidazo[2,1-a]isoquinolines and its Condensed Analogues Using Pd(0)-catalyzed cyclization/C–H Activation. Tetrahedron Lett. 2010, 51, 5294–5297; https://doi.org/10.1016/j.tetlet.2010.07.162.Search in Google Scholar
8. Ooyama, Y.; Nakamura, T.; Yoshida, K. Heterocyclic Quinol-type Fluorophores. Synthesis of Novel Imidazoanthraquinol Derivatives and Their Photophysical Properties in Benzene and in the Crystalline State. New J. Chem. 2005, 29, 447–456; https://doi.org/10.1039/b410311d.Search in Google Scholar
9. Özbey, S.; Kaynak, F. B.; Göker, H.; Kuş, C. Synthesis and Crystal Structure Elucidation of 1-(4-Fluorobenzyl)-2-(4-cyanophenyl)-1h- Benzimidazole-5-Carbonitrile. J. Chem. Crystallogr. 2004, 34, 851–858; https://doi.org/10.1007/s10870-004-7718-0.Search in Google Scholar
10. Yang, X.; Jones, R. A.; Lai, R. J.; Waheed, A.; Oye, M. M.; Holmes, A. L. Supramolecular Assembly of Nanometer-Sized Heterobimetallic 3d-4f Complexes Formed with Benzimidazole Based N,O-donor Ligands. Polyhedron 2006, 25, 881–887; https://doi.org/10.1016/j.poly.2005.09.029.Search in Google Scholar
11. Zheng, L.; Hua, R. Modular Assembly of Ring–Fused and π–Extended Phenanthroimidazoles via C–H Activation and Alkyne Annulation. J.Org. Chem. 2014, 79, 3930–3936; https://doi.org/10.1021/jo500401n.Search in Google Scholar PubMed
12. Bian, Z. –Q.; Wang, K. –Z.; Jin, L. –P. Syntheses, Spectroscopic and Crystal Structural Studies of Novel Imidazo[4,5-f]1,10-phenanthroline Derivatives and their Eu(III) Ternary Complexes with Dibenzoylmethane. Polyhedron 2002, 21, 313–319; https://doi.org/10.1016/s0277-5387(01)00995-0.Search in Google Scholar
13. Chang, M. –Y.; Wu, M. –H.; Chen, Y. –L. Synthesis of Dihydrobenzoimidazo [2,1-a]isoquinolines. Tetrahedron Lett. 2012, 53, 4156–4160; https://doi.org/10.1016/j.tetlet.2012.05.132.Search in Google Scholar
14. Dinnebier, R. E.; Sieger, P.; Nar, H.; Shankland, K.; David, W. I. F. Structural Characterization of Three Crystalline Modifications of Telmisartan by Single Crystal and High–Resolution X–Ray Powder Diffraction. J. Pharm. Sci. 2000, 89, 1465–1479; https://doi.org/10.1002/1520-6017(200011)89:11<1465::aid-jps9>3.0.co;2-c.10.1002/1520-6017(200011)89:11<1465::AID-JPS9>3.0.CO;2-CSearch in Google Scholar
15. Gilbert, J. G.; Addison, A. W.; Prabakaran, P.; Butcher, R. J.; Bocelli, G. A Novel Paradigm for Metal-induced Ring Flipping in the Copper(II) Complex of 1,2-bis(N-methylbenzimidazol-2′-yl)benzene Triflate. Inorg. Chem. Commun. 2004, 7, 701–704, https://doi.org/10.1016/j.inoche.2004.03.019.Search in Google Scholar
16. Kazak, C.; Yilmaz, V. T.; Goker, H.; Kus, C. 1-n–Butyl-2-(4′-fluorophenyl) -1H-benzimidazole-6-carbonitrile. Acta Crystallogr. Sect. E 2004, 60, m819–m821, https://doi.org/10.1107/s1600536804011468.Search in Google Scholar
17. Ramesh, D.; Gangadhar, M.; Babu Nanubolu, J.; Adiyala, P.-R. Visible-light-induced Deaminative Alkylation/Cyclization of Alkyl Amines with N-Methacryloyl-2-phenylbenzoimidazoles in Continuous-Flow Organo-Photocatalysis. Ð. Org. Chem. 2021, 86, 12908–12921. https://doi.org/10.1021/acs.joc.1c01555.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

