Abstract
C20H7Cl2F8N5O3S, monoclinic, P21/n (no. 14), a = 7.78993(14) Å, b = 11.8299(2) Å, c = 25.8640(5) Å, β = 93.1491(17)°, V = 2379.87(7) Å3, Z = 4, Rgt(F) = 0.0540, wRref(F2) = 0.1306, T = 100 K.
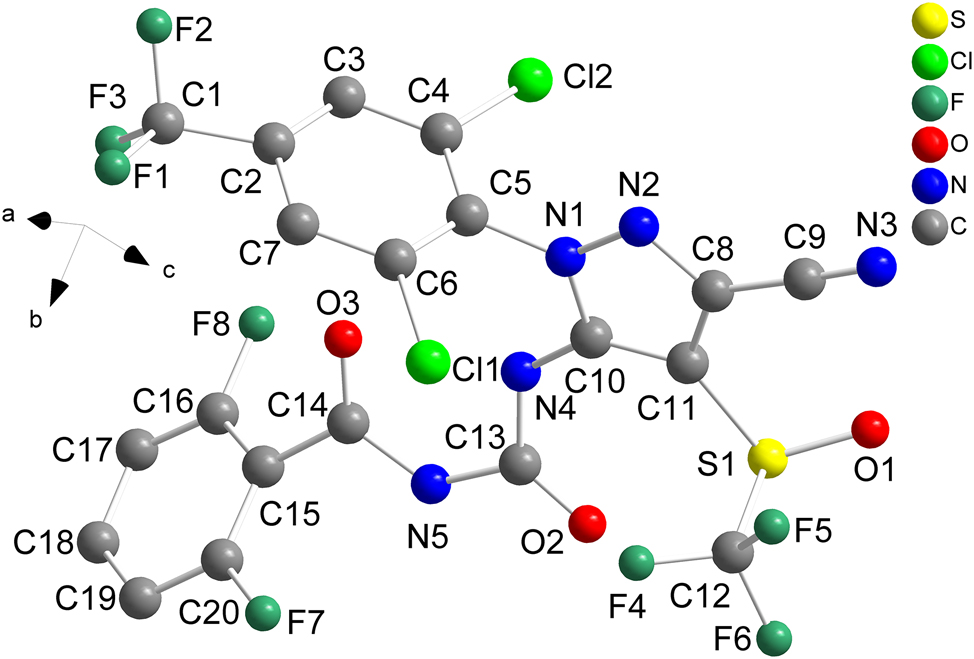
The title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Clear light colourless block |
| Size: | 0.12 × 0.10 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 4.20 mm−1 |
| Diffractometer, scan mode: | Rigaku, Synergy, HyPix, φ and ω scans |
| θmax, completeness: | 71.1°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 11582, 4508, 0.030 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4,223 |
| N(param)refined: | 390 |
| Programs: | Rigaku, 1 Olex2, 2 SHELX 3 , 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| Cl1 | 0.69034 (13) | 0.63675 (8) | 0.73260 (4) | 0.0499 (3) |
| Cl2 | 0.24050 (11) | 0.37725 (8) | 0.62286 (3) | 0.0443 (2) |
| S1 | 0.04661 (8) | 0.64347 (5) | 0.80877 (2) | 0.02156 (17) |
| F4 | 0.3240 (3) | 0.77674 (18) | 0.82028 (9) | 0.0490 (6) |
| F5 | 0.2931 (3) | 0.66252 (19) | 0.88237 (8) | 0.0462 (5) |
| F6 | 0.1264 (3) | 0.8039 (2) | 0.87404 (12) | 0.0710 (9) |
| F7 | 0.3911 (3) | 1.04613 (16) | 0.63247 (7) | 0.0373 (4) |
| F8 | 0.1436 (3) | 0.80823 (16) | 0.50333 (8) | 0.0482 (6) |
| O1 | −0.0107 (3) | 0.56185 (17) | 0.84797 (8) | 0.0297 (5) |
| O2 | 0.1018 (3) | 0.81029 (19) | 0.73317 (8) | 0.0372 (6) |
| O3 | 0.3245 (3) | 0.73556 (18) | 0.59696 (8) | 0.0351 (5) |
| N1 | 0.3704 (3) | 0.50706 (19) | 0.71503 (9) | 0.0243 (5) |
| N2 | 0.3853 (3) | 0.42874 (19) | 0.75328 (9) | 0.0268 (5) |
| N3 | 0.2520 (4) | 0.3464 (2) | 0.87025 (10) | 0.0400 (7) |
| N4 | 0.2365 (3) | 0.6745 (2) | 0.68766 (9) | 0.0279 (5) |
| H4 | 0.2681 (3) | 0.6580 (2) | 0.65640 (9) | 0.0334 (7)* |
| N5 | 0.1874 (3) | 0.8514 (2) | 0.65244 (9) | 0.0291 (6) |
| H5 | 0.1453 (3) | 0.9200 (2) | 0.65527 (9) | 0.0349 (7)* |
| C2 | 0.6821 (4) | 0.5164 (3) | 0.58868 (13) | 0.0355 (8) |
| C3 | 0.5320 (4) | 0.4549 (3) | 0.58467 (12) | 0.0332 (7) |
| H3 | 0.4988 (4) | 0.4163 (3) | 0.55351 (12) | 0.0399 (8)* |
| C4 | 0.4301 (4) | 0.4503 (2) | 0.62700 (11) | 0.0260 (6) |
| C5 | 0.4800 (4) | 0.5059 (2) | 0.67268 (11) | 0.0236 (6) |
| C6 | 0.6333 (4) | 0.5660 (2) | 0.67617 (12) | 0.0303 (7) |
| C7 | 0.7354 (4) | 0.5717 (3) | 0.63410 (15) | 0.0387 (8) |
| H7 | 0.8403 (4) | 0.6128 (3) | 0.63629 (15) | 0.0464 (10)* |
| C8 | 0.2802 (4) | 0.4667 (2) | 0.78822 (11) | 0.0248 (6) |
| C9 | 0.2632 (4) | 0.4010 (2) | 0.83436 (11) | 0.0291 (7) |
| C10 | 0.2590 (4) | 0.5924 (2) | 0.72492 (11) | 0.0247 (6) |
| C11 | 0.1975 (4) | 0.5695 (2) | 0.77282 (11) | 0.0232 (6) |
| C12 | 0.2102 (4) | 0.7278 (2) | 0.84775 (13) | 0.0295 (7) |
| C13 | 0.1693 (4) | 0.7797 (2) | 0.69484 (12) | 0.0282 (6) |
| C14 | 0.2634 (4) | 0.8275 (2) | 0.60674 (11) | 0.0274 (6) |
| C15 | 0.2676 (4) | 0.9231 (2) | 0.56947 (11) | 0.0256 (6) |
| C16 | 0.2114 (5) | 0.9091 (3) | 0.51785 (12) | 0.0317 (7) |
| C17 | 0.2169 (5) | 0.9941 (3) | 0.48153 (12) | 0.0356 (8) |
| H17 | 0.1745 (5) | 0.9823 (3) | 0.44682 (12) | 0.0427 (9)* |
| C18 | 0.2861 (5) | 1.0974 (3) | 0.49707 (12) | 0.0359 (8) |
| H18 | 0.2937 (5) | 1.1565 (3) | 0.47242 (12) | 0.0431 (9)* |
| C19 | 0.3441 (5) | 1.1159 (3) | 0.54774 (13) | 0.0350 (7) |
| H19 | 0.3914 (5) | 1.1869 (3) | 0.55818 (13) | 0.0420 (9)* |
| C20 | 0.3320 (4) | 1.0292 (3) | 0.58280 (11) | 0.0283 (6) |
| F1 | 0.9688 (12) | 0.5866 (11) | 0.5652 (4) | 0.060 (3)a |
| F2 | 0.8555 (18) | 0.4413 (8) | 0.5299 (5) | 0.063 (3)a |
| F3 | 0.754 (4) | 0.603 (2) | 0.5115 (11) | 0.045 (4)a |
| C1 | 0.820 (3) | 0.5394 (17) | 0.5507 (8) | 0.047 (3)a |
| F1A | 0.7452 (6) | 0.4406 (3) | 0.50546 (15) | 0.0542 (13) |
| F2A | 0.9496 (5) | 0.5117 (6) | 0.55385 (17) | 0.0751 (17) |
| F3A | 0.7586 (17) | 0.6199 (8) | 0.5166 (4) | 0.0453 (18) |
| C1A | 0.7835 (9) | 0.5205 (6) | 0.5401 (3) | 0.0383 (14) |
-
aOccupancy: 0.289 (8).
1 Source of materials
2,6-Difluorobenzoyl isocyanate (0.1 mol, 18.3 g) was added to a three-neck flask and heated to 40 °C. Drop 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl-4-((trifluoromethyl)sulfinyl)-1h–pyrazole-3-carbonitrile (0.1 mol, 43.7 g) of dichloroethane (100 g) solution with stirring at 40–45 °C. The suspension was stirred at 80 °C for 2 h and slowly cooled to 0 °C. Then the mixture was filtered and dried at 70–80 °C to get white powder. The white powder was dissolved in a mixed solution (ethyl acetate: n-heptane = 2:1, v/v) and then slowly volatilized at room temperature to obtain the single crystal.
2 Experimental details
Absorption corrections were used by using multi-scan program. 1 The structure was solved with SHELX. 3 , 4 Hydrogen atoms were placed in their geometrically idealized positions. Hydrogen atoms were constrained to ride on their parent atoms.
3 Comment
Urea-based organic compounds are pivotal in the realm of drug discovery and development, owing to their distinct chemical architectures and broad spectrum of biological activities. Recent studies have shown that the classic drug phenobarbital, which contains a urea structure, has great potential to replace conventional benzodiazepine in the treatment of alcohol withdrawal syndrome (AWS). 5 Glimepiride containing sulfonylurea structure is the third generation of long-acting anti-diabetic drugs, the main mechanism of its hypoglycemic effect is to stimulate the secretion of insulin by islet bet which is very important in the treatment of diabetes. 6 Levastinib, a versatile anticancer drug containing urea structure, is often used in clinical studies in combination with other medicines and has shown excellent results. 7 In this paper, a new compound was synthesized by referring to the phenylpyrazole structure of the analgesic celecoxib and the structure of the drug urea described above. 8
The title molecule in the crystal structure is a flexible molecule and each title molecule contains two phenyl rings, one pyrazole ring and one urea group. Intermolecular and intramolecular connectivity are achieved through hydrogen bonds. First hydrogen bond is N4–H4⃛O3, the distance of N4–H4 bond is 0.88 Å, the distance of H4⃛O3 bond is 1.86 Å, the angle of N4–H4⃛O3 is 138°, its an intramolecular hydrogen bond. 9 Second hydrogen bond is N5–H5⃛F7, the distance of N5–H5 bond is 0.88 Å, the distance of H5⃛F7 bond is 2.52 Å, the angle of N5–H5⃛F7 is 104°, its an intramolecular hydrogen bond. 10 Third hydrogen bond is N4–H5⃛O1, the distance of N4–H5 bond is 0.95 Å, the distance of H5⃛O1 bond is 1.98 Å, the angle of N4–H5⃛O1 is 168°.
In addition, there are two intermolecular C–F⋯π interactions and one intramolecular C–O⋯π interaction among the aromatic rings from neighboring molecules. The first C–F⋯π interaction is C12–F4⃛Cg i (C2–C7; symmetry code i: 1 − x, 1/2 + y, 3/2 − z), the shortest distance between F and center of Cg i is 3.118(3) Å. 11 The second C–F⋯π interaction is C12–F6⃛Cg i , the shortest distance between F and center of Cg i is 3.346(3) Å. The first C–O⋯π interaction is C14–O3⃛Cg ii (C2–C7; symmetry code i: x, y, z), the shortest distance between F and center of Cg ii is 3.414(3) Å. 12 All geometric parameters are in the expected ranges. 13
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CRYSALISPro Software System. Version 1.171.43.98, 2023.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXT Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Deanna, M.; Blair, N. C.; Dawn, M.; Mashael, A.-H.; Julie, T.; Yuriy, B. Phenobarbital versus Benzodiazepines in Alcohol Withdrawal Syndrome. Neuropsychopharmacol. Rep. 2023, 43, 532–541.10.1002/npr2.12347Search in Google Scholar PubMed PubMed Central
6. Abhishek, S.; Jothydev, K.; Viswanathan, M.; Banshi, S.; Dina, S.; Anuj, M.; Brij Mohan, M.; Kirtikumar, D. M.; Ashok Kumar, D. Clinical Evidence and Practice-Based Guidelines on the Utility of Basal Insulin Combined Oral Therapy (Metformin and Glimepiride) in the Current Era. Curr. Diabetes Rev. 2023, 19, e090123212444.10.2174/1573399819666230109104300Search in Google Scholar PubMed PubMed Central
7. Josep, M. L.; Masatoshi, K.; Philippe, M.; Tim, M.; Shukui, Q.; Masafumi, I.; Ruocai, X.; Julien, E.; Baek-Yeol, R.; Zhenggang, R.; Gianluca, M.; Mariusz, K.; Ho, Y. L.; Jee, H. K.; Valeriy, B.; Hiromitsu, K.; Ann-Lii, C.; Peter, R. G.; Shuichi, K.; Anran, W.; Kalgi, M.; Corina, D.; Leonid, D.; Abby, B. S.; Richard, S. F. Lenvatinib Plus Pembrolizumab versus Lenvatinib Plus Placebo for Advanced Hepatocellular Carcinoma (LEAP-002): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2023, 24, 1399–1410.Search in Google Scholar
8. Richard, L.; Cesar, M.; Adelaida, M.; Jesús, C.; Mariano, S.; Esther, O.; José María Giménez, A.; Oscar, de L. Co-crystal of Tramadol–Celecoxib (CTC) for Acute Moderate-To-Severe Pain. Curr. Med. Res. Opin. 2024, 40, 455–468.10.1080/03007995.2023.2276118Search in Google Scholar PubMed
9. Holehundi, J.; Shankara, P.; Haleyur, G.; Anil, K.; Thaluru, M.; Mohan, K.; Channappa, N. K. D.; Hemmige, S. Y.; Sean, R. P.; Lilianna, C. Supramolecular Characterisation of Salts of 4-(2-methoxyphenyl) Piperazin-1-Ium and 4-Phenylpiperazin-1-Ium Cations with Simple-Organic Anions; A Closer Look at the Binding Energies of Cation-Anion Pairs Formed by Charge-Assisted (+)N–H…O(−) and (+)N–H…O Hydrogen Bonds. J. Mol. Struct. 2023, 1292, 136193.10.1016/j.molstruc.2023.136193Search in Google Scholar
10. Anas, A.; Mousa, A.-N.; Abeer, A.; Hussien Ahmed, K.; Abdelkader, Z.; Karthik, K.; Ismail, W.; Shaukath Ara, K. Jahn–Teller Distortion in SP-like [Cu(bipy)(triamin Complexes with Novel N–H…F/C–H…F Synthon: XRD/HSA–Interactions, Physicochemical, Electrochemical, DFT, Docking and COX/LOX Inhibition. J. Mol. Liq. 2023, 387, 122689.10.1016/j.molliq.2023.122689Search in Google Scholar
11. Babak, M.; Amirhossein, K.; Faeze, M.; Hassan Hosein, M.; Rahman, B.; Zdirad, Z.; Hadi, E.; Sapana, J.; Ajay Kumar, M. Exploring C–F…π Interactions: Synthesis, Characterization, and Surface Analysis of Copper β-Diketone Complexes. ACS Omega 2024, 9, 5563–5575.10.1021/acsomega.3c07639Search in Google Scholar PubMed PubMed Central
12. Murugesan, P.; Hiregange, A.; Archita, P. Reciprocity of C=O…π Interactions with the Dominant Anion…π on Fullerene (C60)-Aminebased Organocatalysts: A Mechanistic Elucidation for Addition vs. Decarboxylation Reaction. Phys. Chem. Chem. Phys. 2023, 25, 10647–10660.10.1039/D2CP06017ESearch in Google Scholar
13. Chen, L.; Tang, C.; Long, Z.; Wu, Z. The synthesis and crystal structure of N-(3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1H-pyrazol-5-yl)-2-phenylacetamide. Z. Kristallogr. - New Crystal Struct. 2020, 235, 721; https://doi.org/10.1515/ncrs-2019-0919.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

