Abstract
C5H14KN5O9, monoclinic, P21/c, a = 9.3820(3) Å, b = 9.3372(3) Å, c = 14.1593(5) Å, β = 98.916°, V = 1225.39 Å3, Z = 4, Rgt(F) = 0.0249, wRref(F2) = 0.0640, T = 100 K.
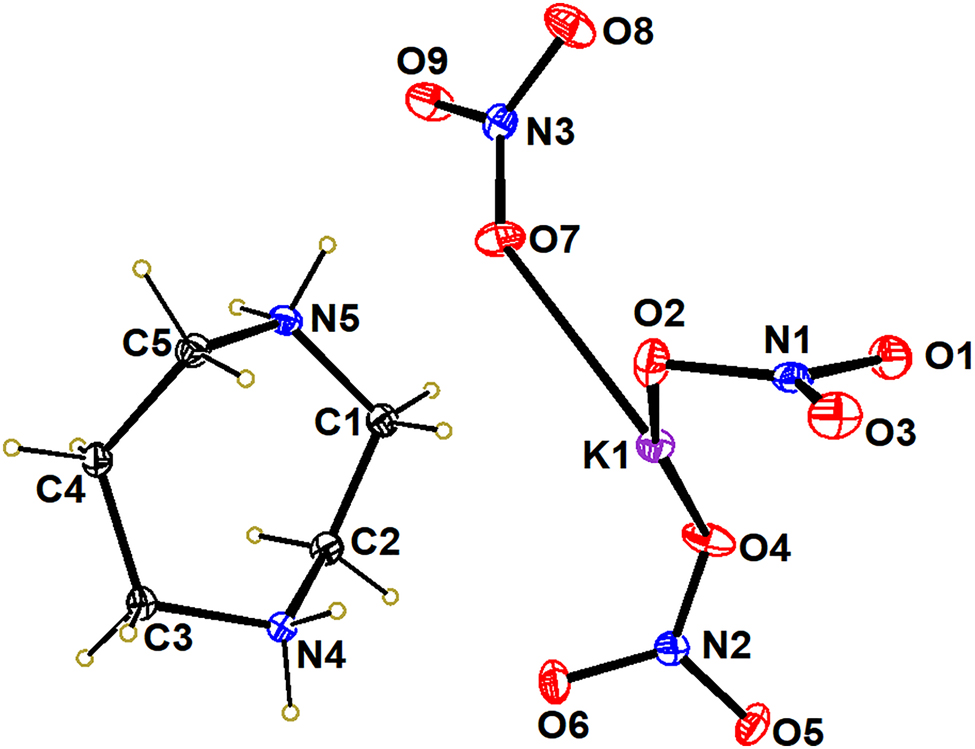
The figure shows the thermal ellipsoid plot of asymmetric unit of a crystal structure with an ellipsoid ratio of 50 %.
1 Source of materials
1.002 g Homopiperazine (10 mmol), 1.011 g KNO3 (10 mmol), and 1.983 g nitric acid (20 mmol) solids were dissolved into the 40 ml H2O and stirred continuously at 50 °C for 30 min. The above mixture solution was slowly evaporated at room temperature for 2 weeks to get colorless and clear crystals.
2 Experimental details
A summary of the crystal parameters, data acquisition, and refinement procedures can be found in Table 1 and the list of the atoms including atomic coordinates and displacement parameters can be found in Table 2. The multi-scan absorption correction was carried out by the SADABS 1 software. The initial structure determination was conducted via the Intrinsic Phasing technique within the OLEX2 2 interface by ShelXT. 3 Subsequent refinement was performed using the ShelXL. 4 Hydrogen atoms were positioned on the basis of riding model while their equivalent temperature factors (Ueq) set to 1.2 or 1.5 times those of respective parent atoms. These hydrogen atoms were then refined to convergence alongside the isotropic and anisotropic non-hydrogen atoms.
Data collection and handling.
| Crystal: | Block |
| Size: | 0.20 × 0.17 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.49 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy R, φ and ω scans |
| θmax, completeness: | 25.0°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 7141, 2152, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 1,984 |
| N(param)refined: | 181 |
| Programs: | Rigaku 1 , SHELX 3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| K1 | 0.75066 (3) | 0.76843 (3) | 0.71592 (2) | 0.01114 (11) |
| O1 | 1.06577 (11) | 0.84480 (11) | 0.79407 (7) | 0.0137 (2) |
| O2 | 0.91516 (11) | 1.01687 (11) | 0.74704 (7) | 0.0145 (2) |
| O3 | 1.09278 (11) | 1.05463 (11) | 0.86241 (7) | 0.0149 (2) |
| O4 | 0.62558 (12) | 0.50245 (12) | 0.71879 (8) | 0.0182 (3) |
| O5 | 0.52965 (11) | 0.37818 (12) | 0.82235 (7) | 0.0161 (2) |
| O6 | 0.40946 (11) | 0.55150 (12) | 0.74667 (8) | 0.0178 (3) |
| O7 | 0.66752 (12) | 0.78384 (12) | 0.52272 (7) | 0.0182 (3) |
| O8 | 0.82679 (11) | 0.77248 (12) | 0.42626 (8) | 0.0197 (3) |
| O9 | 0.60395 (11) | 0.72022 (12) | 0.37493 (8) | 0.0180 (3) |
| N1 | 1.02530 (13) | 0.97248 (13) | 0.80123 (9) | 0.0103 (3) |
| N2 | 0.52172 (13) | 0.47851 (13) | 0.76220 (9) | 0.0110 (3) |
| N3 | 0.70080 (13) | 0.75982 (13) | 0.44227 (9) | 0.0111 (3) |
| N4 | 0.20570 (13) | 0.72462 (13) | 0.62830 (9) | 0.0101 (3) |
| H4A | 0.298045 | 0.747168 | 0.653762 | 0.012* |
| H4B | 0.160077 | 0.695140 | 0.677098 | 0.012* |
| N5 | 0.32914 (13) | 0.71445 (13) | 0.43029 (9) | 0.0108 (3) |
| H5A | 0.254849 | 0.688982 | 0.384147 | 0.013* |
| H5B | 0.411584 | 0.710281 | 0.403971 | 0.013* |
| C1 | 0.33965 (15) | 0.60694 (16) | 0.50904 (10) | 0.0119 (3) |
| H1A | 0.426192 | 0.628677 | 0.556321 | 0.014* |
| H1B | 0.353545 | 0.510945 | 0.482206 | 0.014* |
| C2 | 0.20934 (16) | 0.60190 (15) | 0.56038 (10) | 0.0111 (3) |
| H2A | 0.120475 | 0.603556 | 0.512483 | 0.013* |
| H2B | 0.210519 | 0.510804 | 0.596269 | 0.013* |
| C3 | 0.13251 (16) | 0.85772 (15) | 0.58571 (10) | 0.0122 (3) |
| H3A | 0.167891 | 0.940234 | 0.626641 | 0.015* |
| H3B | 0.027611 | 0.848746 | 0.586690 | 0.015* |
| C4 | 0.15577 (15) | 0.88981 (16) | 0.48338 (10) | 0.0114 (3) |
| H4C | 0.088019 | 0.829531 | 0.439799 | 0.014* |
| H4D | 0.128505 | 0.990888 | 0.469315 | 0.014* |
| C5 | 0.30638 (16) | 0.86704 (15) | 0.45881 (11) | 0.0117 (3) |
| H5C | 0.320836 | 0.931585 | 0.405640 | 0.014* |
| H5D | 0.378858 | 0.892078 | 0.514890 | 0.014* |
3 Comment
Molecular perovskite high-energetic materials are synthesized by simple and green methods without organic solvents, heavy metals, toxic organic components, or any explosive precursors. 5 , 6 , 7 They have a good oxygen balance, high crystal density, good stability, high packing coefficient, excellent environmental tolerance and low mechanical sensitivity, which shows great potential in the field of energetic materials. 8 , 9 , 10 , 11 Perovskite materials have excellent structural tunability. By regulating the A, B, and X sites of their structure, the photoelectric, catalytic, energy, and electromagnetic properties of the material can be controlled. 11 In this work, we regulated the A-site of perovskite structure to obtain better oxygen balance characteristics of high-energetic materials. The structure of the synthesized sample has a different A-site compared to the literature, while the B and X sites are the same. 12
The molecular perovskite energetic materials take 1,4-diazepane-1,4-diium as the A-site, potassium ions as the B-site, and nitrate as the X-site, respectively. The 1,4-diazepane-1,4-diium consists of a seven-membered ring with five carbon atoms and two nitrogen atoms. In the 1,4-diazepane-1,4-diium cation, the bond lengths of C1–C2, C4–C5 and C4–C3 are 1.517, 1.522 and 1.528 Å, respectively. Meanwhile, the three nitrate groups serve as the bridges, forming a framework of perovskite with K ions.
References
1. Krause, L.; Herbst-Irmer, R.; Sheldrick, G. M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48 (1), 3–10. https://doi.org/10.1107/s1600576714022985.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42 (2), 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXT Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71 (Pt 1), 3–8. https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71 (1), 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Feng, Y.; Zhang, J.; Cao, W.; Zhang, J.; Shreeve, J. M. A Promising Perovskite Primary Explosive. Nat. Commun. 2023, 14 (1), 7765. https://doi.org/10.1038/s41467-023-43320-0.Search in Google Scholar PubMed PubMed Central
6. Chen, S. L.; Yang, Z. R.; Wang, B. J.; Shang, Y.; Sun, L. Y.; Zhou, H. L.; Zhang, W. X.; Chen, X. M. Molecular Perovskite High-Energetic Materials. Sci. China Mater. 2018, 1123–1128. https://doi.org/10.1007/s40843-017-9219-9.Search in Google Scholar
7. Xu, J.; Zheng, S.; Huang, S.; Tian, Y.; Liu, Y.; Zhang, H.; Sun, J. Host-Guest Energetic Materials Constructed by Incorporating Oxidizing Gas Molecules into an Organic Lattice Cavity Toward Achieving Highly-Energetic and Low-Sensitivity Performance. Chem. Commun. 2019, 55 (7), 909–9012. https://doi.org/10.1039/c8cc07347c.Search in Google Scholar PubMed
8. Shang, Y.; Huang, R. K.; Chen, S. L.; He, C. T.; Yu, Z. H.; Ye, Z. M.; Zhang, W. X.; Chen, X. M. Metal-Free Molecular Perovskite High-Energetic Materials. Cryst. Growth Des. 2020, 20 (3), 1013–1018. https://doi.org/10.1021/acs.cgd.9b01592.Search in Google Scholar
9. Zhou, J.; Ding, L.; Zhao, F.; Wang, B.; Zhang, J. Thermal Studies of Novel Molecular Perovskite Energetic Material (C6H14N2)NH4(ClO4)3. Chin. Chem. Lett. 2019, 31 (2), 554–558. https://doi.org/10.1016/j.cclet.2019.05.008.Search in Google Scholar
10. Shang, Y.; Chen, S. L.; Yu, Z. H.; Huang, R. K.; Ye, Z. M.; Zhang, W. X.; Chen, X. M. Silver(I)-Based Molecular Perovskite Energetic Compounds with Exceptional Thermal Stability and Energetic Performance. Inorg. Chem. 2022, 61, 4143–4149. https://doi.org/10.1021/acs.inorgchem.1c03958.Search in Google Scholar PubMed
11. Chen, S. L.; Shang, Y.; He, C. T.; Sun, L. Y.; Ye, Z. M.; Zhang, W. X.; Chen, X. M. Optimizing the Oxygen Balance by Changing the A-Site Cations in Molecular Perovskite High Energetic Materials. CrystEngComm 2018, 20 (46), 7458–7463. https://doi.org/10.1039/c8ce01350k.Search in Google Scholar
12. Chen, S.; Shang, Y.; Jiang, J.; Huang, M.; Ren, J. T.; Guo, T.; Yu, C. X.; Zhang, W. X.; Chen, X. M. A New Nitrate-Based Energetic Molecular Perovskite as a Modern Edition of Black Powder. Energ. Mater. Front. 2022, 3 (3), 122–127. https://doi.org/10.1016/j.enmf.2022.07.003.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

