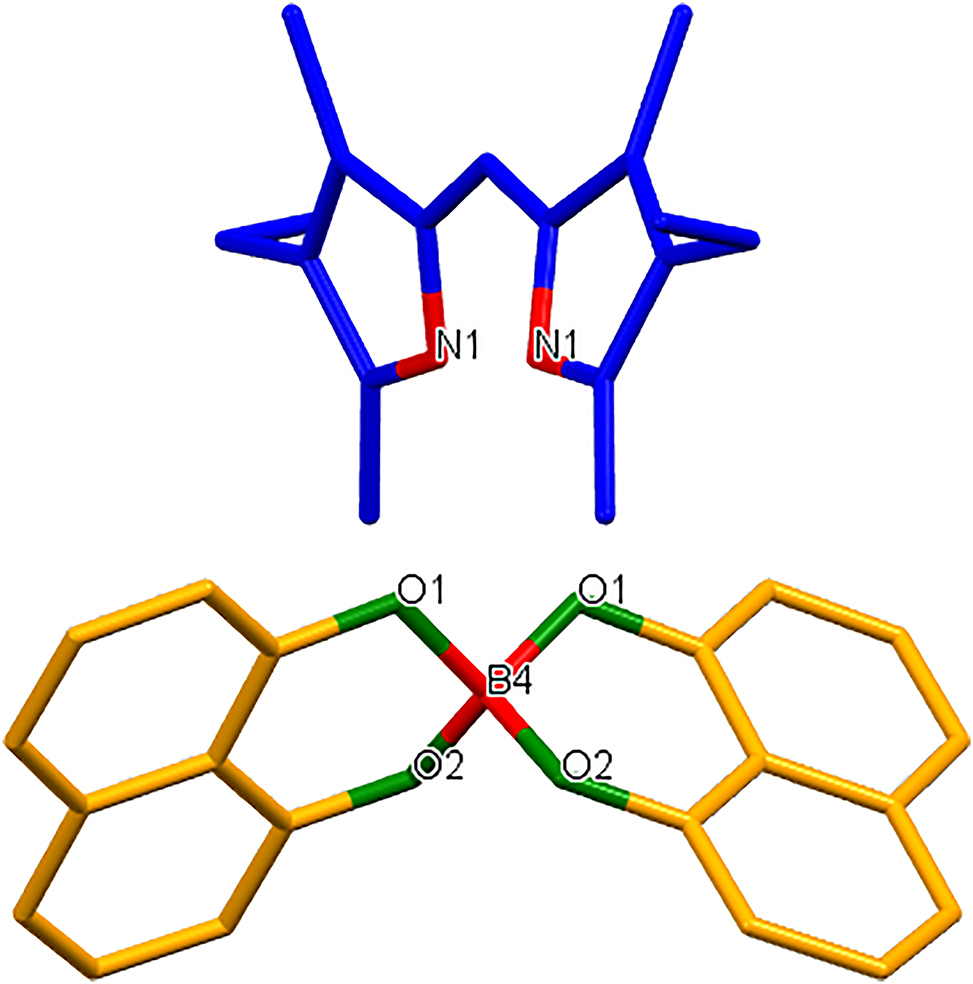
The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
Abstract
C37H37BN2O4, monoclinic, I2/a (no. 15), a = 13.8030(3) Å, b = 17.6109(2) Å, c = 14.1059(3) Å, β = 117.524(3)°, V = 3040.83(11) Å3, Z = 4, Rgt (F) = 0.0340, wRref (F 2) = 0.0867, T = 150 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange plate |
| Size: | 0.43 × 0.08 × 0.05 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.65 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θ max, completeness: | 66.9°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 10,896, 2696, 0.027 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2410 |
| N(param)refined: | 206 |
| Programs: | CrysAlisPro [1], SHELX [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| O1 | 0.67809 (6) | 0.70100 (5) | 0.41408 (6) | 0.0275 (2) |
| O2 | 0.69001 (7) | 0.79929 (5) | 0.54066 (6) | 0.0295 (2) |
| N1 | 0.78953 (8) | 0.56589 (6) | 0.41272 (8) | 0.0238 (2) |
| H1 | 0.7457 (12) | 0.6017 (8) | 0.4184 (11) | 0.029* |
| C1 | 0.86661 (9) | 0.45483 (7) | 0.40453 (9) | 0.0237 (3) |
| C2 | 0.89341 (9) | 0.50969 (7) | 0.34997 (9) | 0.0252 (3) |
| C3 | 0.84312 (9) | 0.57779 (7) | 0.35489 (9) | 0.0251 (3) |
| C8 | 0.7500 | 0.45713 (9) | 0.5000 | 0.0232 (3) |
| H8 | 0.7500 | 0.4032 | 0.5000 | 0.028* |
| C9 | 0.79956 (9) | 0.49010 (7) | 0.44387 (9) | 0.0223 (3) |
| C10 | 0.90618 (10) | 0.37450 (7) | 0.42373 (10) | 0.0281 (3) |
| H10A | 0.8740 | 0.3467 | 0.3559 | 0.042* |
| H10B | 0.8845 | 0.3503 | 0.4736 | 0.042* |
| H10C | 0.9860 | 0.3738 | 0.4543 | 0.042* |
| C11 | 0.96002 (11) | 0.50007 (8) | 0.29149 (11) | 0.0329 (3) |
| H11A | 1.0079 | 0.4551 | 0.3202 | 0.039* |
| H11B | 1.0073 | 0.5452 | 0.3043 | 0.039* |
| C12 | 0.88885 (13) | 0.49009 (9) | 0.17155 (11) | 0.0440 (4) |
| H12A | 0.9355 | 0.4838 | 0.1368 | 0.066* |
| H12B | 0.8425 | 0.5350 | 0.1424 | 0.066* |
| H12C | 0.8427 | 0.4450 | 0.1583 | 0.066* |
| C13 | 0.84431 (11) | 0.65271 (7) | 0.30741 (10) | 0.0327 (3) |
| H13A | 0.7873 | 0.6540 | 0.2329 | 0.049* |
| H13B | 0.9158 | 0.6608 | 0.3101 | 0.049* |
| H13C | 0.8307 | 0.6928 | 0.3479 | 0.049* |
| C14 | 0.57882 (9) | 0.72763 (7) | 0.34104 (9) | 0.0239 (3) |
| C15 | 0.52492 (10) | 0.69411 (7) | 0.24262 (10) | 0.0283 (3) |
| H15 | 0.5577 | 0.6534 | 0.2237 | 0.034* |
| C16 | 0.42069 (11) | 0.72048 (8) | 0.16981 (10) | 0.0346 (3) |
| H16 | 0.3836 | 0.6975 | 0.1013 | 0.041* |
| C17 | 0.37170 (11) | 0.77857 (8) | 0.19572 (11) | 0.0365 (3) |
| H17 | 0.3008 | 0.7949 | 0.1455 | 0.044* |
| C18 | 0.37849 (11) | 0.87430 (8) | 0.32951 (11) | 0.0374 (3) |
| H18 | 0.3076 | 0.8925 | 0.2820 | 0.045* |
| C19 | 0.43468 (12) | 0.90563 (8) | 0.42898 (11) | 0.0366 (3) |
| H19 | 0.4015 | 0.9449 | 0.4500 | 0.044* |
| C20 | 0.54027 (11) | 0.88116 (7) | 0.50087 (10) | 0.0313 (3) |
| H20 | 0.5779 | 0.9041 | 0.5693 | 0.038* |
| C21 | 0.58888 (10) | 0.82405 (7) | 0.47205 (9) | 0.0257 (3) |
| C23 | 0.53162 (10) | 0.78921 (7) | 0.36981 (9) | 0.0248 (3) |
| C24 | 0.42564 (10) | 0.81482 (7) | 0.29707 (10) | 0.0304 (3) |
| B4 | 0.7500 | 0.75178 (11) | 0.5000 | 0.0268 (4) |
1 Source of materials
The 2,6-diethyl-3,5-dimethyl-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (50 mg, 0.16 mmol) and TMSOTf (0.11 mL, 0.57 mmol) were dissolved in toluene (5 mL) and the mixture was heated under reflux for 30 min. The solution was cooled to room temperature, and 1,8-dihydroxynaphthalene (132 mg, 0.82 mmol) was added followed by diisopropylethylamine (0.10 mL, 0.57 mmol). The reaction mixture was stirred for 2 h at room temperature, the solvent was removed under reduced pressure, and the crude product was purified by column chromatography to give the title compound in 20 % yield. Good quality single crystal suitable for single crystal diffraction analysis was obtained by slow evaporation from a mixture of chloroform and hexane (1:3).
2 Experimental details
The structure of the as title compound was solved using SHELXT [2] and refined by SHELXL program [3] through the Olex2 interface [4]. All hydrogen atoms were positioned at calculated coordinates and refined isotropically.
3 Comment
Noncovalent interactions (NCIs) are ubiquitous in nature, playing a crucial role in the cohesion of chemical systems. The last decades have witnessed increased interest in the rational design of supramolecular architectures based on the self-assembly of various suitable building blocks using NCIs [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Amongst the various strategies, the utilization of intermolecular hydrogen bond interactions in the design and self-assembly of well-defined supramolecular structures has become a promising synthetic approach with potential in host–guest chemistry, gas storage, and crystal engineering [16], [17], [18], [19], [20]. The synthesized complex was crystallized in the monoclinic space group I12/a1. Single-crystal structure analysis revealed the product as a salt in which the boron centre had been abstracted from the diypyrromethene core followed by formation of an anion with two molecules of the naphthalene-1,8-bis(olate). The nitrogen atoms of the cation were hydrogen-bonded to the oxygen atoms of the boron containing anion.
Funding source: Princess Nourah bint Abdulrahman University
Award Identifier / Grant number: PNURSP2024R65
Funding source: King Abdullah International Medical Research Center (KAIMRC)
Award Identifier / Grant number: NRC23R/746/11
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was financially supported by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R65), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. A. K thanks the support of King Abdullah International Medical Research Center (KAIMRC) through start up grant NRC23R/746/11.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2015.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Desiraju, G. R. Supramolecular synthons in crystal engineering? A new organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327; https://doi.org/10.1002/anie.199523111.Search in Google Scholar
6. Hosseini, M. W. Molecular tectonics: from simple tectons to complex molecular networks. Acc. Chem. Res. 2005, 38, 313–323; https://doi.org/10.1021/ar0401799.Search in Google Scholar PubMed
7. Khan, A., Bushnak, I., Jiang, Z., Usman, R., Choudhari, P. B., Feng, C., Al-Kaysi, R. O., Rahman, F.-U. Novel jellyfish-shaped resorcin[4]arene macrocyclic cocrystal via collaboration of macrocyclic chemistry and co-crystal engineering: insight into structural, noncovalent interactions and computational chemistry. J. Mol. Struct. 2024, 1298, 137034; https://doi.org/10.1016/j.molstruc.2023.137034.Search in Google Scholar
8. Feng, Q., Wang, M., Xu, C., Khan, A., Wu, X., Lu, J., Wei, X. Investigation of molecular arrangements and solid-state fluorescence properties of solvates and cocrystals of 1-acetyl-3-phenyl-5-(9-anthryl)-2-pyrazoline. CrystEngComm 2014, 16, 5820–5826; https://doi.org/10.1039/c3ce42210k.Search in Google Scholar
9. Khan, A., Liu, M., Usman, R., He, N., Li, R., Sayed, S. M., Li, S., Sun, W., Chen, H., Zhang, L. Solid emission color tuning of organic charge transfer cocrystals based on planar p-conjugated donors and TCNB. J. Solid State Chem. 2019, 272, 96–101; https://doi.org/10.1016/j.jssc.2019.02.005.Search in Google Scholar
10. Khan, A., Usman, R., Li, R., Hajji, M., Tang, H., Ma, D. Polycyclic motif engineering in cyanostilbene-based donors towards highly efficient modulable emission properties in two-component systems. CrystEngComm 2021, 23, 8462–8470; https://doi.org/10.1039/d1ce00959a.Search in Google Scholar
11. Khan, A., Usman, R., Sayed, S. M., Li, R., Chen, H., He, N. Supramolecular design of highly efficient two-component molecular hybrids toward structure and emission properties tailoring. Cryst. Growth Des. 2019, 19, 2772–2778; https://doi.org/10.1021/acs.cgd.8b01922.Search in Google Scholar
12. Khan, A., Wang, M., Usman, R., Lu, J., Sun, H., Du, M., Zhang, R., Xu, C. Organic charge-transfer complexes for the selective accommodation of aromatic isomers using anthracene derivatives and TCNQ. New J. Chem. 2016, 40, 5277–5284; https://doi.org/10.1039/c5nj03442f.Search in Google Scholar
13. Khan, A., Wang, M., Usman, R., Sun, H., Du, M., Xu, C. Molecular marriage via charge transfer interaction in organic charge transfer co-crystals toward solid-state fluorescence modulation. Cryst. Growth Des. 2017, 17, 1251–1257; https://doi.org/10.1021/acs.cgd.6b01636.Search in Google Scholar
14. Sun, H., Wang, M., Wei, X., Zhang, R., Wang, S., Khan, A., Usman, R., Feng, Q., Du, M., Yu, F. Understanding charge-transfer interaction mode in cocrystals and solvates of 1-phenyl-3-(pyren-1-yl) prop-2-en-1-one and TCNQ. Cryst. Growth Des. 2015, 15, 4032–4038; https://doi.org/10.1021/acs.cgd.5b00656.Search in Google Scholar
15. Usman, R., Khan, A., Sun, H., Wang, M. Study of charge transfer interaction modes in the mixed donor-acceptor cocrystals of pyrene derivatives and TCNQ: a combined structural, thermal, spectroscopic, and hirshfeld surfaces analysis. J. Solid State Chem. 2018, 266, 112–120; https://doi.org/10.1016/j.jssc.2018.07.009.Search in Google Scholar
16. Usman, R., Khan, A., Tang, H., Ma, D., Alsuhaibani, A. M., Refat, M. S., Adnan, Ara, N., Fan, H.-J. S. Charge transfer and hydrogen bonding motifs in organic cocrystals derived from aromatic diamines and TCNB. J. Mol. Struct. 2022, 1254, 132360; https://doi.org/10.1016/j.molstruc.2022.132360.Search in Google Scholar
17. Usman, R., Khan, A., Wang, M. Study of H-bonded assemblies of the solvates of anthracene derivatives: guest effect on the crystal symmetry and spectroscopic properties. Supramol. Chem. 2017, 29, 497–505; https://doi.org/10.1080/10610278.2017.1284324.Search in Google Scholar
18. Usman, R., Khan, A., Wang, M., Luo, Y., Sun, W., Sun, H., Du, C., He, N. Investigation of charge-transfer interaction in mixed stack donor-acceptor cocrystals toward tunable solid-state emission characteristics. Cryst. Growth Des. 2018, 18, 6001–6008; https://doi.org/10.1021/acs.cgd.8b00852.Search in Google Scholar
19. Torres-Huerta, A., Velásquez-Hernández, M. D. J., Martínez-Otero, D., Höpfl, H., Jancik, V. Structural induction via solvent variation in assemblies of triphenylboroxine and piperazine-potential application as self-assembly molecular sponge. Cryst. Growth Des. 2017, 17, 2438–2452; https://doi.org/10.1021/acs.cgd.6b01845.Search in Google Scholar
20. Patalag, L. J., Jones, P. G., Werz, D. B. BOIMPYs: rapid access to a family of red-emissive fluorophores and NIR dyes. Angew. Chem. Int. Ed. 2016, 55, 13340–13344; https://doi.org/10.1002/anie.201606883.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3


