Abstract
C18H17N3O2S, monoclinic, P21/c (no. 14), a = 10.9713(5) Å, b = 15.4445(8) Å, c = 9.8221(4) Å, β = 98.275(4)°, V = 1646.99(13) Å3, Z = 4, Rgt (F) = 0.0401, wRref (F 2) = 0.0990, T = 296(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.23 × 0.17 × 0.14 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.21 mm−1 |
| Diffractometer, scan mode: | D8/APEX2, φ and ω |
| θ max, completeness: | 25.0°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 9313, 2906, 0.019 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2430 |
| N(param)refined: | 226 |
| Programs: | Bruker [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 1.1379 (2) | 0.1369 (2) | 1.5252 (3) | 0.0835 (9) |
| H1A | 1.1233 | 0.1180 | 1.6145 | 0.125* |
| H1B | 1.1824 | 0.0930 | 1.4838 | 0.125* |
| H1C | 1.1853 | 0.1894 | 1.5342 | 0.125* |
| C2 | 1.0259 (2) | 0.17954 (14) | 1.3117 (2) | 0.0481 (5) |
| C3 | 0.8980 (2) | 0.2046 (2) | 1.2460 (2) | 0.0571 (6) |
| C4 | 0.85857 (17) | 0.07197 (13) | 1.06705 (18) | 0.0389 (4) |
| C5 | 0.83748 (17) | −0.05848 (13) | 0.9907 (2) | 0.0430 (5) |
| H5 | 0.8198 | −0.1059 | 0.9329 | 0.052* |
| C6 | 0.75820 (16) | 0.05789 (12) | 0.82003 (18) | 0.0349 (4) |
| C7 | 0.63682 (15) | 0.09122 (11) | 0.81232 (17) | 0.0322 (4) |
| C8 | 0.56984 (17) | 0.08977 (12) | 0.92520 (19) | 0.0396 (5) |
| H8 | 0.6058 | 0.0666 | 1.0088 | 0.048* |
| C9 | 0.45342 (18) | 0.12197 (14) | 0.9127 (2) | 0.0470 (5) |
| H9 | 0.4103 | 0.1200 | 0.9876 | 0.056* |
| C10 | 0.39791 (18) | 0.15789 (14) | 0.7888 (2) | 0.0500 (5) |
| H10 | 0.3188 | 0.1807 | 0.7822 | 0.060* |
| C11 | 0.45914 (17) | 0.15966 (13) | 0.6774 (2) | 0.0432 (5) |
| H11 | 0.4212 | 0.1838 | 0.5954 | 0.052* |
| C12 | 0.57983 (16) | 0.12522 (11) | 0.68472 (18) | 0.0342 (4) |
| C13 | 0.64333 (16) | 0.12171 (11) | 0.56667 (18) | 0.0349 (4) |
| C14 | 0.75834 (17) | 0.08656 (13) | 0.58105 (19) | 0.0417 (5) |
| H14 | 0.7990 | 0.0834 | 0.5044 | 0.050* |
| C15 | 0.81785 (17) | 0.05486 (13) | 0.70790 (19) | 0.0414 (5) |
| H15 | 0.8970 | 0.0321 | 0.7149 | 0.050* |
| C16 | 0.58272 (18) | 0.15701 (13) | 0.43202 (18) | 0.0414 (5) |
| H16 | 0.5770 | 0.2203 | 0.4283 | 0.050* |
| C17 | 0.4766 (2) | 0.11063 (16) | 0.3497 (2) | 0.0597 (6) |
| H17A | 0.4519 | 0.0554 | 0.3840 | 0.072* |
| H17B | 0.4098 | 0.1454 | 0.3029 | 0.072* |
| C18 | 0.5974 (2) | 0.11506 (18) | 0.2987 (2) | 0.0682 (7) |
| H18A | 0.6040 | 0.1524 | 0.2207 | 0.082* |
| H18B | 0.6461 | 0.0625 | 0.3019 | 0.082* |
| H3A | 0.834 (2) | 0.1770 (16) | 1.292 (2) | 0.071 (8)* |
| H3B | 0.894 (2) | 0.2632 (18) | 1.252 (3) | 0.075 (8)* |
| N1 | 0.88388 (15) | −0.06394 (12) | 1.11983 (18) | 0.0506 (5) |
| N2 | 0.89793 (15) | 0.02069 (12) | 1.16988 (16) | 0.0467 (4) |
| N3 | 0.81796 (13) | 0.02530 (10) | 0.95072 (15) | 0.0373 (4) |
| O1 | 1.11751 (16) | 0.18538 (12) | 1.26099 (17) | 0.0684 (5) |
| O2 | 1.02109 (14) | 0.15273 (11) | 1.43938 (14) | 0.0606 (4) |
| S1 | 0.86185 (6) | 0.18445 (4) | 1.06464 (5) | 0.0578 (2) |
1 Source of materials
Intermediate 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (45.0 g, 0.169 mol) and triethylamine (18.7 g, 0.183 mol) of were added into absolute ethanol (400 mL) at room temperature. Cooling to 0 °C, dropping 150 mL of absolute ethanol solution of ethyl bromoacetate (29.6 g, 0.18 mol), and controlling the temperature to 0 °C for 1 h after dropping. Most of the solvent was evaporated under reduced pressure, and the reaction solution was poured into 250 mL of water, extracted with dichloromethane (100 mL × 3), and the organic layers were combined. Wash with 200 mL water and 200 mL saturated sodium chloride solution, and dry the organic layer with anhydrous sodium sulfate. 52.4 g yellow oil product was obtained with a yield of 88.2 %.
2 Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with U iso(H) = 1.5 U eq(C) for methyl H atoms and 1.2 U eq(C) for all other H atoms.
3 Comment
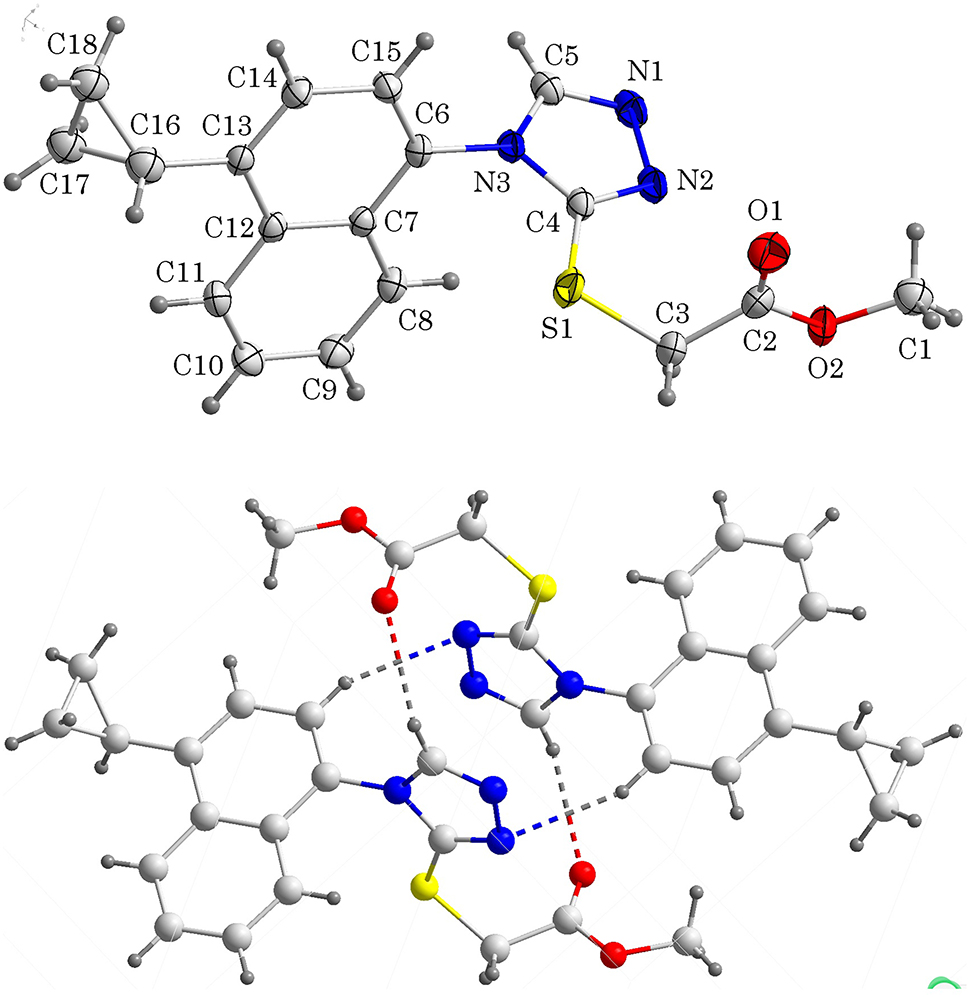
Lesinurad sodium is the first inhibitor of uric acid selective reabsorption transporter-1 (URAT1), which was first developed by Aread Biosciences and then continued to be developed by Astra Zeneca [5]. The chemical name of lesinurad sodium is 2-{[5-bromo-4-(4-cyclopropylnaphthalene-1-yl)-4h-1,2,4-triazol-3-yl]thio} acetic acid, which is mainly used for treating hyperuricemia related to gout in combination with xanthine oxidase inhibitors (such as allopurinol and febuxostat) [6], [7], [8]. Optimizing the synthesis process of lesinurad sodium and its derivatives has attracted the attention of researchers [9], [10], [11]. Recently, we have been focusing on the synthesis of esinurad sodium and optimizing its synthesis process. Here we reveal the synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, the key intermediate of lesinurad sodium.
In the molecules of the title structure bond lengths and angles are very similar to those given in the literature [12–15]. In the title structure, the dihedral angle formed by the naphthalene ring plane, the 1,2,4-triazole-3-thione plane and the cyclopropyl group plane are 54.6°, 71.5(73)° and 86.9°, respectively. These parameters are different from the data reported before [13]. The dihedral angle between the 1,2,4-triazole-3-thione plane and the ester plane to which it is connected is 89.0(213)°. The torsion angles of N2–C4–S1–C3 and C4–S1–C3–C2 are −12.798(207)° and 69.286(186)°. Intermolecular C–H⋯O hydrogen bonds and C–H⋯N hydrogen bonds connect two adjacent compounds into a dimer.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 22168018
Funding source: National Natural Science Foundation of Jiangxi, China
Award Identifier / Grant number: 20232BAB203048; 20202BABL206111
Funding source: Science and Technology Plan Project Fund of Jiangxi Provincial Health Planning Commission
Award Identifier / Grant number: 202411004
Funding source: Science and Technology Plan Project of Ji’an
Award Identifier / Grant number: 20233–043398, 20233–043995
Acknowledgment
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by the National Natural Science Foundation of China (22168018), the National Natural Science Foundation of Jiangxi, China (20232BAB203048; 20202BABL206111), The Science and Technology Plan Project Fund of Jiangxi Provincial Health Planning Commission (202411004), the Science and Technology Plan Project of Ji’an (20233–043398, 20233–043995).
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Hoy, S. M. Lesinurad: first global approval. Drugs 2016, 76, 509–516. https://doi.org/10.1007/s40265–016–0550-y.10.1007/s40265-016-0550-ySearch in Google Scholar PubMed
6. Sattui, S. E., Gaffo, A. L. Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther. Adv. Muscoskel. Dis. 2016, 8, 145–159. https://doi.org/10.1177/1759720X16646703.Search in Google Scholar PubMed PubMed Central
7. Shen, Z., Yeh, L. T., Wallach, K., Zhu, N., Kerr, B., Gillen, M. In vitro and in vivo interaction studies between lesinurad, a selective urate reabsorption inhibitor, and major liver or kidney transporters. Clin. Drug Invest. 2016, 36, 443–452. https://doi.org/10.1007/s40261–016–0386-y.10.1007/s40261-016-0386-ySearch in Google Scholar PubMed PubMed Central
8. Miner, J. N., Tan, P. K., Hyndman, D., Liu, S., Iverson, C., Nanavati, P., Hagerty, D. T., Manhard, K., Shen, Z., Girardet, J. L., Yeh, L. T., Terkeltaub, R., Quart, B. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res. Ther. 2016, 18, 214–236. https://doi.org/10.1186/s13075–016–1107-x.10.1186/s13075-016-1107-xSearch in Google Scholar PubMed PubMed Central
9. Zou, L., Liu, Y., Yao, K., Li, J. Q., Zhang, Z. X. Synthesis of lesinurad. Chin. J. Pharm. 2017, 48, 488–491. https://doi.org/10.16522/j.cnki.cjph.2017.04.003.Search in Google Scholar
10. Pan, W., Wan, T., Jiang, N., Gong, P., Zhai, X. Improved synthetic process of lesinurad sodium. Chin. J. Med. Chem. 2019, 29, 363–367. https://doi.org/10.14142/j.cnki.cn21–1313/r.2019.05.005.Search in Google Scholar
11. Chen, J., Su, W. Q., Chen, N. G., Wang, Y. M. Study on synthesis process of lesinurad. Chin. J. Guangzhou Chem. Ind. 2023, 51, 102–104.Search in Google Scholar
12. Drumright, R. E., Mas, R. H., Merola, J. S., Tanko, J. M. Interplay between conjugative and steric effects in cyclopropylarenes. J. Org. Chem. 1990, 55, 4098–4102. https://doi.org/10.1021/jo00300a029.Search in Google Scholar
13. Zeng, H. L., He, Y., Zhang, D. W., Yi, X. G., Yi, Z. Q. Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 415–417. https://doi.org/10.1515/NCRS-2024–0025.10.1515/ncrs-2024-0025Search in Google Scholar
14. Palanisamy, V., Sanphui, P., Prakash, M., Chernyshev, V. Multicomponent solid forms of the uric acid reabsorption inhibitor lesinurad and cocrystal polymorphs with urea: DFT simulation and solubility study. Acta Crystallogr. 2019, C75, 1102–1117. https://doi.org/10.1107/s2053229619008829.Search in Google Scholar PubMed
15. Terruzzi, S., Bellomi, S., Marras, G., Barreca, G., Ventimiglia, G., Cervellino, A., Masciocchi, N. Disclosing the rich crystal chemistry of lesinurad by ab initio laboratory X-ray powder diffraction methods. Cryst. Growth Des. 2018, 18, 6863–6872. https://doi.org/10.1021/acs.cgd.8b01083.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

