Abstract
C10H11ClN4O2, monoclinic, P21/c (no. 14), a = 12.7802(17) Å, b = 7.6211(12) Å, c = 12.0486(17) Å, β = 95.551(4)°, V = 1168.0(3) Å3, Z = 4, R gt (F) = 0.0562, wR ref (F 2) = 0.1172, T = 273(2) K.
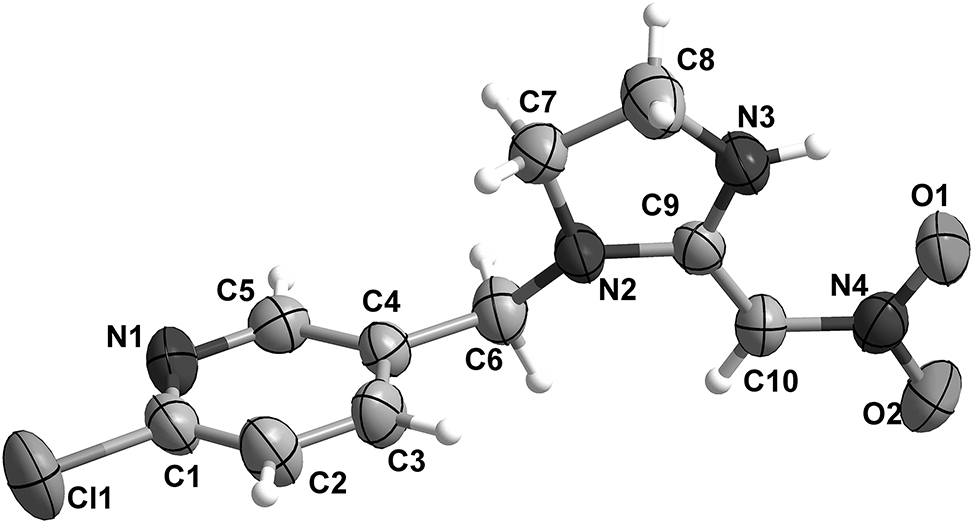
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.32 mm−1 |
| Diffractometer, scan mode: | Bruker Smart Apex II, φ and ω |
| θ max, completeness: | 25.0°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 17,187, 2047, 0.071 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ (I obs), 1242 |
| N(param)refined: | 154 |
| Programs: | Bruker [1], Olex2 [2], Shelx [3, 4], Diamond [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Cl1 | −0.05579 (8) | −0.73366 (15) | 0.85293 (8) | 0.0838 (4) |
| O1 | 0.53016 (17) | −0.8124 (3) | 0.28131 (19) | 0.0634 (7) |
| O2 | 0.5971 (2) | −0.5955 (3) | 0.3834 (2) | 0.0741 (8) |
| N1 | 0.1315 (2) | −0.8538 (4) | 0.8340 (2) | 0.0520 (7) |
| N2 | 0.31630 (19) | −0.9053 (3) | 0.5092 (2) | 0.0477 (7) |
| N3 | 0.3704 (2) | −0.9947 (4) | 0.3525 (2) | 0.0540 (7) |
| H3 | 0.407054 | −0.999009 | 0.296406 | 0.065* |
| N4 | 0.5304 (2) | −0.7188 (4) | 0.3678 (2) | 0.0533 (7) |
| C1 | 0.0537 (3) | −0.7680 (5) | 0.7795 (3) | 0.0518 (9) |
| C2 | 0.0527 (3) | −0.7055 (5) | 0.6729 (3) | 0.0618 (10) |
| H2 | −0.006112 | −0.649300 | 0.637910 | 0.074* |
| C3 | 0.1421 (3) | −0.7291 (4) | 0.6195 (3) | 0.0560 (9) |
| H3A | 0.145117 | −0.686529 | 0.547542 | 0.067* |
| C4 | 0.2275 (2) | −0.8162 (4) | 0.6730 (2) | 0.0425 (8) |
| C5 | 0.2170 (2) | −0.8778 (4) | 0.7788 (2) | 0.0463 (8) |
| H5 | 0.272945 | −0.940372 | 0.814573 | 0.056* |
| C6 | 0.3296 (2) | −0.8386 (5) | 0.6230 (2) | 0.0533 (9) |
| H6A | 0.365395 | −0.726346 | 0.623439 | 0.064* |
| H6B | 0.373882 | −0.919001 | 0.668914 | 0.064* |
| C7 | 0.2547 (3) | −1.0645 (4) | 0.4823 (3) | 0.0552 (9) |
| H7A | 0.179953 | −1.041521 | 0.480825 | 0.066* |
| H7B | 0.273775 | −1.157219 | 0.535618 | 0.066* |
| C8 | 0.2846 (3) | −1.1123 (5) | 0.3672 (3) | 0.0741 (12) |
| H8A | 0.306815 | −1.233801 | 0.364420 | 0.089* |
| H8B | 0.226358 | −1.093064 | 0.310714 | 0.089* |
| C9 | 0.3868 (2) | −0.8800 (4) | 0.4345 (2) | 0.0419 (8) |
| C10 | 0.4627 (2) | −0.7474 (4) | 0.4453 (3) | 0.0488 (8) |
| H10 | 0.466792 | −0.676188 | 0.508198 | 0.059* |
1 Source of materials
We started the synthesis of 2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine from 1,1-dimethylthio-2-nitroethylene and N-2(chloro-5-pyridylmethyl)ethylenediamine according to the literature reported by Li [6]. A mixture of 1,1-dimethylthio-2-nitroethylene (662 mg 4.0 mmol) prepared from nitromethane and carbon disulfide and N-2(chloro-5-pyridylmethyl)ethylenediamine (754 mg 4.1 mmol) in anhydrous ethanol (9 mL) was refluxed for 8 h affording a clear yellow solution. The solvent was removed in vacuo after cooling to ambient temperature. The crude product was purified by silica gel chromatography using dichloromethane:acetone (4:1, v/v) as eluant to give a white power. Then a certain amount of white powder was dissolved in ethyl acetate. After solvent slowly evaporated at room temperature, the crystals were obtained.
2 Experimental details
The structure was solved by Direct Methods with the SHELX program system [3]. All H-atoms were positioned geometrically and refined using a riding model with d(N–H) = 0.86 Å, d(C–H) = 0.93 Å (aromatic and olefin CH), 0.97 Å (methylene CH2). For all H atoms, isotropic displacement parameters were assigned as U iso (H) = 1.2 U eq (N, C).
3 Comment
Nitrogen-containing heterocyclic insecticide has successful established its place in the field of insecticides research and development [7]. Especially, the neonicotinic insecticides are widely concerned and studied in the field of crop protection [8]. In addition, how to construct nitrogen heterocyclic ring and polycyclic compounds is also a classical subject in the field of synthesis. Title compound (I) is a key intermediate in the synthesis of complicated neonicotinic insecticides [8], [9], [10], [11]. It is not only a nitrogen-containing heterocyclic compound in itself, but also a precursor to construct polycyclic compounds through cyclization with unsaturated aldehydes. In view of the excellent potential of this compound, it is worth studying its synthesis optimization and structural characteristics.
The asymmetric unit of (I) is shown in the figure. The C=C bond length is 1.398(4) Å and the C=C double bond in compound (I) will lead to geometrical isomerism. Single crystal X-ray diffraction confirmed that the title compound is the (E)-diastereomer. The C–Cl bond is 1.747(3) Å. The C–N bond lengths are between 1.312(4) and 1.466(4) Å. The N–O bond lengths are between 1.262(3) and 1.270(3) Å. The O–N–O bond angle is 119.2(3)°. All these values are in the normal range [12, 13]. The dihedral angle between the mean planes passing through the pyridine ring and the imidazoline ring is 81.79(3). The C4–C6–N2 bond angle 113.024(6)° is smaller than the value for this bond angle in the similar structure (E)-2-[1-[(6–chloropyridin-3-yl)methyl]-2-imidazolidinylidene]-2-nitroacetonitrile [14]. The torsion angle of C4–C6–N2–C7 is −52.253(9)° which is obviously different with the value 88.1° observed in similar structure 2(E)-2-[1-[(6–chloropyridin-3-yl)methyl]-2-imidazolidinylidene]-2-nitroacetonitrile, which may be due to the space effect and interaction of cyano group in comparative structure. There exist intramolecular interactions [N3⋯O1 = 2.684(4) Å and N3–H3⋯O1 = 120.147(7)°] and intermolecular interactions [N3⋯O2 i = 3.012(3) Å and N3–H3⋯O2 i = 142.543(8)° (i = 1 − X, −1/2 + Y, 1/2 − Z)] between N–H donors from imidazoline ring and the oxygen atoms from nitro group. The presence of intermolecular hydrogen bonds results in the formation of one-dimensional chain-like structure along the (010) direction. There are four non-classical hydrogen bonds between C–H donors and the oxygen atoms from nitro group. The intermolecular interactions [C6⋯O2 ii = 3.441(4) Å and C6–H6A⋯O2 ii = 162.998(6)° (ii = 1 − X, −1 − Y, 1 − Z)] and [C6⋯O1 iii = 3.349(4) Å and C6–H6B⋯O1 iii = 157.636(6)° (iii = 1 − X, −2 − Y, 1 − Z)] lead the extension of one-dimensional chain structure along the (001) direction to two-dimensional (2D) supramolecular layer. The existence of intermolecular interactions [C10⋯O2 ii = 3.462(4) Å and C10–H10⋯O2 ii = 150.724(6)° and C7⋯O2 iii = 3.511(4) Å and C7–H7B⋯O2 iii = 151.176(6)°] enhanced the stability of this structure.
Funding source: Scientific and Technological Research Project of Institutions of Higher Education in Hebei Province
Award Identifier / Grant number: ZC2024070
Funding source: S&T Program of Chengde
Award Identifier / Grant number: 202205B090, HZLC2024020
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The work was supported by Scientific and Technological Research Project of Institutions of Higher Education in Hebei Province (ZC2024070) and S&T Program of Chengde (No. 202205B090, No. HZLC2024020).
References
1. Bruker. SADABS, SAINT and SMART; Bruker AXS Inc.: Madison, Wisconsin, USA, 2008.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. Olex2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. A short history of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
6. Li, C., Xu, X. Y., Li, J. Y., Ye, Q. F., Li, Z. Synthesis and chiral purification of 14C-labeled novel neonicotinoids, paichongding. J. Label. Compd. Radiopharm. 2011, 54, 775–779; https://doi.org/10.1002/jlcr.1921.Search in Google Scholar
7. Higasio, Y. S., Shoji, T. Heterocyclic compounds such as pyrroles, pyridines, pyrollidins, piperdines, indoles, imidazol and pyrazins. Appl. Catal. A Gen. 2001, 221, 197–207; https://doi.org/10.1016/s0926-860x(01)00815-8.Search in Google Scholar
8. Shao, X. S., Ye, Z. J., Bao, H. B., Liu, Z. W., Xu, X. Y., Li, Z., Qian, X. H. Advanced research on cis-neonicotinoids. Chimia Int. J. Chem. 2011, 65, 957–960; https://doi.org/10.2533/chimia.2011.957.Search in Google Scholar PubMed
9. Cui, S. X., Zhang, G. Y., Tian, Z. Z. 1-[(6–Chloropyridin-3-yl)methyl]-10-nitro-1,2,3,5,6,7,8,9-octahydro-5,9-methanoimidazo[1,2-a]azocin-5-ol. Acta Crystallogr. 2013, E69, o1015; https://doi.org/10.1107/s1600536813014402.Search in Google Scholar PubMed PubMed Central
10. Tian, Z. Z., Li, D. M., Li, Z. 1-[(6–Chloro-3-pyridyl)methyl]-5-ethoxy-8-nitro-1,2,3,5,6,7-hexahydroimidazo-[1,2-a]pyridine. Acta Crystallogr. 2009, E65, o2517; https://doi.org/10.1107/s1600536809037660.Search in Google Scholar
11. Shao, X. S., Fu, H., Xu, X. Y., Xu, X. L., Liu, Z. W., Li, Z., Qian, X. H. Divalent and oxabridged neonicotinoids constructed by dialdehydes and nitromethylene analogues of imidacloprid: design, synthesis, crystal structure, and insecticidal activities. J. Agric. Food Chem. 2010, 58, 2696–2702; https://doi.org/10.1021/jf902531y.Search in Google Scholar PubMed
12. Tian, Z., Dong, H., Li, D. Wang, G. (E)-1-[(1,3–Dioxan-4-yl)methyl]-2-(nitromethylidene)imidazolidine. Acta Crystallogr. 2010, E66, o2359.10.1107/S1600536810032691Search in Google Scholar PubMed PubMed Central
13. Wang, G., Li, D., Li, H. 2-[(E)-2-(Nitromethylidene)imidazolidin-1-yl]ethanol. Acta Crystallogr. 2010, E66, o2759; https://doi.org/10.1107/s1600536810039280.Search in Google Scholar
14. Wang, K. W., Qian, X. H., Cui, J. N. Design, synthesis, and bioactivity of cyanonitrovinyl neonicotinoids as potential insecticides. Monatsh. Chem. 2010, 141, 1117–1122; https://doi.org/10.1007/s00706-010-0375-4.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

