Abstract
C6H6N2O2, monoclinic P21/c (no. 14), a = 6.7909(2) Å, b = 23.9261(7) Å, c = 7.5103(2) Å, β = 95.265(2)°, V = 1215.12(6) Å3, Z = 8, R gt(F) = 0.0574, wR ref(F 2) = 0.1439, T = 293.
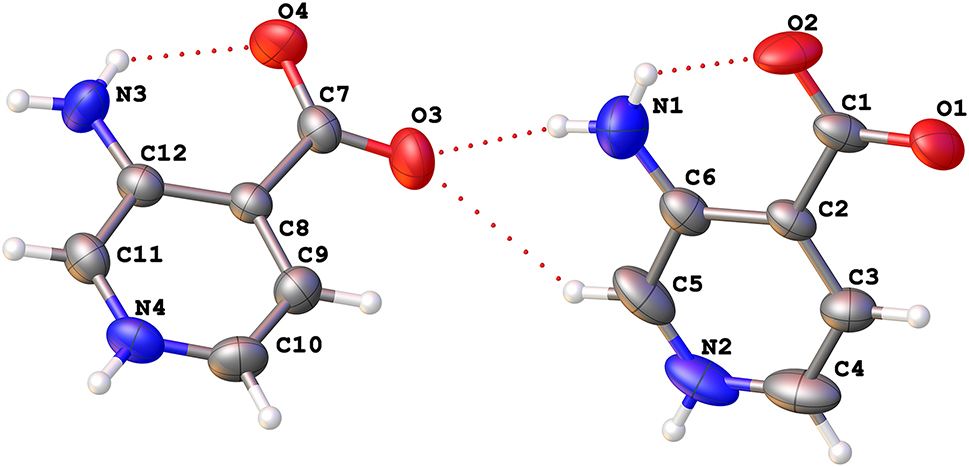
The crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow plate |
| Size: | 0.15 × 0.13 × 0.09 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.99 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω-scans |
| θ max, completeness: | 71,5°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 5419, 2278, 0.033 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 1330 |
| N(param)refined: | 181 |
| Programs: | CrysAlisPRO [1], OLEX2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | U iso*/U eq | |
|---|---|---|---|---|
| O1 | 0.7517 (3) | 0.78844 (11) | 1.1216 (3) | 0.0529 (6) |
| O2 | 0.7655 (3) | 0.69649 (11) | 1.1192 (3) | 0.0599 (7) |
| N1 | 0.4589 (5) | 0.63808 (13) | 0.9789 (4) | 0.0659 (9) |
| H1A | 0.393541 | 0.608200 | 0.949171 | 0.079* |
| H1B | 0.575262 | 0.635733 | 1.034047 | 0.079* |
| N2 | 0.1042 (3) | 0.73829 (14) | 0.8086 (3) | 0.0583 (8) |
| H2 | −0.012341 | 0.737810 | 0.753127 | 0.070* |
| C1 | 0.6792 (4) | 0.74113 (14) | 1.0819 (4) | 0.0386 (7) |
| C2 | 0.4735 (3) | 0.73988 (13) | 0.9852 (3) | 0.0329 (6) |
| C3 | 0.3766 (4) | 0.78897 (14) | 0.9390 (4) | 0.0432 (7) |
| H3 | 0.436982 | 0.823053 | 0.968251 | 0.052* |
| C4 | 0.1877 (4) | 0.78778 (19) | 0.8483 (4) | 0.0601 (9) |
| H4 | 0.121445 | 0.820798 | 0.816118 | 0.072* |
| C5 | 0.1904 (4) | 0.69034 (18) | 0.8498 (4) | 0.0560 (10) |
| H5 | 0.124523 | 0.657174 | 0.818855 | 0.067* |
| C6 | 0.3790 (4) | 0.68844 (14) | 0.9391 (4) | 0.0398 (7) |
| O3 | 0.2262 (3) | 0.54899 (10) | 0.8283 (3) | 0.0542 (6) |
| O4 | 0.2557 (4) | 0.45760 (10) | 0.8737 (3) | 0.0542 (6) |
| N3 | 0.2149 (4) | 0.38967 (11) | 0.5879 (3) | 0.0510 (7) |
| H3A | 0.206906 | 0.358277 | 0.531879 | 0.061* |
| H3B | 0.210339 | 0.390469 | 0.701898 | 0.061* |
| N4 | 0.2533 (3) | 0.48043 (12) | 0.2136 (3) | 0.0430 (6) |
| H4A | 0.257206 | 0.476959 | 0.099979 | 0.052* |
| C7 | 0.2422 (4) | 0.50017 (12) | 0.7751 (3) | 0.0348 (6) |
| C8 | 0.2440 (3) | 0.49113 (12) | 0.5756 (3) | 0.0298 (6) |
| C9 | 0.2558 (4) | 0.53756 (14) | 0.4669 (4) | 0.0381 (6) |
| H9 | 0.261222 | 0.573126 | 0.517130 | 0.046* |
| C10 | 0.2597 (4) | 0.53129 (15) | 0.2837 (4) | 0.0458 (7) |
| H10 | 0.266691 | 0.562496 | 0.210724 | 0.055* |
| C11 | 0.2413 (4) | 0.43485 (14) | 0.3112 (4) | 0.0395 (7) |
| H11 | 0.237271 | 0.400159 | 0.255112 | 0.047* |
| C12 | 0.2343 (4) | 0.43735 (13) | 0.4974 (3) | 0.0335 (6) |
1 Source of materials
Method-1: A mixture of FeSO4·7H2O (0.1190 g) and 3-aminoisonicotinic acid (0.0690 g) was stirred in 15 mL aqueous solution for 6 h at 60 °C. Then the resulting mixture slowly cooled to room temperature was filtrated and volatilized in the air. The yellow stick crystals of the titled compound were isolated directly.
Method-2: Cadmium acetate dihydrate (0.0533 g) and 3-aminoisonicotinic acid (0.0552 g) in a mixture of H2O (6 mL) and ethanol (3 mL) were sonicated for 1 h, then two drops of 1 mol/L HNO3 were added into the mixture. The homogeneous mixture was sealed in a 20 mL solvothermal vial, and heated at 120 °C for 48 h, then cooled to room temperature at 5 °C/h. The powder XRD of obtained yellow stick crystals is identical to that prepared in the method-1.
2 Experimental details
Crystal data, data collection and structure refinement details are provided in Table 1. All hydrogen atoms were placed in calculated positions and refined using a riding-model approximation with C–H = 0.93 Å and Uiso(H) = 1.2Ueq(C), N–H = 0.86 Å and Uiso(H) = 1.2Ueq(N).
3 Comment
Amino-functionalized isonicotinic acids are important medical intermediates and isoniazid derivatives in the synthesis and pharmacology [5]. In addition, as the one-center-acceptor–donor ligand they can react with the metal ion to construct a series of unique diamondoid network metal-organic frameworks (MOFs). For example, the acentric framework reported by Zhou et al. [6] exhibits potential 2nd-harmonic-generation response, ferroelectric behaviors and photoluminescence. Although the crystal structures of nicotinic acid and isonicotinic acid as pure organic room-temperature phosphorescences have been reported in the neutral form and zwitterion [7, 8], however, the crystal structure of amino-functionalized isonicotinic acid is not available. In our attempt to synthesize the MOFs containing the 3-aminoisonicotinic acid, we unexpectedly obtained the single-crystal of 3-aminoisonicotinic acid.
The determined results indicated that 3-aminoisonicotinic acid crystallizes as a zwitterion in the space group P21/c without any other solvent molecules, which often were directly involved in the formation of hydrogen bonds or as the filler of void space. Its asymmetric unit includes two independent 3-aminoisonicotinic acids. The pyridine ring, the carboxylate group, and the –NH2 group are in the same plane, similar to bis(4-aminopyridin-1-ium) iodide triiodide [9]. The existence of the zwitterionic form of 3-aminoisonicotinic acid is confirmed by the presence of an H atom bonded to the N1 or N4 atom in the difference Fourier map. All of the C–O band distances, less than 1.27 Å, also agree with this result. Specifically, the C1–O1, C1–O2, C7–O4, and C7–O3 bond distances are 1.259(4), 1.238(4), 1.258(3), and 1.242(3) Å, respectively. In the crystal of 3-aminoisonicotinic acid, all nitrogen and oxygen atoms are involved in N⋯O hydrogen bonds with distances in the range between 2.612(3) Å and 3.317(3) Å. The head-to-tail N2–H2⋯O2 and N2–H2⋯O1 interactions form one chain running parallel to the a axis, and the N⋯O distances are 3.025(4) Å and 2.738(4) Å, respectively. Another chain formed by very strong head-to-tail N4–H4A⋯O4 interactions with a N⋯O distance of 2.612(3) Å extends along the c axis. These two kinds of chains are mutually perpendicular and further interact by the N–H⋯O hydrogen bonds between carboxyl groups and neighboring amino groups. In addition, the weak C–H⋯O hydrogen bonds are also found in the crystal packing. The combination of these interactions builds an intricate three-dimensional assembly of a hydrogen bond network. Theoretical calculations at the ωB97XD/6–311++G** level have shown that in the gas phase the energy of the neutral form of 3-aminonicotinic acid is lower by about 92 kJ/mol than that of inner salt. However, in water the zwitterion becomes the favorable form with a difference in energy of 14 kJ/mol. From a structural point of view, zwitterion is stabilized by the strong head-to-tail N–H⋯O hydrogen bonds between the –NH2 and carboxyl group. This is in agreement with the calculated changes in bond distance and other reported spectrophoto-chemical results in the case of 2-aminonicotinic acid with a similar structure [10].
Funding source: The School-level Innovation Fund project of Xinxiang Institute of Engineering
Award Identifier / Grant number: (2023ZK-1)
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The School-level Innovation Fund project of Xinxiang Institute of Engineering (2023ZK-1) is gratefully acknowledged.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton: Oxfordshire, England, 2017.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXT – integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
5. Ni, T., Xie, F., Li, L., Hao, Y., Chi, X., Yan, L., Zhang, D., Jiang, Y., Lv, Q. Design, synthesis and structure-activity relationship studies of nicotinamide derivatives as potent antifungal agents by disrupting cell wall. Molecules 2023, 28, 1135; https://doi.org/10.3390/molecules28031135.Search in Google Scholar PubMed PubMed Central
6. Zhou, W. W., Wei, B., Wang, F. W., Fang, W. Y., Liu, D. F., Wei, Y. J., Xu, M., Zhao, X., Zhao, W. An acentric 3-D metal-organic framework with threefold interpenetrated diamondoid network: second-harmonic-generation response, potential ferroelectric property and photoluminescence. RSC Adv. 2015, 5, 100956–100959; https://doi.org/10.1039/c5ra20719c.Search in Google Scholar
7. Chen, Y., Xie, Y., Li, Z. Room-temperature phosphorescence of nicotinic acid and isonicotinic acid: efficient intermolecular hydrogen-bond interaction in molecular array. J. Phys. Chem. Lett. 2022, 13, 1652–1659; https://doi.org/10.1021/acs.jpclett.2c00118.Search in Google Scholar PubMed
8. Takusagawa, F., Shimada, A. Isonicotinic acid. Acta Crystallogr. 1976, B32, 1925–1927; https://doi.org/10.1107/s0567740876006754.Search in Google Scholar
9. Reis, G. J., Leske, P. B. The twinned crystal structure of bis(4-aminopyridin-1-ium) iodide triiodide. Z. Kristallogr. N. Cryst. Struct. 2014, 229, 452–454.10.1515/ncrs-2014-0193Search in Google Scholar
10. Hirai, E. The behavior of 4-amino-5-carboxy-2-methylpyrimidine in aqueous solution. Chem. Pharm. Bull. 1966, 14, 861–865; https://doi.org/10.1248/cpb.14.861.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

