Abstract
C17H18F6N6O, monoclinic, P21/n (no. 14), a = 9.9491(11) Å, b = 18.1523(17) Å, c = 11.7703(15) Å, β = 113.473(14)°, V = 1949.8(4) Å3, Z = 4, Rgt(F) = 0.0606, wRref(F2) = 0.1876, T = 169.99(10) K.
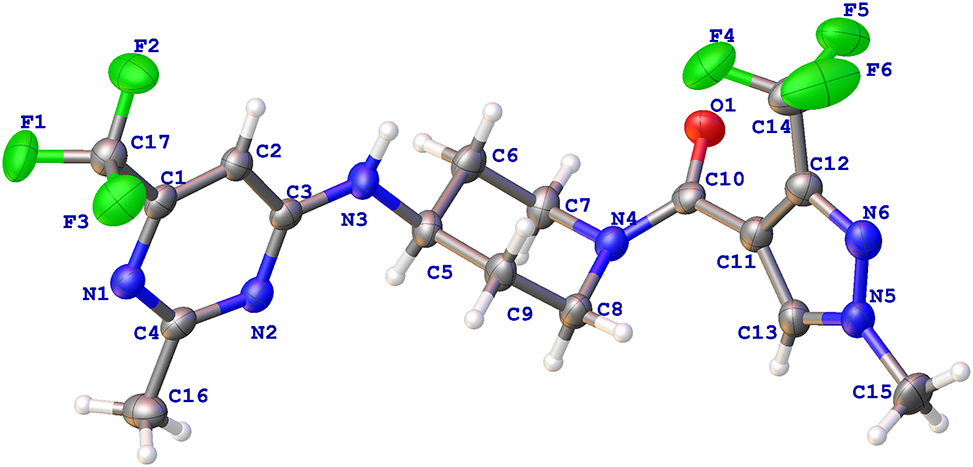
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.15 × 0.13 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.14 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 25.0°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 7948, 3409, 0.052 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2543 |
| N(param)refined: | 278 |
| Programs: | Olex2 [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| F1 | 0.2465 (2) | 0.51045 (11) | −0.11940 (17) | 0.0504 (5) |
| F2 | 0.24580 (19) | 0.61700 (10) | −0.04247 (18) | 0.0522 (6) |
| F3 | 0.12910 (17) | 0.52834 (11) | −0.00436 (18) | 0.0523 (6) |
| F4 | 0.7062 (2) | 0.82670 (12) | 0.69787 (19) | 0.0599 (6) |
| F5 | 0.8248 (2) | 0.89407 (10) | 0.8558 (2) | 0.0589 (6) |
| F6 | 0.6012 (2) | 0.86766 (14) | 0.8133 (3) | 0.0855 (9) |
| O1 | 1.0177 (2) | 0.75617 (11) | 0.7954 (2) | 0.0397 (6) |
| N1 | 0.4115 (2) | 0.45207 (12) | 0.1128 (2) | 0.0292 (6) |
| N2 | 0.6078 (2) | 0.48127 (12) | 0.3068 (2) | 0.0284 (6) |
| N3 | 0.6610 (3) | 0.60361 (14) | 0.3682 (2) | 0.0318 (6) |
| N4 | 0.9274 (2) | 0.64138 (12) | 0.7422 (2) | 0.0293 (6) |
| N5 | 0.7848 (3) | 0.70002 (13) | 1.0376 (2) | 0.0368 (7) |
| N6 | 0.7239 (3) | 0.76384 (13) | 0.9829 (2) | 0.0357 (6) |
| C1 | 0.3853 (3) | 0.52515 (15) | 0.0957 (3) | 0.0268 (6) |
| C2 | 0.4649 (3) | 0.57809 (15) | 0.1748 (3) | 0.0263 (6) |
| H2 | 0.445537 | 0.627914 | 0.157563 | 0.032* |
| C3 | 0.5787 (3) | 0.55357 (15) | 0.2848 (3) | 0.0253 (6) |
| C4 | 0.5240 (3) | 0.43438 (15) | 0.2191 (3) | 0.0285 (7) |
| C5 | 0.7685 (3) | 0.58740 (15) | 0.4919 (3) | 0.0288 (6) |
| H5 | 0.805980 | 0.537410 | 0.492713 | 0.035* |
| C6 | 0.8961 (3) | 0.64198 (16) | 0.5253 (3) | 0.0330 (7) |
| H6A | 0.946610 | 0.634125 | 0.470772 | 0.040* |
| H6B | 0.857985 | 0.691854 | 0.512488 | 0.040* |
| C7 | 1.0039 (3) | 0.63324 (17) | 0.6586 (3) | 0.0320 (7) |
| H7A | 1.049562 | 0.585058 | 0.669935 | 0.038* |
| H7B | 1.080190 | 0.670224 | 0.678041 | 0.038* |
| C8 | 0.8122 (3) | 0.58636 (15) | 0.7195 (3) | 0.0333 (7) |
| H8A | 0.764903 | 0.594110 | 0.776438 | 0.040* |
| H8B | 0.855022 | 0.537460 | 0.734036 | 0.040* |
| C9 | 0.6995 (3) | 0.59187 (16) | 0.5874 (3) | 0.0317 (7) |
| H9A | 0.647243 | 0.638177 | 0.577052 | 0.038* |
| H9B | 0.628865 | 0.552307 | 0.572129 | 0.038* |
| C10 | 0.9399 (3) | 0.70520 (14) | 0.8037 (3) | 0.0272 (6) |
| C11 | 0.8597 (3) | 0.71327 (15) | 0.8863 (3) | 0.0304 (7) |
| C12 | 0.7713 (3) | 0.77274 (15) | 0.8935 (3) | 0.0320 (7) |
| C13 | 0.8640 (3) | 0.66829 (16) | 0.9820 (3) | 0.0344 (7) |
| H13 | 0.913321 | 0.623606 | 1.004146 | 0.041* |
| C14 | 0.7260 (3) | 0.83939 (19) | 0.8157 (3) | 0.0459 (9) |
| C15 | 0.7510 (4) | 0.67171 (19) | 1.1392 (3) | 0.0509 (9) |
| H15A | 0.708849 | 0.710276 | 1.170267 | 0.076* |
| H15B | 0.839309 | 0.654452 | 1.204384 | 0.076* |
| H15C | 0.682554 | 0.631751 | 1.109862 | 0.076* |
| C16 | 0.5581 (3) | 0.35447 (16) | 0.2419 (3) | 0.0413 (8) |
| H16A | 0.569145 | 0.333180 | 0.171406 | 0.062* |
| H16B | 0.479630 | 0.330232 | 0.254854 | 0.062* |
| H16C | 0.647498 | 0.348494 | 0.313937 | 0.062* |
| C17 | 0.2527 (3) | 0.54602 (16) | −0.0180 (3) | 0.0332 (7) |
| H3 | 0.631 (4) | 0.652 (2) | 0.360 (3) | 0.057 (10)* |
1 Source of materials
4-chloro-2-methyl-6-(trifluoromethyl)pyrimidine (10 mmol), and tert-butyl 4-aminopiperidine-1-carboxylate (8 mmol) were dissolved in isopropanol (10 mL). Then N,N-diisopropylethylamine (DIPEA, 10 mmol) was added slowly. The reaction was refluxed for 5 h. After completion, the reaction was cooled to room temperature, and dispersed with ethyl acetate. The solution was washed with distilled water (30 mL) for three times. The organic layers were collected and dried with anhydrous sodium sulfate. After filtering, the solvents were evaporated. The residue was purified by silica gel column chromatography. The obtained solid was deprotected in HCl/ethanol system to get 2-methyl-N-(piperidin-4-yl)-6-(trifluoromethyl)pyrimidin-4-amine. By using 2-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU, 1.5 mmol) as condensing agent, 2-methyl-N-(piperidin-4-yl)-6-(trifluoromethyl)pyrimidin-4-amine (1 mmol) was further reacted with 1-methyl-3-(trifluoromethyl)-1H-pyrazole-4-carboxylic acid (1 mmol) under DIPEA/DMF (2.5 mmol/10 mL) at room temperature. After completion, the distilled water was added to the reaction, and extracted with ethyl acetate for two times. The combined organic layers were further washed with ethyl acetate for five times. Then the solution was dried with anhydrous sodium sulfate. After filtering, the solvents were removed by rotary evaporation. The residue was purified by silica gel column chromatography to obtain a white solid. After slow volatilization of methanol at room temperature, colourless crystals of the title compound were obtained.
2 Experimental details
Coordinates of hydrogen atoms were set. Their Uiso values were set to 1.2Ueq of the parent atoms. Using Olex2 [1], the structure was solved with the Shelxt [2] structure solution program using and refined with the Shelxl [3] refinement package.
3 Comment
Pyrimidines are extensively presented in fungicides, such as Fenarimol, Mepanioyrim, and Pyrimethanil. Several pyrimidine compounds have been utilized to control plant fungal diseases [4], [5], [6], [7], [8], [9]. Pyrazole as a classical five-membered heterocyclic compound, has been an vital intermediate skeleton of agrochemicals [10], [11], [12]. Many compounds containing pyrazole [13, 14] or pyrimidine [15, 16] moiety were developed. Compared to the reported crystal [17], the title structure was constructed by linking pyrimidine and pyrazole with a bridge group of aminopiperidine [18]. The characteristic C–N bond length of aminopiperidine linked to pyrimidine and pyrazole are 1.348(4) Å (N3–C3) and 1.345(4) Å (N4–C10). The torsion angle of C5–N3–C3–N2 is −9.7(4)°, and the torsion angle of N4–C10–C11–C13 is 54.1(4)°. The dihedral angle between pyrimidine plane and pyrazole plane is 73.75(1)°. The piperidine ring is in a relatively stable chair configuration (cf. the Figure), and the bond angles of N3–C5–C6 and C10–N4–C7 are 109.2(2)° and 119.0(2)°. In addition, the characteristic bond angles in the three rings of N2–C4–N1, C13–N5–N6, and C8–N4–C7 are 126.6(2)°, 112.1(2)°, and 112.7(2)°, respectively. Intermolecular hydrogen bonding exists in the title crystal structure. The oxygen atom O1 provides intermolecular hydrogen bonds to fluoro atom F2 (O1–F2 = 3.424 Å), hydrogen atom H3(O1–H3 = 3.424 Å) and hydrogen atom H15A (O1–H15A = 3.424 Å).
Funding source: Guiyang University Graduate Project
Award Identifier / Grant number: GYU–YJS[2021]-33
Funding source: Guiyang University Initial Funding Project
Award Identifier / Grant number: GYU–ZRD[2018]-023
Funding source: Guizhou Provincial Science and Technology Projects
Award Identifier / Grant number: QKHPR–CXTD[2022]002
Funding source: Education Department of Guizhou Province-Natural Science Research Project
Award Identifier / Grant number: QJJ[2023]042
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Guiyang University Graduate Project (NO. GYU-YJS[2021]-33), Guiyang University Initial Funding Project (NO. GYU-ZRD[2018]-023), Guizhou Provincial Science and Technology Projects (NO. QKHPR-CXTD[2022]002), and Education Department of Guizhou Province-Natural Science Research Project (No. QJJ[2023]042).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Wu, W. N., Jiang, Y. M., Fei, Q., Du, H. T. Synthesis and fungicidal activity of novel 1,2,4-triazole derivatives containing a pyrimidine moiety. Phosphorus, Sulfur, Silicon Relat. Elem. 2019, 194, 1171–1175; https://doi.org/10.1080/10426507.2019.1633321.Search in Google Scholar
5. Zhang, P., Guan, A., Xia, X., Sun, X., Wei, S., Yang, J., Wang, J., Li, Z., Lan, J., Liu, C. Design, synthesis, and structure-activity relationship of new arylpyrazole pyrimidine ether derivatives as fungicides. J. Agric. Food Chem. 2019, 67, 11893–11900; https://doi.org/10.1021/acs.jafc.9b05185.Search in Google Scholar PubMed
6. Hai, D. S., Ha, N., Tung, D. T., Giang, N., Huong, N., Anh, H. H., Van, H., Toan, D., Thanh, N. Synthesis, biological evaluation and induced fit docking simulation study of d-glucose-conjugated 1H-1,2,3-triazoles having 4H-pyrano[2,3-d]pyrimidine ring as potential agents against bacteria and fungi. New J. Chem. 2022, 46, 8303–8323; https://doi.org/10.1039/d1nj05330b.Search in Google Scholar
7. Desai, N. C., Vaghani, H. V., Jethawa, A. M., Khedkar, V. M. In silico molecular docking studies of oxadiazole and pyrimidine bearing heterocyclic compounds as potential antimicrobial agents. Arch. Pharm. 2021, 354, 2100134; https://doi.org/10.1002/ardp.202100134.Search in Google Scholar PubMed
8. Ghoneim, A. A., Bilel, H., Moustafa, S. M. N. Design, synthesis, and antifungal activity of some new thiazolo[4,5-d] pyrimidine-5-thione derivatives. Russ. J. Org. Chem. 2020, 56, 2148–2152; https://doi.org/10.1134/s1070428020120167.Search in Google Scholar
9. Blokhina, S. V., Sharapova, A. V., Ol’khovich, M. V., Doroshenko, I. A., Levshin, I. B., Perlovich, G. L. Synthesis and antifungal activity of new hybrids thiazolo[4,5-d]pyrimidines with (1H-1,2,4)triazole. Bioorg. Med. Chem. Lett. 2021, 40, 127944; https://doi.org/10.1016/j.bmcl.2021.127944.Search in Google Scholar PubMed
10. Xia, D. G., Cheng, X., Liu, X. H., Zhang, C. Q., Wang, Y. X., Liu, Q. Y., Zeng, Q., Huang, N. Q., Cheng, Y., Lv, X. H. Discovery of novel pyrazole carboxylate derivatives containing thiazole as potential fungicides. J. Agric. Food Chem. 2021, 69, 8358–8365; https://doi.org/10.1021/acs.jafc.1c01189.Search in Google Scholar PubMed
11. Halim, K. N. M., Ramadan, S. K., Rizk, S. A., EI-Hashash, M. A. Synthesis, DFT study, molecular docking and insecticidal evaluation of some pyrazole-based tetrahydropyrimidine derivatives. Synth. Commun. 2020, 50, 1159–1175; https://doi.org/10.1080/00397911.2020.1720739.Search in Google Scholar
12. Ahmed, W., Yan, X. J., Hu, D. K., Adnan, M., Tang, R. Y., Cui, Z. N. Synthesis and fungicidal activity of novel pyrazole derivatives containing 5-phenyl-2-furan. Bioorg. Med. Chem. 2019, 27, 115048; https://doi.org/10.1016/j.bmc.2019.115048.Search in Google Scholar PubMed
13. Wei, W., Wu, Q., Jin, T. Y., Zhang, Y. Z., Xuan, Z. K., Zhang, H. Q. Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl) methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 527–529; https://doi.org/10.1515/ncrs-2022-0086.Search in Google Scholar
14. Li, N., Li, Z., Wang, T. C., Miao, J., Wu, H. X., Gao, J. L., Shan, J. C. Crystal structure of N-2,6-difluorobenzoyl-N′-[1-(3-chloro-4-methyl-phenyl)-4-cyano-1H-pyrazol-5-carbamoyl]urea, C19H12ClF2N5O2. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 193–195; https://doi.org/10.1515/ncrs-2022-0569.Search in Google Scholar
15. Shin, S. Y., Koh, D. The crystal structure of 2-(7-(2,3-dimethoxyphenyI)-[1,2,4]triazolo[1,5-a]-pyrimidin-5-yl)-3-methoxyphenol, C20H18N4O4. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 167–169; https://doi.org/10.1515/ncrs-2022-0534.Search in Google Scholar
16. Lou, X. H. Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yD)pyrimidine, C20H15N3. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1037–1038; https://doi.org/10.1515/ncrs-2019-0247.Search in Google Scholar
17. Fang, B., Meng, J. P., Gan, L. L. Crystal structure of methyl 2-(4-(3-(1-methyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidin-6-yI)pheny)acetate, C19H17N5O2. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 575–577; https://doi.org/10.1515/ncrs-2018-0580.Search in Google Scholar
18. Yin, L., Zhang, M. X., He, T. G. Design and development of novel thiazole-sulfonamide derivatives as a protective agent against diabetic cataract in Wistar rats via inhibition of aldose reductase. Heterocycl. Commun. 2021, 27, 63–70; https://doi.org/10.1515/hc-2020-0124.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

