Abstract
BaSO4, orthorhombic, Pnma (no. 62), a = 8.8806(8) Å, b = 5.4539(5) Å, c = 7.1570(7) Å, V = 346.64(6) Å3, Z = 4, Rgt (F 2) = 0.0141, wRref (F 2) = 0.0329, T = 293 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
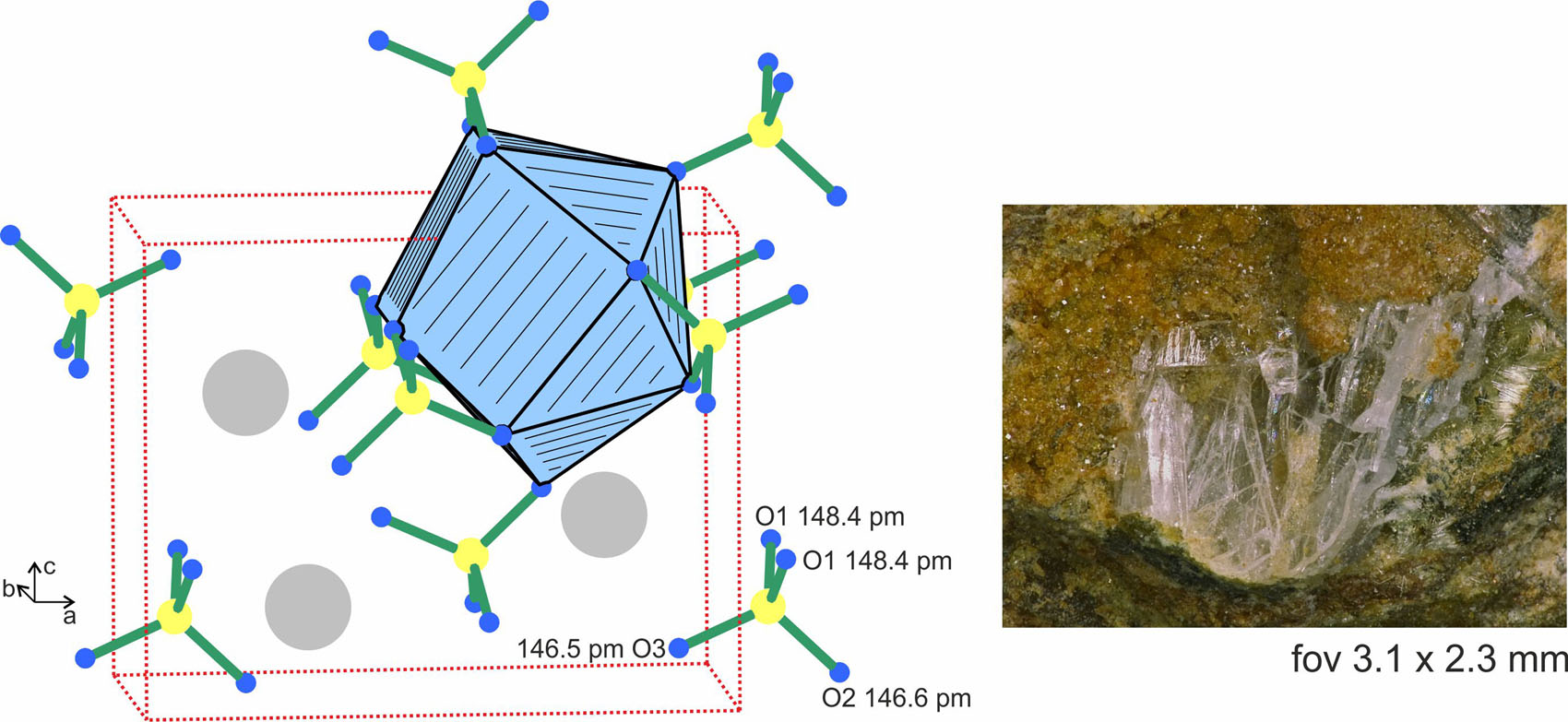
The crystal structure of BaSO4 is shown in the left-hand part of the figure, emphasizing the sulfate groups and the barium coordination. The mineral sample is presented at the right.
Data collection and handling.
| Crystal: | Colorless platelets |
| Size: | 0.05 × 0.04 × 0.03 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 11.9 mm−1 |
| Diffractometer, scan mode: | IPDS-Ⅱ, Stoe |
| θ max, completeness: | 33.4°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 4105, 729, 0.032 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 559 |
| N(param)refined: | 35 |
| Programs: | X-Area [1], JANA2006 [2], SUPERFLIP [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Ba | 0.18452 (2) | 0.25 | 0.65843 (3) | 0.01054 (5) |
| S | 0.06250 (9) | 0.25 | 0.19095 (10) | 0.00902 (17) |
| O1 | 0.08056 (18) | 0.0301 (3) | 0.3111 (2) | 0.0130 (4) |
| O2 | 0.1824 (3) | 0.25 | 0.0501 (3) | 0.0154 (6) |
| O3 | 0.4120 (3) | 0.25 | 0.3927 (4) | 0.0223 (7) |
1 Source of material
Transparent, platelet-shaped baryte crystals (see Figure 1) were selected from a specimen of Mine du Pradet, Cap Garonne (France), which was collected in the Salle du Grand Bassin in the north part of the mine (Mine du Nord). The baryte crystals were accompanied by olivenite and pharmacosiderite.
2 Experimental details
The BaSO4 crystals were selected from the carefully, mechanically fragmented sample. The crystal quality was tested by Laue photographs, using a Buerger camera, which was equipped with an image plate detection system. Single crystal X-ray diffraction data was collected at room temperature on a Stoe IPDS–II diffractometer (graphite monochromatized Mo–K α radiation; oscillation mode). A numerical absorption correction was applied. The starting atomic parameters were deduced with the charge-flipping algorithm [3] implemented in Superflip [4] and the structure was refined on F 2 with the Jana2006 software package [2]. For the final refinement we used the standardized setting listed in the Pearson database [5]. An EDX analyses of the studied crystal gave no hint for contamination with other cations, especially Sr2+, Pb2+ or Cd2+.
3 Comment
Baryte is one of the frequently occurring minerals in Mine du Pradet at Cap Garonne in France [6]. It was already mentioned in the early work of Ferdinand [7] and Lacroix [8]. The present single crystal data are of high quality and fully confirm previous refinements (powder and single crystal X-ray data) on natural [9], [10], [11], [12], [13], [14], [15] and synthetic barium sulfate [16], [17], [18], [19]. The BaSO4 structure (see Figure 1) contains three crystallographically independent oxygen atoms, which lead to S–O distances in the range of 146.5–148.4 pm. The slight orthorhombic distortion is also expressed in the O–S–O angles (107.8–112.4°), which slightly deviate from the ideal tetrahedron angle. The barium atoms are coordinated by 12 oxygen atoms from seven sulfate tetrahedra. Five of these tetrahedra coordinate side-on as chelate ligands and two end-on. The Ba–O distances cover a broader range from 277.5 to 331.4 pm with an average of 295 pm, a consequence of the orthorhombic distortion. It is worthwhile to note, that the BaSO4 structure can geometrically be derived from the NaCl type. The barium and sulfur atoms (i.e. the centers of the sulfate tetrahedra) correspond to the sodium and chlorine sites. The room temperature modification (Pnma phase) shows a strong orthorhombic distortion. Above 1425 ± 5 K [20], BaSO4 transforms to its cubic polymorph with space group
Acknowledgements
We thank Dipl.–Ing. J. Kösters for the intensity data collection.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. X-Area. The Stoe Single Crystal Diffraction Software Package; STOE & Cie GmbH: Darmstadt, Germany.Search in Google Scholar
2. Petříček, V., Dušek, M., Palatinus, L. Crystallographic computing system JANA2006: general features. Z. Kristallogr. 2014, 229, 345–352.10.1515/zkri-2014-1737Search in Google Scholar
3. Palatinus, L. The charge-flipping algorithm in crystallography. Acta Crystallogr. 2013, B69, 1–16; https://doi.org/10.1107/s0108768112051361.Search in Google Scholar
4. Palatinus, L., Chapuis, G. SUPERFLIP – a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790; https://doi.org/10.1107/s0021889807029238.Search in Google Scholar
5. Villars, P., Cenzual, K. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (release 2022/23); ASM International®: Materials Park, Ohio (USA), 2022.Search in Google Scholar
6. Favreau, G., Galéa-Clolus, V. Cap Garonne, Association des Amis de la Mine de Cap Garonne (AAMCG)–Association Française de Microminéralogie (AFM), Couleur et Impression Les Arcades; Castelnau-Le-Lez: France, 2014.Search in Google Scholar
7. Ferdinand, G. Addition aux minéraux de la mine de Cap Garonne (Var). Bull. Minéral. 1893, 16, 40–42.10.3406/bulmi.1893.2308Search in Google Scholar
8. Lacroix, A. Minéralogie de la France et de ces colonies, Vol. IV; Librairie polytechnique Baudry et Cie: Paris, 1910; p. 516.Search in Google Scholar
9. James, R. W., Wood, W. A. The crystal structure of barytes, celestine and anglesite. Proc. R. Soc. Lond. 1925, 109A, 598–620.10.1098/rspa.1925.0148Search in Google Scholar
10. Jellinek, F. On barium manganate and some related compounds. J. Inorg. Nucl. Chem. 1960, 13, 329–331; https://doi.org/10.1016/0022-1902(60)80316-8.Search in Google Scholar
11. Sahl, K. Die Verfeinerung der Kristallstrukturen von PbCl2 (Cotunnit), BaCl2, PbSO4 (Anglesit) und BaSO4 (Baryt). Beiträge Mineral. Petrogr. 1963, 9, 111–132.10.1007/BF02679983Search in Google Scholar
12. Colville, A. A., Staudhammer, K. A refinement of the structure of barite. Am. Mineral. 1967, 52, 1877–1880.Search in Google Scholar
13. Miyake, M., Minato, I., Morikawa, H., Iwai, S.-I. Crystal structures and sulfate force constants of barite, celestite, and anglesite. Am. Mineral. 1978, 63, 506–510.Search in Google Scholar
14. Sawada, H., Takéuchi, Y. The crystal structure of barite, β–BaSO4, at high temperatures. Z. Kristallogr. 1990, 191, 161–171; https://doi.org/10.1524/zkri.1990.191.14.161.Search in Google Scholar
15. Crichton, W. A., Merlini, M., Hanfland, M., Müller, H. The crystal structure of barite, BaSO4, at high pressure. Am. Mineral. 2011, 96, 364–367; https://doi.org/10.2138/am.2011.3656.Search in Google Scholar
16. Frevel, L. K. Indexing powder diffraction patterns of isomorphous substances. J. Appl. Phys. 1942, 13, 109–112; https://doi.org/10.1063/1.1714835.Search in Google Scholar
17. Matsuno, T., Takayanagi, H., Koishi, M., Kurematsu, K., Ogaru, H. The crystal structure of synthetic barium sulfate. Zair. Gijutsu 1986, 4, 409–414.Search in Google Scholar
18. Santamaría-Pérez, D., Gracia, L., Garbarino, G., Beltrán, A., Chuliá-Jordán, R., Gomis, O., Errandonea, D., Ferrer-Roca, Ch., Martínez-Garcíaa, D., Segura, A. High-pressure study of the behavior of mineral barite by X-ray diffraction. Phys. Rev. B 2011, 84, 054102; https://doi.org/10.1103/physrevb.84.054102.Search in Google Scholar
19. Ye, Z., Li, B., Chen, W., Tang, R., Huang, S., Xu, J., Fan, D., Zhou, W., Ma, M., Xie, H. Phase transition and thermoelastic behavior of barite-group minerals at high-pressure and high-temperature conditions. Phys. Chem. Miner. 2019, 46, 607–621; https://doi.org/10.1007/s00269-019-01026-0.Search in Google Scholar
20. Butler, J. C., Sorrell, C. A. Thermal expansion and the orthorhombic-cubic transition of BaSO4 and SrSO4. High Temp. Sci. 1971, 3, 389–400.Search in Google Scholar
21. Sawada, H., Takéuchi, Y. Structure of the high-temperature forms of the barite-type compounds. I: α–BaSO4. Z. Kristallogr. 1987, 181, 179–186; https://doi.org/10.1524/zkri.1987.181.1-4.179.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2

