Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
Abstract
[C40H28F3IrN5O]PF6, monoclinic, P21/c (no. 14), a = 20.2282(19) Å, b = 14.5095(11) Å, c = 12.6091(10) Å, β = 96.937(3)°, V = 3673.7(5) Å3, Z = 4, Rgt(F) = 0.0498, wRref(F2) = 0.1250, T = 107(2) K.
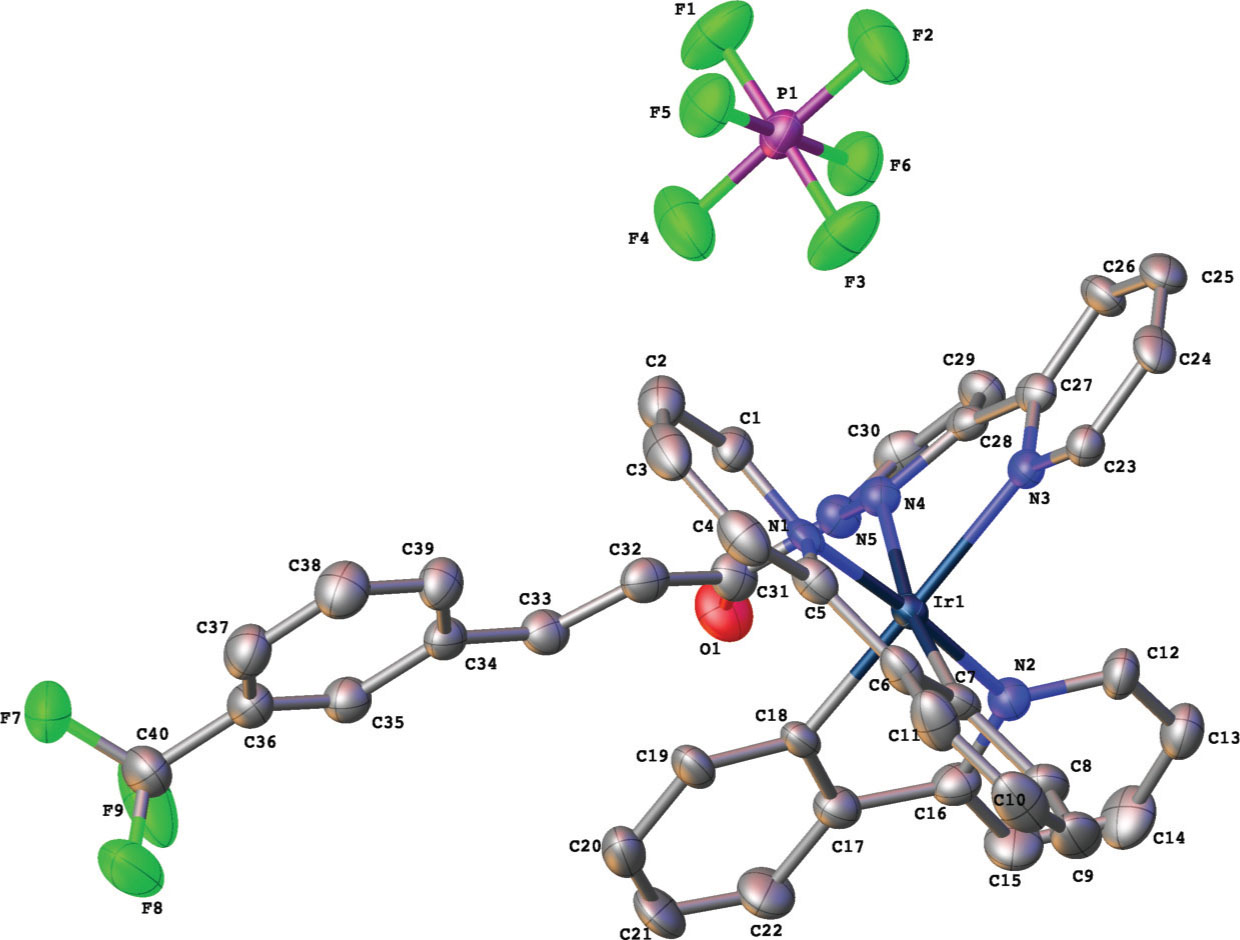
The crystal structure is shown in the figure (hydrogen atoms are omitted for clarity). Table 1 contains crystallographic data and Table 2 shows the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange plate |
| Size: | 0.30 × 0.18 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.77 mm−1 |
| Diffractometer, scan mode: | Bruker D8 QUEST PHOTON 100, φ and ω-scans |
| θmax, completeness: | 28.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 112848, 9126, 0.182 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 6926 |
| N(param)refined: | 496 |
| Programs: | Bruker programs [1], SHELX [2], SHELXT [3], SHELXL [4] and Olex2 [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Ir1 | 0.22452(2) | 0.42037(2) | 0.46589(2) | 0.02025(8) |
| P1 | 0.10114(10) | 0.78265(12) | 0.43177(14) | 0.0346(4) |
| F1 | 0.4711(3) | 0.5441(4) | −0.1143(4) | 0.0725(11) |
| F2 | 0.5112(2) | 0.6570(4) | −0.0172(4) | 0.0638(10) |
| F3 | 0.4420(3) | 0.6840(4) | −0.1540(4) | 0.0725(11) |
| F4 | 0.1493(2) | 0.7922(4) | 0.5429(4) | 0.0638(10) |
| F5 | 0.1008(3) | 0.8932(3) | 0.4195(4) | 0.0665(10) |
| F6 | 0.0532(3) | 0.7725(3) | 0.3191(4) | 0.0665(10) |
| F7 | 0.1022(2) | 0.6740(3) | 0.4440(4) | 0.0509(11) |
| F8 | 0.1649(3) | 0.7771(4) | 0.3704(4) | 0.0704(16) |
| F9 | 0.0364(3) | 0.7871(5) | 0.4928(4) | 0.0777(18) |
| O1 | 0.4095(2) | 0.6461(4) | 0.5037(4) | 0.0459(12) |
| N1 | 0.1617(2) | 0.4429(3) | 0.3292(4) | 0.0225(10) |
| N2 | 0.2915(2) | 0.3892(4) | 0.5963(4) | 0.0260(10) |
| N3 | 0.1432(2) | 0.4576(3) | 0.5522(4) | 0.0232(10) |
| N4 | 0.2473(2) | 0.5635(3) | 0.5201(4) | 0.0231(10) |
| N5 | 0.3060(3) | 0.6123(4) | 0.5394(4) | 0.0273(11) |
| C1 | 0.1477(3) | 0.5262(5) | 0.2859(5) | 0.0294(13) |
| H1 | 0.167010 | 0.579251 | 0.321376 | 0.035* |
| C2 | 0.1057(3) | 0.5376(6) | 0.1906(5) | 0.0389(16) |
| H2 | 0.096323 | 0.597160 | 0.161165 | 0.047* |
| C3 | 0.0784(3) | 0.4599(6) | 0.1407(5) | 0.0435(18) |
| H3 | 0.050881 | 0.465149 | 0.074322 | 0.052* |
| C4 | 0.0906(3) | 0.3745(5) | 0.1863(5) | 0.0388(16) |
| H4 | 0.070655 | 0.321279 | 0.152202 | 0.047* |
| C5 | 0.1319(3) | 0.3659(4) | 0.2817(5) | 0.0280(13) |
| C6 | 0.1484(3) | 0.2813(4) | 0.3409(5) | 0.0305(14) |
| C7 | 0.1947(3) | 0.2910(4) | 0.4335(5) | 0.0268(12) |
| C8 | 0.2114(4) | 0.2122(4) | 0.4949(6) | 0.0365(15) |
| H8 | 0.242767 | 0.216636 | 0.557168 | 0.044* |
| C9 | 0.1827(4) | 0.1275(5) | 0.4657(7) | 0.0445(18) |
| H9 | 0.194787 | 0.074523 | 0.507914 | 0.053* |
| C10 | 0.1368(4) | 0.1197(5) | 0.3760(7) | 0.051(2) |
| H10 | 0.117098 | 0.061644 | 0.356780 | 0.061* |
| C11 | 0.1196(4) | 0.1961(5) | 0.3144(6) | 0.0412(17) |
| H11 | 0.087688 | 0.190632 | 0.253001 | 0.049* |
| C12 | 0.2755(3) | 0.3808(5) | 0.6968(5) | 0.0325(14) |
| H12 | 0.230190 | 0.386402 | 0.708716 | 0.039* |
| C13 | 0.3228(4) | 0.3645(5) | 0.7822(5) | 0.0387(16) |
| H13 | 0.310367 | 0.358296 | 0.852184 | 0.046* |
| C14 | 0.3886(4) | 0.3572(6) | 0.7648(6) | 0.0487(19) |
| H14 | 0.422329 | 0.346798 | 0.822753 | 0.058* |
| C15 | 0.4045(4) | 0.3651(6) | 0.6619(6) | 0.0476(19) |
| H15 | 0.449641 | 0.360014 | 0.648718 | 0.057* |
| C16 | 0.3553(3) | 0.3805(4) | 0.5780(5) | 0.0315(14) |
| C17 | 0.3651(3) | 0.3856(5) | 0.4643(5) | 0.0327(14) |
| C18 | 0.3059(3) | 0.3957(4) | 0.3930(5) | 0.0256(12) |
| C19 | 0.3113(4) | 0.3906(4) | 0.2832(5) | 0.0326(14) |
| H19 | 0.272104 | 0.393484 | 0.233446 | 0.039* |
| C20 | 0.3726(4) | 0.3813(5) | 0.2461(6) | 0.0452(18) |
| H20 | 0.375261 | 0.378041 | 0.171542 | 0.054* |
| C21 | 0.4304(4) | 0.3769(6) | 0.3182(7) | 0.053(2) |
| H21 | 0.472531 | 0.373867 | 0.292291 | 0.064* |
| C22 | 0.4271(4) | 0.3769(6) | 0.4260(7) | 0.0485(19) |
| H22 | 0.466570 | 0.371002 | 0.474653 | 0.058* |
| C23 | 0.0857(3) | 0.4111(4) | 0.5534(5) | 0.0248(12) |
| H23 | 0.079891 | 0.354648 | 0.515373 | 0.030* |
| C24 | 0.0349(3) | 0.4422(5) | 0.6077(5) | 0.0342(15) |
| H24 | −0.004850 | 0.407261 | 0.607790 | 0.041* |
| C25 | 0.0425(3) | 0.5248(5) | 0.6618(5) | 0.0361(15) |
| H25 | 0.007766 | 0.547742 | 0.698943 | 0.043* |
| C26 | 0.1008(3) | 0.5739(5) | 0.6618(5) | 0.0320(14) |
| H26 | 0.106896 | 0.630743 | 0.699077 | 0.038* |
| C27 | 0.1502(3) | 0.5393(4) | 0.6068(4) | 0.0247(12) |
| C28 | 0.2125(3) | 0.5862(4) | 0.5992(5) | 0.0246(12) |
| C29 | 0.2475(3) | 0.6510(4) | 0.6686(5) | 0.0317(14) |
| H29 | 0.232979 | 0.679306 | 0.729698 | 0.038* |
| C30 | 0.3062(3) | 0.6640(4) | 0.6293(5) | 0.0345(14) |
| H30 | 0.341533 | 0.702626 | 0.659435 | 0.041* |
| C31 | 0.3547(3) | 0.6205(4) | 0.4665(5) | 0.0313(14) |
| C32 | 0.3323(3) | 0.6062(4) | 0.3529(5) | 0.0319(14) |
| H32 | 0.287208 | 0.590623 | 0.330190 | 0.038* |
| C33 | 0.3752(3) | 0.6149(4) | 0.2821(5) | 0.0319(13) |
| H33 | 0.420118 | 0.627154 | 0.309571 | 0.038* |
| C34 | 0.3606(3) | 0.6077(4) | 0.1661(5) | 0.0303(13) |
| C35 | 0.4135(3) | 0.6175(5) | 0.1033(5) | 0.0347(14) |
| H35 | 0.457633 | 0.626638 | 0.136739 | 0.042* |
| C36 | 0.4009(3) | 0.6139(5) | −0.0062(5) | 0.0352(14) |
| C37 | 0.3378(4) | 0.5999(5) | −0.0565(6) | 0.0411(16) |
| H37 | 0.330251 | 0.596262 | −0.132188 | 0.049* |
| C38 | 0.2852(4) | 0.5913(5) | 0.0030(7) | 0.0440(17) |
| H38 | 0.241244 | 0.583123 | −0.031720 | 0.053* |
| C39 | 0.2966(3) | 0.5945(5) | 0.1137(6) | 0.0348(15) |
| H39 | 0.260253 | 0.587542 | 0.154236 | 0.042* |
| C40 | 0.4571(4) | 0.6230(5) | −0.0728(6) | 0.0429(17) |
Source of material
3-(Dimethylamino)-1-(2-pyridyl)-2-propen-1-one, hydrazine hydrate, 3-(trifluoromethyl)cinnamoyl chloride, iridium trichloride hydrate, 2-phenylpyridine, ammonium hexafluorophosphate and solvents were purchased and used without further purification.
The synthesis of the complex involves a total of four steps.
In the first step, 2-(1H-pyrazol-3-yl)pyridine (PyPzH) was synthesised according to the literature [5]. Subsequently, PyPzH was reacted with 3-(trifluoromethyl)cinnamoyl chloride to produce 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl)pyrazole (3-CF3CnPyPz) [6], [7], [8].
Next, iridium trichloride hydrate (0.352 g, 1.0 mmol) with 2-phenylpyridine (0.388 g, 2.5 mmol) were dissolved in a mixture of 2-ethoxyethanol (30 mL) and water (10 mL), and then refluxed for 24 h. The solution was cooled to room temperature, and the resulting yellow precipitate was collected by filtration through a Büchner funnel. The precipitate was washed with an excess of water and dried under vacuum. The crude cyclometalated chlorido-bridged dimer was directly used for the next step without purification.
In the final step, the aforementioned chlorido-bridged dimer (1.0 mmol) and 3-CF3CnPyPz ligand (2.5 mmol) were dissolved in dichloromethane (30 mL). The solution was refluxed under argon for 7 h. After cooling to room temperature, ammonium hexafluorophosphate (NH4PF6) in methanol (5 mL) was added and the mixture was stirred for 15 min. The solvent was then reduced under atmospheric pressure and the crude product was purified by column chromatography on silica gel with CH2Cl2/CH3OH (98:2) as eluent. The complex was obtained as orange powders. Yield: 67%. IR (ATR, cm−1): 1716 [ν(C=O)], 1605 [ν(C=Npyridine)], 1476 [ν(C=Cpyridine)], 832 [ν(PF6)]. 1H NMR (CDCl3, 400 MHz) δ (ppm): 8.35 (d, J = 7.6 Hz, 1H), 8.31 (d, J = 3.2 Hz, 1H), 8.16 (td, J = 8.0 Hz, 1H), 8.05 (d, J = 5.6 Hz, 1H), 8.02 (d, J = 8.0 Hz, 1H), 7.87–7.93 (m, 2H), 7.70–7.77 (m, 3H), 7.65 (d, J = 7.6 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H), 7.49–7.52 (m, 3H), 7.42–7.45 (m, 1H), 7.36–7.38 (m, 2H), 7.21–7.26 (m, 2H), 7.03–7.10 (m, 2H), 6.90 (td, J = 7.6 Hz, 1H), 6.82 (td, J = 8.0 Hz, 1H), 6.44–6.49 (m, 2H), 6.19 (d, J = 7.6 Hz, 1H), 6.12 (d, J = 7.6 Hz, 1H). MS (ESI): Found: 844.2006. C40H28F3IrN5O requires 844.1880.
Experimental details
All hydrogen atoms were positioned geometrically and allowed to ride on their respective parent atoms with C—H distances = 0.95 Å, and with Uiso(H) = 1.2Ueq for aryl and alkene H atoms. The PF6− anion molecule was refined with geometrical constraints (SADI) and displacement parameter constraints (EADP). The large R(int) parameter results from a non-optimal data collection. The resulting R-factors however verifies the structure assignments.
Comment
Phosphorescent cyclometalated Ir(III) complexes have attracted extensive interest due to their broad range of emission colours and high phosphorescence quantum efficiencies [9], [10]. These unique properties are quintessential for a complex to be explored as a promising candidate in the applications of organic light-emitting diodes (OLEDs) [11], [12] and light-emitting electrochemical cells (LEECs) [13], [14]. The archetypal Ir(III) phenylpyridine based complexes are widely employed in OLEDs and LEECs due to their high quantum yields, stability and facile colour tunability [15], [16]. The photophysical properties of these Ir(III) complexes can be tuned by utilising different ancillary ligands [17], [18] and also by employing various substituents on the phenylpyridine moieties and ancillary ligands [19], [20]. The photophysical properties of Ir(III) phenylpyridine complex with pyridylpyrazole moieties as an ancillary ligand has been studied for LEECs application [21]. Herein, we tune the emission of the aforementioned Ir(III) complex by attaching a cinnamoyl group to the pyridylpyrazole moieties. The crystal structure and the photophysical properties of the title complex were investigated.
In the crystal structure of the title compound, the Ir(III) metal centre adopts a distorted octahedral geometry, and is coordinated by two 2-phenylpyridyl (PPy) ligands and one 3-CF3CnPyPz ligand. The PPy ligands are arranged in a cis-C,C and trans-N,N chelate dispositions, which resemble those of previously reported [Ir(PPy)2(N^N)]+ complexes (N^N = polypyridine chelating ligand) [9], [22]. The bond lengths and angles around the iridium centre are similar to those related structures of Ir(C^N)2(N^N)]+ complexes (C^N = C-,N-donor cyclometalated ligand and N^N = polypyridine chelating ligand) [23], [24]. The Ir—N bond distances between the Ir centre and the pyridylpyrazole moiety, namely Ir(1)—N(3) and Ir(1)—N(4), are longer than those between the Ir centre and the PPy ligands, i.e. Ir(1)—N(1) and Ir(1)—N(2), which reflects the stronger trans influence of the phenyl groups of the PPy cyclometalated ligand [9], [25]. The pyridine and pyrazole moieties in the same ancillary ligand are slightly distorted from each other with an angle of 22.0(3)°. The trifluoromethyl-cinnamoyl fragment is tilted with respect to the pyridylpyrazole mean plane with a dihedral angle of 133.3(13)°. The vinyl hydrogens, —CH=CH— showed a trans configuration, with the H(32)—C(32)=C(33)—H(33) torsion angle of −176.6(6)°.
An extraordinary phenomenon is observed for the packing of the title complex, in which the PF6− counter ions play a crucial role to form the packing scheme of this structure. Two adjacent molecules form a centrosymmetric dimer via a non-classical C(22)—H(22)⋯O(1) hydrogen bond and these dimers are connected by five C—H⋯F contacts, generating a two-dimensional network when viewed along the c axis. Interestingly, there were no significant π⋯π interactions formed between the heterocylic rings of the neighbouring complexes despite the presence of 7 heterocyclic rings. However, a weak π⋯π interaction (between the pyrazole and pyridine rings of the ancillary ligand) is observed.
The absorption and photoluminescence spectra of the titled complex in CH3CN solution were investigated. Intense absorption bands between 210–300 nm was assigned to the spin allowed π–π* intraligand transitions [26], [27]. The weaker and broad absorption band in the range of 350–500 nm were assigned to the mixture of metal-to-ligand charge transfer (spin allowed 1MLCT and spin forbidden 3MLCT) [22], [28] and ligand-centered (LC) transitions [23], [29]. The complex displayed a broad emission band at 493 nm. The broad and featureless photoluminescence spectrum indicates that the emissive excited states have predominantly 3MLCT characters [30], [31].
Acknowledgements
The authors thank the Ministry of Higher Education for MyPhD support for CYY, Universiti Kebangsaan Malaysia (UKM) for GGP-2017-091, MI-2018-012 and Universiti Putra Malaysia (UPM) for GP-IPM/2020/9683100 research grant. We are also grateful to the Centre for Advanced Materials and Renewable Resources, Faculty of Science and Technology (UKM) for their provision of experimental facilities and Center for Research and Instrumentation Management (UKM) for the X-ray analysis provided.
References
1. Bruker. APEX3, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2016).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
5. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
6. Mark-Lee, W. F.; Chong, Y. Y.; Law, K. P.; Ahmad, I.; Kassim M. B.: Synthesis, structure and density functional theory (DFT) study of a rhenium(I) pyridylpyrazol complex as a potential photocatalyst for CO2 reduction. Sains Malaysiana. 47 (2018) 1491–1499.10.17576/jsm-2018-4707-17Suche in Google Scholar
7. Mark-Lee, W. F.; Chong, Y. Y.; Kassim, M. B.: Supramolecular structures of rhenium(I) complexes mediated by ligand planarity via the interplay of substituents. Acta Cryst. C. 74 (2018) 997–1006.10.1107/S2053229618010586Suche in Google Scholar PubMed
8. Shelton, A. H.; Stephenson, A.; Ward, M. D.; Kassim, M. B.: 1-Benzoyl-3-(pyridin-2-yl)-1H-pyrazole. Acta Crystallogr. E67 (2011) o2445.10.1107/S1600536811033368Suche in Google Scholar PubMed PubMed Central
9. Zhang, F.; Li, W.; Wei, D.; Dong, X.; Li, S.; Li, Z.; Zhang, F.; Wei, X.; Wei, B.; Cao, G.; Zhai, B.: Solution-processed organic light-emitting diodes based on a blue-emitting cationic iridium(III) complex using 2-(1H-pyrazol-1-yl)pyridine as ancillary ligand. Inorg. Chim. Acta 453 (2016) 115–121.10.1016/j.ica.2016.07.052Suche in Google Scholar
10. Tang, M.; Zhu, S.; Liu, R.; Wang, J.; Zhang, Z.; Zhu, H.: Synthesis, characterization and optical properties of novel Ir(III) complexes bearing N-heterocycle substituents. J. Organomet. Chem. 880 (2019) 363–367.10.1016/j.jorganchem.2018.11.031Suche in Google Scholar
11. Bolink, H. J.; Coronado, E.; Garcia Santamaria, S.; Sessolo, M.; Evans, N.; Klein, C.; Baranoff, E.; Kalyanasundaram, K.; Gräetzel, M.; Nazeeruddin, M. K.: Highly phosphorescent perfect green emitting iridium(III) complex for application in OLEDs. ChemComm. 31 (2007) 3276–3278.10.1039/b707879jSuche in Google Scholar PubMed
12. Yang, C.-H.; Fang, K.-H.; Chen, C.-H.; Sun, I.: High efficiency mer-iridium complexes for organic light-emitting diodes. ChemComm. 19 (2004) 2232–2233.10.1039/b406958gSuche in Google Scholar PubMed
13. Sunesh, C. D.; Shanmugasundaram, K.; Subeesh, M. S.; Chitumalla, R. K.; Jang, J.; Choe, Y.: Blue and blue-green light-emitting cationic iridium complexes: Synthesis, characterization, and optoelectronic properties. ACS Appl. Mater. Interfaces 7 (2015) 7741–7751.10.1021/acsami.5b00875Suche in Google Scholar PubMed
14. Costa, R. D.; Ortí, E.; Bolink, H. J.; Graber, S.; Housecroft, C. E.; Constable, E. C.: Intramolecular π-stacking in a phenylpyrazole-based iridium complex and its use in light-emitting electrochemical cells. J. Am. Chem. Soc. 132 (2010) 5978–5980.10.1021/ja1010674Suche in Google Scholar PubMed
15. Coppo, P.; Plummer, E. A.; De Cola, L.: Tuning iridium(III) phenylpyridine complexes in the ”almost blue” region. ChemComm. 15 (2004) 1774–1775.10.1039/B406851CSuche in Google Scholar PubMed
16. He, L.; Duan, L.; Qiao, J.; Zhang, D.; Wang, L.; Qiu, Y.: Enhanced stability of blue-green light-emitting electrochemical cells based on a cationic iridium complex with 2-(1-phenyl-1H-pyrazol-3-yl)pyridine as the ancillary ligand. ChemComm. 47 (2011) 6467–6469.10.1039/c1cc11263eSuche in Google Scholar PubMed
17. Shang, X.; Han, D.; Li, D.; Guan, S.; Wu, Z.: Theoretical study on the electronic structures and photophysical properties of a series of Ir(III) complexes based on substituted 2-(pyrazol-3-yl)pyridine ligand. Chem. Phys. Lett. 588 (2013) 68–75.10.1016/j.cplett.2013.10.041Suche in Google Scholar
18. Chen, L.; Wu, Z.; Yang, J.; Zhang, S.: The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir. Z. Kristallogr. NCS 234 (2019) 1173–1176.10.1515/ncrs-2019-0308Suche in Google Scholar
19. Ho, C.-L.; Lam, C.-S.; Sun, N.; Ma, D.; Liu, L.; Yu, Z.-Q.; Xue, L.; Lin, Z.; Li, H.; Lo, Y. H.; Wong, W.-Y.: Synthesis, characterization, and electroluminescent properties of iridium(III) 2-phenylpyridine-type complexes containing trifluoromethyl substituents and various main-group moieties. Isr. J. Chem. 54 (2014) 999–1014.10.1002/ijch.201400076Suche in Google Scholar
20. Zanoni, K. P. S.; Kariyazaki, B. K.; Ito, A.; Brennaman, M. K.; Meyer, T. J.; Murakami Iha, N. Y.: Blue-green iridium(III) emitter and comprehensive photophysical elucidation of heteroleptic cyclometalated iridium(III) complexes. Inorg. Chem. 53 (2014) 4089–4099.10.1021/ic500070sSuche in Google Scholar PubMed
21. He, L.; Duan, L.; Qiao, J.; Wang, R.; Wei, P.; Wang, L.; Qiu, Y.: Blue-emitting cationic iridium complexes with 2-(1H-pyrazol-1-yl)pyridine as the ancillary ligand for efficient light-emitting electrochemical cells. Adv. Funct. Mater. 18 (2008) 2123–2131.10.1002/adfm.200701505Suche in Google Scholar
22. Stagni, S.; Colella, S.; Palazzi, A.; Valenti, G.; Zacchini, S.; Paolucci, F.; Marcaccio, M.; Albuquerque, R. Q.; De Cola, L.: Essential role of the ancillary ligand in the color tuning of iridium tetrazolate complexes. Inorg. Chem. 47 (2008) 10509–10521.10.1021/ic801157kSuche in Google Scholar PubMed
23. He, L.; Ma, D.; Duan, L.; Wei, Y.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y.: Control of intramolecular π–π stacking interaction in cationic iridium complexes via fluorination of pendant phenyl rings. Inorg. Chem. 51 (2012) 4502–4510.10.1021/ic2021325Suche in Google Scholar PubMed
24. Zubaidi, Z. N.; Metherell, A. J.; Baggaley, E.; Ward, M. D.: Ir(III) and Ir(III)/Re(I) complexes of a new bis(pyrazolyl-pyridine) bridging ligand containing a naphthalene-2,7-diyl spacer: Structural and photophysical properties. Polyhedron 133 (2017) 68–74.10.1016/j.poly.2017.05.017Suche in Google Scholar
25. Orselli, E.; Kottas, G. S.; Konradsson, A. E.; Coppo, P.; Fröhlich, R.; De Cola, L.; Van Dijken, A.; Büchel, M.; Börner, H.: Blue-emitting iridium complexes with substituted 1,2,4-triazole ligands: Synthesis, photophysics, and devices. Inorg. Chem. 46 (2007) 11082–11093.10.1021/ic701110pSuche in Google Scholar PubMed
26. Sykes, D.; Parker, S. C.; Sazanovich, I. V.; Stephenson, A.; Weinstein, J. A.; Ward, M. D.: d→f Energy transfer in Ir(III)/Eu(III) dyads: Use of a naphthyl spacer as a spatial and energetic “stepping stone”. Inorg. Chem. 52 (2013) 10500–10511.10.1021/ic401410gSuche in Google Scholar PubMed PubMed Central
27. Zhang, K. Y.; Lo, K. K.-W.: Synthesis, properties, and live-cell imaging studies of luminescent cyclometalated iridium(III) polypyridine complexes containing two or three biotin pendants. Inorg. Chem. 48 (2009) 6011–6025.10.1021/ic900412nSuche in Google Scholar PubMed
28. Connell, T. U.; White, J. M.; Smith, T. A.; Donnelly, P. S.: Luminescent iridium(III) cyclometalated complexes with 1,2,3-triazole “click” ligands. Inorg. Chem. 55 (2016) 2776–2790.10.1021/acs.inorgchem.5b02607Suche in Google Scholar PubMed
29. Song, Y.-H.; Chiu, Y.-C.; Chi, Y.; Cheng, Y.-M.; Lai, C.-H.; Chou, P.-T.; Wong, K.-T.; Tsai, M.-H.; Wu, C.-C.: Phosphorescent iridium(III) complexes with nonconjugated cyclometalated ligands. Chem. Eur. J. 14 (2008) 5423–5434.10.1002/chem.200800050Suche in Google Scholar PubMed
30. Jayabharathi, J.; Thanikachalam, V.; Srinivasan, N.; Perumal, M. V.: Evidence for strong mixing between the LC and MLCT excited states in some heteroleptic iridium(III) complexes. J. Fluoresc. 21 (2011) 1585–1597.10.1007/s10895-011-0847-xSuche in Google Scholar PubMed
31. Jayabharathi, J.; Thanikachalam, V.; Sathishkumar, R.: Highly phosphorescent green emitting iridium(III) complex for application in OLEDs. New J. Chem. 39 (2015) 235–245.10.1039/C4NJ01334DSuche in Google Scholar
©2020 Yan Yi Chong et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4
Artikel in diesem Heft
- Frontmatter
- Crystal structure of bis [1-(phenylsulfonyl)-2-(1-(pyrazin-2-yl)ethylidene)hydrazin-1-ido-κ3N,N′,O]cobalt(II), C24H22N8O4S2Co
- The crystal structure of 1,3-bis(4-(methoxycarbonyl)benzyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide, C26H25BrN2O4
- Crystal structure of {tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′}-(nitrito-κ2O,O′)nickel(II) perchlorate – ethanol (1/1), C26H27ClN8NiO7
- Crystal structure of catena-poly[aqua[(μ2-4,5-dicarboxylato-2-(2-carboxylatophenyl)imidazol-1-ido-κ4N,O,O′:N′)](μ2-4,4′-bipyridine-κ2N:N′)dicopper(II)], C22H14Cu2N4O7
- Crystal structure of chlorido-tris(4-methylbenzyl-κC)-(triphenylarsine oxide-κO)tin(IV), C42H42AsClOSn
- The crystal structure of 4,4′-bipyridinium bis(3-carboxy-2-nitrobenzoate) tetrahydrate, C13H13N2O8
- Crystal structure of 1-(3-chlorophenyl)-4-(4-(((2,3-dihydro-1H-inden-5-yl)oxy)methyl)phenethyl)piperazine, C28H31ClN2O
- Crystal structure of catena-poly[diaqua-bis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′:N′′,N′′′)magnesium], C10H8N20O2Mg
- The crystal structure of (E)-2-((2-hydroxy-4-ethoxybenzylidene)amino)-2-methylpropane-1,3-diol monohydrate, C13H21NO5
- Crystal structure of catena-poly[diaqua-(μ2-bipyridine-κ2N:N′)-bis(3,5-dichloroisonicotinato-κO)cadmium(II)] dihydrate, C22H20CdCl4N4O8
- The crystal structure of 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid, C13H15ClO2
- Redetermination of the crystal structure of yttrium(III) trinitrate(V) pentahydrate, Y(NO3)3 ⋅ 5 H2O, H10N3O14Y
- Crystal structure of catena-poly[di-μ2-chlorido-1,10-phenanthroline-κ2N,N′-cadmium(II)], C12H8Cl2CdN2
- Crystal structure of 4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)benzoic acid, C13H9F3N2O3
- Crystal structure of 3-acetyl-4-hydroxybenzoic acid, C18H16O8
- Crystal structure of bis(N,2-bis(4-ethoxybenzylidene)hydrazine-1-carbohydrazonothioato-κ2N,S)nickel(II) — N,N-dimethylformamide (1/2), C44H56N10S2O6Ni
- The crystal structure of 5-chloro-4,6-dimethoxypyrimidin-2-amine, C6H8ClN3O2
- Crystal structure of poly[aqua-(μ4-benzene-1,2,4,5-tetracarboxylato-κ4O,O′,O′′,O′′′)bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N)dinickel(II)], NiC17H14N5O5
- Crystal structure of poly[aqua(5-dimethylamino)naphthalene-1-sulfonato-κ2N:O)(μ2-4,4′-bipyridyl -κ2N:N′)silver(I)], C44H44Ag2N6O8S2
- Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN)pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N)iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6
- Crystal structure of catena-poly[aqua(μ6-piperazine-1,4-bisethanesulfonato-κ6N:N′:O:O′:O′′:O′′′)(μ2-pyrazinyl-κ2N:N′)disilver(I)sesquihydrate], C12H30Ag2N4O11S2
- Crystal structure of (E)-1-(2-nitrophenyl)-N-(o-tolyl)methanimine, C14H12N2O2
- Crystal structure of 4′-amino-3′,5′-diisopropyl-(1,1′-biphenyl)-4-carbonitrile, C19H22N2
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)-tetrakis(μ2-cyanido-κ2C:N)dinickel(II)], C10H14N6O2Ni2
- Crystal structure of rac-trans-N,N′-bis(3-bromo-5-chlorosalicylidene)-1,2-cyclohexanediamine, C20H18Br2Cl2N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2
- The crystal structure of (dichromato-κ2O,O′)bis(1,10-phenanthroline-κ2N,N′)nickel(II), C12H16N4O7Cr2Ni
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridozincate(II) monohydrate, C10H18Cl4ZnN2O
- Crystal structure of bis(μ2-azido-k2N,N)-bis(2-amino-1-(N-(3-bromosalicylaldiminato))ethane)-dicopper(II), C20H18Br4N2O2
- Crystal structure of (η6-1-methyl-4-isopropylbenzene)-[5-bromo-2-(2-pyridyl)phenyl-κ2C,N]-chloro-ruthenium(II), C21H21BrClNRu
- Crystal structure of N-(methyl(oxo)(1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)-λ6-sulfanylidene)cyanamide, C10H10F3N3OS
- Crystal structure of 6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromo-4-chlorophenolato-κ4N,N′,O,O′)nickel(II), C20H16Br2Cl2NiN2O2
- Redetermination of the crystal structure of catena-poly[aqua-(1,10-phenanthroline-κ2N,N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)manganese(II) monohydrate, C12H12N2O6MoMn
- The crystal structure tetrakis(μ2-o-chlorobenzoato-κ2O:O′)-bis(methanol-κ1O)dirhodium(II), C30H24Cl4O10Rh2
- Crystal structure of bis(2,3-diphenyltetrazolidine-5-thione-κ1S)-(nitrato-κ1O)-(nitrato-κ2O,O′)lead(II), C26H20N10O6S2Pb
- Crystal structure of bis(3-bromo-N-(1-(3-methylpyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′)cadmium(II) hemihydrate, C28H25N8O2.5Br2Cd
- Crystal structure of catena-poly[tetrakis(μ2-trifluoroacetato-κ2O:O′)(μ2-2,5-dimethylpyrazine-κ2N,N′)dicopper(II)], C7H4CuF6NO4
- The crystal structure of catena-poly[bis[3-azoniapentane-1,5-diammonium][bis(μ4-oxo)-tetrakis(μ3-oxo)-heptakis(μ2-oxo)-tetradecaoxo-octa-molybdenum] dihydrate], (C8H36N6O29Mo8)n
- Crystal structure of tetraaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κO)-nickel(II)—diaqua-bis(2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-nickel(II), C28H24Cl12N4Ni2O18
- The crystal structure of bis(2-hydroxypyrimidinium) pentachloridobismuthate(III), (C4N2H5O)2BiCl5
- The crystal structure of catena-poly[(μ2-4,4′-dipyridine-κ2N,N′)-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-bis(ethanol-κO)nickel(II)], C28H26Cl6N4NiO8
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3,5-bis(4-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C26H20Cl3F2NO3S
- The crystal structure of 5-bromopicolinic acid monohydrate, C6H6BrNO3
- The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S
- The crystal structure of catena-poly[(μ2-2-((3-bromo-2-oxidobenzylidene)amino)acetato-κ4O,N,O′:O′′)-(dimethylformamide-κ1O)]zinc(II), C12H13N2O4BrZn
- Crystal structure of aqua-azido-κ1N-(6,6′-((propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(3-bromophenolato)-κ4N,N′,O,O′iron(III), C17H16Br2FeN5O3
- The crystal structure of tris(1-ethylimidazole-κ1N)-(sulfato-κ2O,O′)vanadium(IV), C15H24N6O5SV
- Crystal structure of (E)-3-methoxy-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide, C15H15N3O2
- Crystal structure of dichloro-bis-(1-butyl-1H-benzo[d]imidazole)-nickel(II), C22H28Cl2N4Ni
- The crystal structure of 2-(2,3-dimethoxyphenyl)-3-hydroxy-4H-chromen-4-one, C17H14O5
- The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S⋅C3H7NO
- The crystal structure of 1,8-bis(pyridin-4-ylethynyl)anthracene-1,2,4,5-tetrafluoro-3,6-diiodobenzene (2/1), C62H32F4I2N4
- The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N
- The crystal structure of 8-((4-chlorophenylamino)methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4
- The crystal structure of catena-poly[oktaaqua-bis(μ2-4,4′-ethene-1,2-diyldipyridine-κ2N:N′)-(μ2-3,3′-(1-oxidodiazene-1,2-diyl)diphthalato-κ2O:O′)dicobalt(II)] dihydrate, C28H36N4O19Co2
- Crystal structure of (E)-1-(2-cyano-3-oxo-1-phenylprop-1-en-1-yl)-3,7-diphenylindolizine-6-carbonitrile, C31H19N3O
- Crystal structure of 1,1′-bis(diphenylphosphino)ferrocene-(1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-(O-isobutyl sulfurodithioito-κ2S,S′)copper(I), C39H37CuFeOP2S2
- Crystal structure of poly[(5-bimethylamino-1-naphthalenesulfonato-κO)-(μ3-hexamethylenetetramino-κ3N:N′:N′′)silver(I)] dihydrate, C36H52Ag2N10O8S2
- Crystal structure of poly[μ2-diaqua-(μ2-2-amino-4,5-dicyano-κ2N:N′-imidazol-1-ide)sodium(I)], C5H6N5O2Na
- Crystal structure of (1,3-propanediamine-κ2N,N′)(N-(3-aminopropyl)-α-methyl aspartato-κ4N,N′,O,O′)cobalt(III) chloride, C11H24ClCoN4O4
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S
- Crystal structure of (S)-(+)-1-cyclohexylethylaminium chloride, C8H18NCl
- The crystal structure of tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(o-pyridyl)imidazoline-1-oxyl 3-oxide-κ2N,O)yttrium(III), C24H32N9O13Y
- Hydrogen bonding versus packing effects in the crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetraiodidozincate(II), C10H16I4ZnN2
- Dimerization of 2-[(2-((2-aminophenyl)thio)phenyl)amino]-cyclohepta-2,4,6-trien-1-one through hydrogen bonding, C19H16N2OS
- Crystal structure of 1-(4-chloro-phenyl)-7-ethoxyl-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid, C18H12ClF2NO4
- Crystal structure of 7-ethoxy-6,8-difluoro-4-oxo-1-pyridin-2-ylmethyl-1,4-dihydro-quinoline-3-carboxylic acid, C18H14F2N2O4
- Crystal structure of octahydro-7aR,8′R-dimethylspiro[isobenzofuran-4(1H), 4′ (3′H)-[1H-7,9a]methanocyclohepta[c]pyran]-1′,3, 9′ (3aH,4′aH)-trione, C20H26O5
- Crystal structure of bis(5-ethoxy-2-(((1-hydroxy-2-methyl-3-oxidopropan-2-yl)imino)methyl)phenolato-κ3N,O,O’)manganese(IV) – methanol (1/1), C27H38MnN2O9
- Crystal structure of 8a,8a′′-oxybis(8aH-8,9-dioxa-3a1λ4-aza-8aλ4-borabenzo[fg]tetracene), C34H22B2N2O5
- Crystal structure of bromido-triphenyl-(triphenylarsine oxide-κO)tin(IV), C36H30AsBrOSn
- Crystal structure of catena-poly[chlorido-(μ2-formato-κ2O:O′)-(1,10-phenathroline-κ2N,N′)copper(II)], C26H18Cl2Cu2N4O4
- The crystal structure of poly[(μ10-5-carboxyisophthalato-κ10O)disodium], C9H4Na2O6
- The crystal structure of 3,5-difluoroisonicotinic acid, C6H3F2NO2
- The crystal structure of ethyl-1-(N-(adamantan-1-yl)-carbamothioyl)piperidine-4-carboxylate, C19H30N2O2S
- Crystal structure of 5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydropyridine, C19H21NO2S
- Crystal structure of bis((3-chlorosalicylidene)-ethylenediaminato-κ4N,N′,O,O′)nickel (II), C16H12Cl2NiN2O2
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-4-hydroxybenzohydrazide — dihydrofuran-2(3H)-one (1/1), C18H17ClN2O5
- Crystal structure of bis((3-bromosalicylidene)-ethylenediaminato-κ4N,N′,O,O′) nickel (II), C16H12Br2NiN2O2
- Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH3)3SO]2CoCl4

