Abstract
C23H28O2, monoclinic, P21/n (no. 14), a = 8.9114(3) Å, b = 17.7151(6) Å, c = 12.6087(5) Å, β = 100.0520(10), V = 1959.93(12) Å3, Z = 4, Rgt(F) = 0.061, wRref(F2) = 0.171, T = 296(2) K.
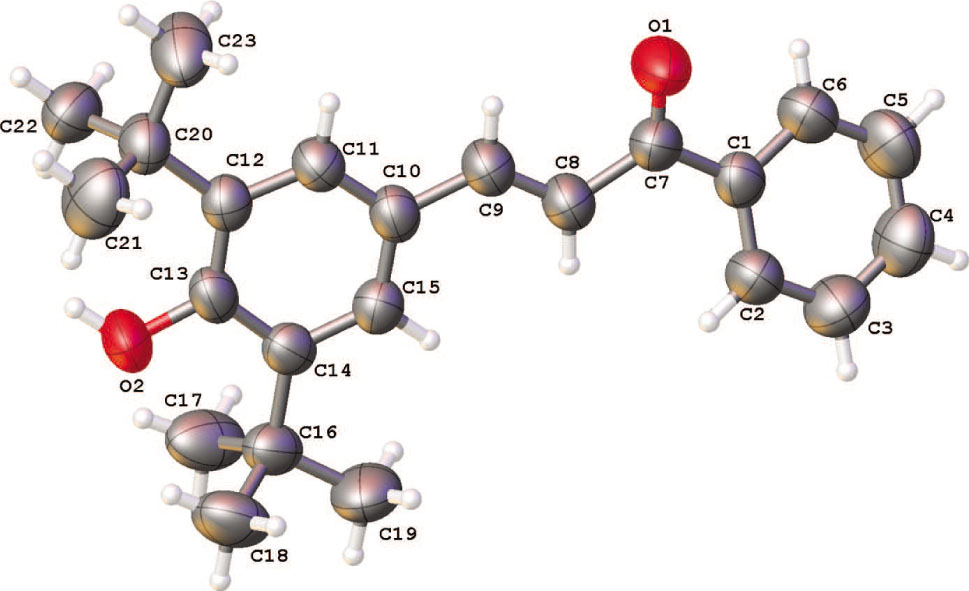
Crystal data, data collection and structure refinement details are summarized in Table 1.
Data collection and handling.
| Crystal: | Prismatic, red |
| Size: | 0.2 × 0.2 × 0.2 mm |
| Wavelength: | Mo Kα radiation (λ =0.71073 Å) |
| μ: | 0.071 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II CCD, Φ and ω-scans |
| 2θmax, completeness: | 27.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 19602, 4266, 0.0193 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3308 |
| N(param)refined: | 229 |
| Programs: | Bruker programs [1], ShelX [2], OLEX2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | −0.1656(2) | 0.06016(10) | 0.22861(18) | 0.0780(6) |
| O2 | 0.2734(2) | 0.29775(9) | −0.20358(14) | 0.0684(5) |
| H2 | 0.2502 | 0.3454 | −0.2181 | 0.103 |
| C1 | 0.0180(2) | −0.03502(11) | 0.26970(16) | 0.0427(4) |
| C2 | 0.1167(3) | −0.08460(13) | 0.23132(18) | 0.0527(5) |
| H2 | 0.1498 | −0.0743 | 0.1668 | 0.063 |
| C3 | 0.1659(3) | −0.14910(14) | 0.2884(2) | 0.0630(6) |
| H3 | 0.2303 | −0.1826 | 0.2615 | 0.076 |
| C4 | 0.1205(3) | −0.16394(14) | 0.3843(2) | 0.0620(6) |
| H4 | 0.1543 | −0.2074 | 0.4225 | 0.074 |
| C5 | 0.0255(3) | −0.11506(15) | 0.42444(19) | 0.0641(7) |
| H5 | −0.0043 | −0.1250 | 0.4902 | 0.077 |
| C6 | −0.0258(3) | −0.05131(13) | 0.36761(18) | 0.0553(6) |
| H6 | −0.0910 | −0.0185 | 0.3951 | 0.066 |
| C7 | −0.0478(3) | 0.03280(11) | 0.20867(18) | 0.0499(5) |
| C8 | 0.0309(3) | 0.06601(12) | 0.12732(18) | 0.0492(5) |
| H8 | 0.1129 | 0.0405 | 0.1074 | 0.059 |
| C9 | −0.0124(3) | 0.13181(12) | 0.08141(18) | 0.0485(5) |
| H9 | −0.0990 | 0.1535 | 0.1005 | 0.058 |
| C10 | 0.0597(2) | 0.17406(11) | 0.00475(16) | 0.0424(4) |
| C11 | −0.0012(2) | 0.24332(11) | −0.03236(16) | 0.0427(4) |
| H11 | −0.0876 | 0.2607 | −0.0080 | 0.051 |
| C12 | 0.0617(2) | 0.28766(11) | −0.10444(16) | 0.0406(4) |
| C13 | 0.1930(2) | 0.25954(11) | −0.13756(16) | 0.0425(4) |
| C14 | 0.2586(2) | 0.18879(11) | −0.10338(16) | 0.0407(4) |
| C15 | 0.1880(2) | 0.14789(11) | −0.03310(16) | 0.0418(4) |
| H15 | 0.2277 | 0.1010 | −0.0100 | 0.050 |
| C16 | 0.4052(2) | 0.16003(12) | −0.13895(18) | 0.0489(5) |
| C17 | 0.5378(3) | 0.21243(16) | −0.0951(3) | 0.0704(8) |
| H17A | 0.6304 | 0.1924 | −0.1128 | 0.106 |
| H17B | 0.5472 | 0.2162 | −0.0183 | 0.106 |
| H17C | 0.5190 | 0.2616 | −0.1267 | 0.106 |
| C18 | 0.3852(3) | 0.15416(17) | −0.2624(2) | 0.0709(7) |
| H18A | 0.4787 | 0.1370 | −0.2823 | 0.106 |
| H18B | 0.3593 | 0.2028 | −0.2938 | 0.106 |
| H18C | 0.3053 | 0.1189 | −0.2881 | 0.106 |
| C19 | 0.4494(3) | 0.08123(15) | −0.0932(3) | 0.0729(8) |
| H19A | 0.5409 | 0.0649 | −0.1168 | 0.109 |
| H19B | 0.3685 | 0.0463 | −0.1183 | 0.109 |
| H19C | 0.4663 | 0.0832 | −0.0160 | 0.109 |
| C20 | −0.0105(2) | 0.36464(12) | −0.14150(18) | 0.0478(5) |
| C21 | −0.0531(3) | 0.36999(16) | −0.2669(2) | 0.0749(8) |
| H21A | −0.0933 | 0.4193 | −0.2868 | 0.112 |
| H21B | −0.1285 | 0.3325 | −0.2928 | 0.112 |
| H21C | 0.0363 | 0.3615 | −0.2982 | 0.112 |
| C22 | 0.0945(3) | 0.42986(12) | −0.0929(2) | 0.0599(6) |
| H22A | 0.0494 | 0.4772 | −0.1180 | 0.090 |
| H22B | 0.1920 | 0.4249 | −0.1146 | 0.090 |
| H22C | 0.1073 | 0.4280 | −0.0157 | 0.090 |
| C23 | −0.1616(3) | 0.37747(16) | −0.1010(3) | 0.0730(8) |
| H23A | −0.2029 | 0.4257 | −0.1255 | 0.109 |
| H23B | −0.1437 | 0.3763 | −0.0237 | 0.109 |
| H23C | −0.2326 | 0.3385 | −0.1285 | 0.109 |
Source of material
The title compound was prepared by the refluxing of 3,5-di-tert-butyl-benzaldehyde (4.3 mmol) with acetophenone (4.3 mmol) in methanol (10 mL) in presence catalytic amount of sulfuric acid during 3 h. Then the reaction mixture was cooled down. After one day the precipitated yellow single crystals were collected, washed with distilled water and dried in desiccator. The yield of 3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenyl-propenone is 67%. The analysis of the title compound was described in literature [4].
Experimental details
H atoms were located in the difference Fourier map, but refined with fixed individual displacement parameters, using a riding model with C—H distances of 0.93 Å (for aromatic rings), 0.96 Å ( CH3 group), with U(H) values of 1.2Ueq(C) (for CH in aromatic moiety), and 1.5Ueq(C) (for CH3) and O—H distance 0.88 Å, with U(H) values of 1.2Ueq(O).
Discussion
Being of 1,3-diphenyl-2-propen-1-one derivatives, chalcones are simple scaffold found in many plant sources. Different synthesis methods and biological activities of chalcones were summarized in recent review article [5]. Also 3,5-di-tert-butyl-4-hydroxy styrene derivatives demonstrate antiinflammatory activity [6], gastroprotective effect [7], and antifungal activity [8].
The conformation about the C=C bond is E, with a C7—C8—C9—C10 torsion angle of 175.88(5)°. In the title compound the dihedral angle between the mean planes of the aromatic rings is 21.77(10)°. In the crystal, the molecules are linked by strong O—H⋯O hydrogen bonds into chain with graph-set notation C(10) along the [101] direction [9]. These supramolecular properties and the geometric and molecular parameters are very similar to 3-(3,5-di-t-butyl-4-hydroxyphenyl)-1-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one, an close related compound [10].
References
Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2005).Search in Google Scholar
Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination and Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.; OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr., 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
Katsumi, I.; Kondo, H.; Fuse, Y.; Yamashita, K.; Hidaka, T.; Hosoe, K.; Takeo, K.; Yamashita, T.; Watanabe, K.: Studies on styrene derivatives. II. Synthesis and antiinflammatory activity of 3,5-di-tert-butyl-4-hydroxystyrenes. Chem. Pharm. Bull. 34 (1986) 1619–1627.10.1002/chin.198639168Search in Google Scholar
Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z.: Chalcone: a privileged Structure in medicinal chemistry. Chem. Rev. 117 (2017) 7762–7810.10.1021/acs.chemrev.7b00020Search in Google Scholar PubMed PubMed Central
Kant, G.; Parate, A.; Chaturvedi, S. C.: QSAR study of substituted 3,5-di-tert-butyl-4-hydroxy styrene; a series with antiinflammatory activity. Indian J. Pharm. Sci. 67 (2005) 116–119.Search in Google Scholar
Rao, P. N. P.; Rao, M.; Rao, M. N. A.: Gastroprotective effect of 1-phenyl-3-(4-hydroxy-3,5-di-tert-butylphenyl)prop-2-en-1-one in rats. J. Pharm. Pharmacol. 50 (1998) 1371–1375.10.1111/j.2042-7158.1998.tb03362.xSearch in Google Scholar PubMed
Arnoldi, A.; Carughi, M.; Farina, G.; Merlini, L.; Parrino, M. G.: Synthetic analogs of phytoalexins. Synthesis and antifungal activity of potential free-radical scavengers. J. Agric. Food. Chem, 37 (1989) 508–512.10.1021/jf00086a052Search in Google Scholar
Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N.-L.: Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 34 (1995) 1555–1573.10.1002/anie.199515551Search in Google Scholar
Cabral, B. L. S.; da Silva, A. C. G.; de Ávila, R. I.; Cortez, A. P.;Luzin, R. M.; Lião, L. M.; de Souza Gil, E.; Sanz, G.; Vaz, B. G.; Sabino, J. R.; Menegatti, R.; Valadares, M. C.: A novel chalcone derivative, LQFM064, induces breast cancer cells death via p53, p21, KIT and PDGFRA. Eur. J. Pharm. Sci. 30 (2017) 1–15.10.1016/j.ejps.2017.06.018Search in Google Scholar
©2018 Ali N. Khalilov et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-amino-4-(2,4-dinitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo-pyran, C18H16N4O6
- Crystal structure of catena-poly[(μ3-5-carboxy-2-(pyridin-4-yl)benzoato-κ5O,O′:O′′,O′′′:O′′′)(1,10-phenanthroline-κ2N,N′)cadmium(II)], C100H60N12O16Cd4
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C19H18N2O6
- Crystal structure of 1-{4-[(2-hydroxy-5-methyl benzylidene)amino]phenyl}ethanone O-ethyl-oxime, C18H20N2O2
- Crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C36H38CuN4O4
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}nickel(II), C34H34N4NiO6
- Crystal structure of poly[μ8-3-carboxyphthalat-κ8-O:O1,O1,O1:O2:O3,O3:O4)silver(I)], C9H4Ag2O6
- Crystal structure of 2-((E)-((4-((E)-1-(hydroxyimino)ethyl)phenyl)iminio)methyl)-5-methoxyphenolate, C16H16N2O3
- Crystal structure of (E)-1-(4-(((E)-2-hydroxy-3-methoxybenzylidene)amino)phenyl)ethan-1-one oxime, C16H16N2O3
- The crystal structure of 2-(tert-butyl)-4-chloro-6-phenyl-1,3,5-triazine, C13H14Cl1N3
- Crystal structure of (6,6′-(((((2-aminoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenolato)-κ6N,N′,N′′,N′′′,O,O′)cadmium(II) – ethanol – water (1/1/1), C22H28CdCl4N4O4
- Crystal structure of 6-chloro-N-methylpyrimidin-4-amine, C5H6ClN3
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)-2-naphtholato-κ2N,O}nickel(II), C40H34N4NiO4
- Crystal structure of (E)-2-(4-bromophenyl)ethenesulfonyl fluoride (C8H6BrFO2S)
- Synthesis and crystal structure of 2,2′-ethylenedioxybis(benzimide)-2,2′-bis[O-(1-propyloxyamide)]oxime-4,4′,6,6′-tetrachlorodiphenol, C36H34Cl4N4O8
- Crystal structure of bis(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)cadmium(II) – methanol (1/1), C26H28N10O4Zn
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)nicotinohydrazonato-κ3N,N′,O)-bis(nitrato-κ2O,O′)samarium(III), C12H14N7O9Sm
- Crystal structure of hexaaqua-{(E)-N′-(1-(pyrazin-2-yl)ethylidene)isonicotinohydrazide-κ3N,N′,O}praseodym(III) trichloride monohydrate, C12H25Cl3N5O8Pr
- Crystal structure of methyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C21H22N2O3
- Crystal structure of ethyl 2-amino-4-(2-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of 2,12-dibromo-5,15-dihexyl-5,15-dihydrobenzo [1,2-b:5,6-c′]dicarbazole, C38H38Br2N2
- Crystal structure of ethyl 2-amino-4-(3-hydroxy-4-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO7
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO5
- Crystal structure of 2-hydroxy-N′-(pyrimidin-2-yl)benzohydrazide, C11H10N4O2
- Crystal structure of 2-(3,4-dimethylphenyl)-1,8-naphthyridine, C16H14N2
- Crystal structure of ethyl 4-(3,4-dimethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C23H29NO3
- Crystal structure of ethyl 4-(3-cyanophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C22H24N2O3
- Crystal structure of 7β,9β-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of Ent-7β,20-epoxy-kaur-16-en-1β,6α,7α,14α,15α-pentaol-20-one, C20H30O8
- Crystal structure of 1,4-bis(benzo[d][1,3]dioxol-5-ylmethyl)dihydro-1H,3H-furo[3,4-c]furan-3a(4H)-yl acetate, C22H20O8
- Crystal structure of methyl 2-((4-((2-nitrophenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl) benzoate, C18H16N4O5
- Crystal structure of diaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O)zinc(II), C12H14N4O10Zn
- Hydrothemal synthesis and crystal structure of triaqua-bis(5-carboxy-1-methyl-1H-imidazole-4-carboxylato-κ2N,O;κ1O)manganese(II), C12H16N4O11Mn
- Redetermination of methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22ClNO3
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imdazol)-κN}-dithiocyano-κN-zinc(II) C24H22N12S2Zn
- Crystal structure of (5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl furan-2-carboxylate, C10H7FN2O5
- Crystal structure of bis(η6-cymene)-tri-μ2-chlorido-ruthenium(II) tetrafluoroborate, C20H28BCl3F4Ru2
- Crystal structure of 3,6-diphenyl-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazine, C16H12N4S
- Synthesis and crystal structure of 1,3-bis[(3,4-dicyano)phenoxy]-4,6-dinitro-benzene, C22H8N6O6
- Crystal structure of ethyl 2-amino-4-(2,6-dichlorophenyl)-7-methyl-5-oxo-4H,5H-pyrano [4,3-b]pyran-3-carboxylate, C18H15Cl2NO5
- Crystal structure of poly[μ3-hydroxy-(μ5-(5-(2-carboxylatophenoxy)isophthalato-κ6O1:O2:O3:O4:O5,O6)-(μ2-1,4-di(1H-imidazol-1-yl)butane-κ2N:N′)dicobalt(II)] hemihydrate, C25H22Co2N4O8.5
- Crystal structure of methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C20H22BrNO3
- Crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium 5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate trihydrate, C35H33NO12
- Crystal structure of 2,5-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,3,4-oxadiazole, C38H24N4OS2
- The crystal structure of (E)-2-(4-hydroxyphenyl)-5-(prop-1-en-1-yl)benzofuran-3-carbaldehyde, C18H14O3
- Crystal structure of bis{5H-dibenzo[c,f][1,5]oxabismocin-12(7H)-yl} carbonate, C29H24O5Bi2
- Crystal structure of ethyl-5-formyl-3,4-dimethylpyrrole-2-carboxylate — N-(5-ethoxycarbonyl-3,4-dimethylpyrrole)-2-methylene-5-nitrobenzene-1,2-diamine (1:1), C26H31N5O7
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-6-bromo-4-chlorophenol, C14H12BrClN2O
- Crystal structure of (Z)-2-(4-chlorophenyl)-4-(furan-2-yl(phenylamino)methylene)-5-methyl-2,4-dihydro-3H-pyrazol-3-one, C21H16ClN3O2
- Crystal structure of N2,N4-dibutyl-6-chloro-N2,N4-bis(1,2,2,6,6-pentamethylpiperidin-4-yl)-1,3,5-triazine-2,4-diamine, C31H58ClN7 – Important intermediate of Chimassorb 119 synthesis
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethanone oxime, C17H15BrN2O2
- Crystal structure of poly-{diaqua-bis[(μ2-3-nitrobenzenesulfonylglycine-κ3N:O:O′)(4,4′-bipyridine)manganese(II)]}-dimethylformamide (1/1), C39H35Mn2N9O15S2
- Crystal structure of 4-(dimethylamino)-1-(prop-2-yn-1-yl)pyridin-1-ium perchlorate, C10H13ClN2O4
- Crystal structure of 2-amino-5-oxo-4-(4-chloro-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C15H11ClN2O2
- Crystal structure of triaqua-(pyridine-2,6-dicarboxylato-κ3O,N,O′)cobalt(II) – 6-phenyl-1,3,5-triazine-2,4-diamine (1/1), C16H18CoN6O7
- Crystal structure of ethyl 2-amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4
- Crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3-nitrophenyl)diazene 1-oxide, C12H5Cl2N5O7
- Crystal structure of 3,4-dimethyl-2,6-dinitrophenol, C8H8N2O5
- Crystal structure of 1,2-dimethyl-3,5-dinitrobenzene, C8H8N2O4
- Crystal structure of ethyl 4-(3-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C21H24ClNO3
- Crystal structure of N-(4-chlorophenyl)-2-(2,6-dichlorophenyl)acetamide, C14H10Cl3NO
- Crystal structure of 2-amino-4-(4-fluorophenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2c] chromene, C19H11FN2O3
- The crystal structure of (4-nitrophenyl) (5-ferrocenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl) methanone, C21H12F3FeN3O3
- Crystal structure of 5,5′-((3-hydroxy-4-methoxyphenyl)methylene)bis(1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one), C24H30N4O6S2
- Crystal structure of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-5-(1-hydroxy-2-((R)-3-hydroxypyrrolidin-1-yl)ethoxy)-4,7,9,12-tetramethyldecahydro-4,9a-propanocyclopenta[8]annulene-3,8-diol – a pleuromutilin derivative, C26H41NO5
- Crystal structure of bis(μ2-3-formyl-5-methoxy-2-oxidobenzoato-κ3O,O′:O′)-hexapyridine-dicadmium(II) – pyridine (1/1), C53H47Cd2N7O10
- Crystal structure of ethyl 2-amino-4-(3-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C22H19NO6
- Crystal structure of methyl 4-(3,5-ditrifluoromethylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate — water (2/1), C22H21F6NO3
- Crystal structure of 2-(4-bromophenyl)-2,3-dihydro-1H-perimidine, C17H13BrN2
- Crystal structure of (5-ethyl-2-(4-methoxyphenyl)-1,3-dioxan-5-yl)methanol, C14H20O4
- Crystal structure of (E)-N-(4-bromo-2-(1-(hydroxyimino)ethyl)phenyl)benzamide, C15H13BrN2O2
- Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4
- Crystal structure of methyl 2-methyl-4-(3-methoxyphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO4
- Crystal structure of (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H28O2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride, C8H18N8Cl2
- The crystal structure of dichlorido-(1,3-bis(2,6-dimethylphenyl)-1H-imidazol-2(3H)-ylidene)-(morpholine-κ1N)palladium(II), C23H29Cl2N3OPd(II)
- The crystal structure of 1-((5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-yl)methyl)-1,3-diphenylurea, C24H21ClN4O
- Crystal structure of 6-(2-bromoacetamido)tetrahydro-2H-pyran-2,3,4,5-Tetrayl tetraacetate, C16H22BrNO10
- Crystal structure of 5-methylpyrazine-2-carbohydrazide, C6H8N4O
- Crystal structure of catena-poly[(μ2-5-(tert-butyl)isophthalato-κ4O,O′:O′′,O′′′)(-4′-(pyridin-4-yl)-2,2′:6′,2′′-terpyridine-κ3N,N′,N′′)manganese(II)], C32H28N4O5Mn

