Abstract
C23H29N3O, orthorhombic P212121 (no. 19), a = 8.62130(10) Å, b = 18.25770(10) Å, c = 25.6873(2) Å, V = 4043.31(6) Å3, Z = 8, Rgt(F) = 0.0305, wRref(F2) = 0.0807, T = 293(2) K.
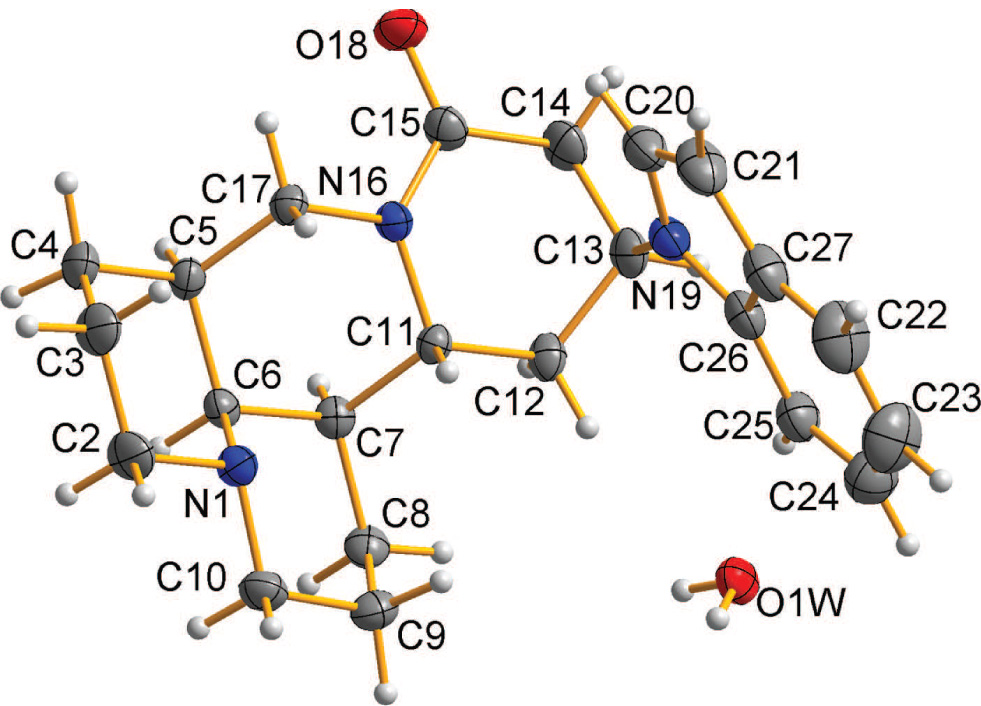
One of the two crystallographically different title molecules and one of the two water molecules of the title crystal structure are shown in the figure. Tables 1 and 2 contain details on the crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.36 × 0.30 × 0.20 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.64 mm−1 |
| Diffractometer, scan mode: | Multiwire, φ and ω-scans |
| 2θmax, completeness: | 73.9°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 38567, 8120, 0.039 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7843 |
| N(param)refined: | 521 |
| Programs: | OLEX2 [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1′ | 0.74083(12) | 0.07011(6) | 0.28353(4) | 0.0258(2) |
| C2′ | 0.89274(16) | 0.06038(9) | 0.30794(6) | 0.0340(3) |
| H2′A | 0.8990 | 0.0116 | 0.3227 | 0.041* |
| H2′B | 0.9039 | 0.0954 | 0.3361 | 0.041* |
| C3′ | 1.02461(15) | 0.07085(9) | 0.26943(6) | 0.0356(3) |
| H3′A | 1.0193 | 0.0334 | 0.2427 | 0.043* |
| H3′B | 1.1232 | 0.0661 | 0.2873 | 0.043* |
| C4′ | 1.01348(15) | 0.14630(9) | 0.24436(6) | 0.0329(3) |
| H4′A | 1.0906 | 0.1504 | 0.2170 | 0.040* |
| H4′B | 1.0352 | 0.1836 | 0.2702 | 0.040* |
| C5′ | 0.85187(14) | 0.15903(7) | 0.22143(5) | 0.0257(3) |
| H5′ | 0.8445 | 0.2105 | 0.2109 | 0.031* |
| C6′ | 0.72494(14) | 0.14408(7) | 0.26169(5) | 0.0225(2) |
| H6′ | 0.7350 | 0.1798 | 0.2900 | 0.027* |
| C7′ | 0.56495(13) | 0.15343(7) | 0.23640(5) | 0.0219(2) |
| H7′ | 0.5562 | 0.2042 | 0.2244 | 0.026* |
| C8′ | 0.44009(14) | 0.14068(8) | 0.27769(5) | 0.0295(3) |
| H8′A | 0.4486 | 0.1777 | 0.3046 | 0.035* |
| H8′B | 0.3383 | 0.1449 | 0.2619 | 0.035* |
| C9′ | 0.45798(15) | 0.06524(10) | 0.30179(6) | 0.0360(3) |
| H9′A | 0.4371 | 0.0280 | 0.2758 | 0.043* |
| H9′B | 0.3837 | 0.0594 | 0.3298 | 0.043* |
| C10′ | 0.62156(16) | 0.05566(9) | 0.32280(5) | 0.0355(3) |
| H10A | 0.6366 | 0.0887 | 0.3520 | 0.043* |
| H10B | 0.6336 | 0.0060 | 0.3356 | 0.043* |
| C11′ | 0.54679(13) | 0.10304(6) | 0.18859(5) | 0.0192(2) |
| H11′ | 0.5604 | 0.0523 | 0.2001 | 0.023* |
| C12′ | 0.38618(13) | 0.11015(7) | 0.16423(5) | 0.0209(2) |
| H12A | 0.3118 | 0.0837 | 0.1854 | 0.025* |
| H12B | 0.3561 | 0.1613 | 0.1640 | 0.025* |
| C13′ | 0.38059(13) | 0.08069(6) | 0.10895(5) | 0.0209(2) |
| H13′ | 0.2762 | 0.0893 | 0.0951 | 0.025* |
| C14′ | 0.49483(14) | 0.12404(6) | 0.07620(5) | 0.0231(2) |
| H14A | 0.5062 | 0.0999 | 0.0428 | 0.028* |
| H14B | 0.4516 | 0.1723 | 0.0698 | 0.028* |
| C15′ | 0.65406(14) | 0.13309(6) | 0.10019(5) | 0.0207(2) |
| N16′ | 0.67150(11) | 0.12046(6) | 0.15120(4) | 0.0219(2) |
| C17′ | 0.82799(14) | 0.11180(8) | 0.17319(5) | 0.0259(3) |
| H17A | 0.9042 | 0.1251 | 0.1471 | 0.031* |
| H17B | 0.8444 | 0.0608 | 0.1822 | 0.031* |
| O18′ | 0.76480(10) | 0.14995(5) | 0.07147(3) | 0.02536(19) |
| N19′ | 0.41092(12) | 0.00158(5) | 0.10717(4) | 0.0204(2) |
| C20′ | 0.54803(14) | −0.03454(7) | 0.09693(5) | 0.0236(2) |
| H20′ | 0.6406 | −0.0120 | 0.0875 | 0.028* |
| C21′ | 0.52813(16) | −0.10826(7) | 0.10261(5) | 0.0268(3) |
| H21′ | 0.6031 | −0.1442 | 0.0976 | 0.032* |
| C22′ | 0.27984(19) | −0.18164(8) | 0.12925(6) | 0.0340(3) |
| H22′ | 0.3231 | −0.2283 | 0.1281 | 0.041* |
| C23′ | 0.12632(19) | −0.17240(8) | 0.14223(6) | 0.0377(3) |
| H23′ | 0.0658 | −0.2133 | 0.1496 | 0.045* |
| C24′ | 0.05930(16) | −0.10259(9) | 0.14465(6) | 0.0339(3) |
| H24′ | −0.0448 | −0.0980 | 0.1536 | 0.041* |
| C25′ | 0.14492(15) | −0.04024(8) | 0.13400(5) | 0.0272(3) |
| H25′ | 0.1008 | 0.0062 | 0.1358 | 0.033* |
| C26′ | 0.30079(14) | −0.04997(7) | 0.12040(5) | 0.0218(2) |
| C27′ | 0.37065(16) | −0.11980(7) | 0.11769(5) | 0.0256(3) |
| N1 | 0.56425(12) | 0.62700(6) | 0.05129(4) | 0.0238(2) |
| C2 | 0.62153(17) | 0.70209(7) | 0.05779(7) | 0.0352(3) |
| H2A | 0.5582 | 0.7271 | 0.0834 | 0.042* |
| H2B | 0.6113 | 0.7281 | 0.0250 | 0.042* |
| C3 | 0.78966(17) | 0.70419(7) | 0.07504(7) | 0.0347(3) |
| H3A | 0.7998 | 0.6811 | 0.1089 | 0.042* |
| H3B | 0.8238 | 0.7546 | 0.0781 | 0.042* |
| C4 | 0.89048(16) | 0.66403(7) | 0.03543(6) | 0.0313(3) |
| H4A | 0.8909 | 0.6910 | 0.0029 | 0.038* |
| H4B | 0.9963 | 0.6616 | 0.0481 | 0.038* |
| C5 | 0.82989(14) | 0.58662(6) | 0.02594(5) | 0.0225(2) |
| H5 | 0.8859 | 0.5666 | −0.0041 | 0.027* |
| C6 | 0.65623(14) | 0.58709(6) | 0.01221(5) | 0.0213(2) |
| H6 | 0.6435 | 0.6117 | −0.0214 | 0.026* |
| C7 | 0.59632(14) | 0.50821(6) | 0.00692(5) | 0.0210(2) |
| H7 | 0.6537 | 0.4852 | −0.0217 | 0.025* |
| C8 | 0.42432(15) | 0.50899(7) | −0.00854(5) | 0.0260(3) |
| H8A | 0.4137 | 0.5290 | −0.0433 | 0.031* |
| H8B | 0.3849 | 0.4592 | −0.0090 | 0.031* |
| C9 | 0.32931(14) | 0.55472(8) | 0.02939(5) | 0.0281(3) |
| H9A | 0.2238 | 0.5593 | 0.0167 | 0.034* |
| H9B | 0.3260 | 0.5306 | 0.0630 | 0.034* |
| C10 | 0.40049(15) | 0.63013(7) | 0.03531(6) | 0.0301(3) |
| H10C | 0.3928 | 0.6560 | 0.0024 | 0.036* |
| H10D | 0.3421 | 0.6576 | 0.0610 | 0.036* |
| C11 | 0.62982(14) | 0.46343(6) | 0.05660(4) | 0.0195(2) |
| H11 | 0.5798 | 0.4879 | 0.0861 | 0.023* |
| C12 | 0.56533(15) | 0.38624(7) | 0.05242(5) | 0.0251(3) |
| H12C | 0.5727 | 0.3701 | 0.0165 | 0.030* |
| H12D | 0.4564 | 0.3869 | 0.0619 | 0.030* |
| C13 | 0.64980(16) | 0.33161(7) | 0.08708(5) | 0.0269(3) |
| H13 | 0.6058 | 0.2829 | 0.0808 | 0.032* |
| C14 | 0.81842(17) | 0.33058(7) | 0.07025(6) | 0.0326(3) |
| H14C | 0.8766 | 0.2999 | 0.0941 | 0.039* |
| H14D | 0.8253 | 0.3085 | 0.0360 | 0.039* |
| C15 | 0.89277(15) | 0.40555(7) | 0.06847(5) | 0.0267(3) |
| N16 | 0.79929(12) | 0.46406(5) | 0.06521(4) | 0.0217(2) |
| C17 | 0.86376(14) | 0.53735(6) | 0.07247(5) | 0.0221(2) |
| H17C | 0.9751 | 0.5337 | 0.0773 | 0.027* |
| H17D | 0.8198 | 0.5591 | 0.1036 | 0.027* |
| O18 | 1.03566(11) | 0.41139(5) | 0.07156(5) | 0.0363(2) |
| N19 | 0.63303(13) | 0.34846(6) | 0.14266(4) | 0.0276(2) |
| C20 | 0.74580(18) | 0.37074(8) | 0.17771(6) | 0.0347(3) |
| H20 | 0.8495 | 0.3787 | 0.1695 | 0.042* |
| C21 | 0.6833(2) | 0.37917(9) | 0.22567(6) | 0.0409(4) |
| H21 | 0.7357 | 0.3938 | 0.2556 | 0.049* |
| C22 | 0.3982(2) | 0.35928(10) | 0.25712(7) | 0.0503(4) |
| H22 | 0.4128 | 0.3723 | 0.2918 | 0.060* |
| C23 | 0.2544(2) | 0.33760(11) | 0.23985(7) | 0.0539(4) |
| H23 | 0.1717 | 0.3365 | 0.2631 | 0.065* |
| C24 | 0.2305(2) | 0.31723(9) | 0.18792(7) | 0.0428(4) |
| H24 | 0.1324 | 0.3021 | 0.1774 | 0.051* |
| C25 | 0.34933(17) | 0.31915(8) | 0.15213(6) | 0.0315(3) |
| H25 | 0.3332 | 0.3058 | 0.1176 | 0.038* |
| C26 | 0.49531(17) | 0.34203(7) | 0.16959(5) | 0.0279(3) |
| C27 | 0.5227(2) | 0.36160(8) | 0.22203(6) | 0.0359(3) |
| O1W | 0.20777(11) | 0.29923(5) | 0.02952(4) | 0.0295(2) |
| O2W | 0.09101(12) | 0.17699(6) | 0.08043(5) | 0.0377(2) |
| H1WA | 0.229(2) | 0.3133(11) | 0.0001(9) | 0.048(5)* |
| H2WA | −0.011(3) | 0.1734(13) | 0.0756(9) | 0.062(6)* |
| H1WB | 0.164(3) | 0.3366(12) | 0.0448(9) | 0.057(6)* |
| H2WB | 0.122(2) | 0.2160(12) | 0.0602(8) | 0.052(6)* |
Source of materials
The synthesis of the title compound was carried out using the michael addition reaction. A mixture of sophocarpine (1.3 g, 5.0 mmol), indole (0.9 g, 8 mmol) and cesium chloride (0.8 g, 5 mmol) was dissolved in 12 mL petroleum ether, and stirred vigorously at 80 °C with nitrogen protection and condensation cycle. After 5 min, tetramethoxysilane (0.5 mL, 2.5 mmol) was added dropwise with continued stirring and reacting for 9 h. The crude reaction mixture was collected after adding 10 mL of dichloromethane and filtrated with filter tissue. A concentrated brown solution was obtained after removing dichloromethane using a rotary evaporator. The concentrated resulting solution was purified by the silica gel column chromatography eluting with ethyl acetate/ethanol = 10/1. The title compound was crystallized from n-hexane/ ethanol(6/1), whereupon a few colorless, rod-shape crystals were obtained.
Experimental details
Hydrogen atoms were placed in calculated positions and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C).
Comment
Matrine is one of the main alkaloid components extracted from the Sophora root, which was first isolated and identified in 1958 from Sophora flavescens Ait, subprostrata and alopecuroides [3], [4], [5], [6]. Matrine and its derivatives are known for their importance in pharmaceutical and agricultural applications because of their biological activities, such as anticancer, anti-inflammatory, antimicrobial, antiviral and insecticidal activity [7], [8], [9], [10], [11]. Considering the good pharmacological and agricultural effects of matrine, we are interested in developing a general and practical strategy for the preparation. The insecticidal activity and pharmacological activity for the title compound are part of an ongoing study in our laboratory.
To explore the synthetic strategy, sophocarpine is a suitable starting material because it contains an a, b-unsaturated carbonyl group that is reactive toward a variety of useful nucleophiles. Therefore, in the present study, sophocarpine was used as starting material to design and synthesize a matrine derivative by introducing indole group to C13, forming a bond (C13—N19) with bond length 1.4685 Å. The planes of the phenyl groups (C12—C13) make dihedral angle with the mean plane of indole group (N19—C20) equal to 111.8 Å. The molecules were packed in the crystal structure without any hydrogen bonds.
Acknowledgement
This work was sponsored by the National Natural Science Foundation of China (no. 201376281–21406274).
References
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–C341.10.1107/S0021889808042726Suche in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. Sect.A 64 (2008) 112–C122.10.1107/S0108767307043930Suche in Google Scholar
Lai, J. P.; He, X. W.; Jiang, Y.; Chen F.: Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens ait by molecularly imprinted solidphase extraction. Anal. Bioanal. Chem. 375 (2003) 264–269.10.1007/s00216-002-1675-2Suche in Google Scholar
Boiteau, L.; Boivin, J.; Liard, A.; Quiclet-Sire, B.; Zard, S. Z.: A short synthesis of (±)-Matrine. Angew. Chem. Int. Ed. 38 (1998) 1128–1131.10.1002/(SICI)1521-3773(19980504)37:8<1128::AID-ANIE1128>3.0.CO;2-PSuche in Google Scholar
Liu, J. Y.; Hu, J. H.; Zhu, Q. G.; Li, F. Q.; Wang, J.; Sun, H. J.: Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int. Immuno. pharmacol. 7 (2007) 816–823.10.1016/j.intimp.2007.02.003Suche in Google Scholar
Zhang, L. J.; Wang, T. T.; Wen, X. M.; Wei, Y.; Peng, X. C.; Li, H.; Wei, L.: Effect of matrine on HeLa cell adhesion and migration. Eur. J. Pharmacol. 563 (2007) 69–76.10.1016/j.ejphar.2007.01.073Suche in Google Scholar
Cheng, H.; Xia, B.; Zhang, L.; Zhou, F.; Zhang, Y. X.; Ye, M.; Hu, Z. G.; Li, J.; Li, J.; Wang, Z. L.; Li, C.; Guo, Q. S.: Matrine improves 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Pharmacol. Res. 53 (2006) 202–208.10.1016/j.phrs.2005.11.001Suche in Google Scholar
Hu, Z. L.; Zhang, J. P.; Qian, D. H.; Lin, W.; Xie, W. F.; Zhang, X. R.; Chen, W. Z.: Effects of matrine on mouse splenocyte proliferation and release of interleukin-1 and -6 from peritoneal macrophages in vitro. Acta pharmacol. Sin. 17 (1996) 259–261.Suche in Google Scholar
Long, Y.; Lin, X. T.; Zeng, K. L.; Zhang L.: Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat. Dis. Int. 3 (2004) 69–72.Suche in Google Scholar
Liu, J. P.; Zhu, M. H.; Shi, R.; Yang, M.: Radix sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. Am. J. Chin. Med. 31 (2003) 337–354.10.1142/S0192415X03001107Suche in Google Scholar
Cai, G. X.; Sun, Y. J.: Toxicity and field control effect of botanical pesticide matrine to mulberry pests. Sci. Sericulture. 37 (2011) 0538–0543.Suche in Google Scholar
©2017 Cheng Xing-An et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of potassium 1-methyl-1H-1,2,3,4-tetrazole-5-thiolate, C2H3N4SK
- Crystal structure of bis(3-(3-ethylureido)-N,N-dimethylpropan-1-aminium) bis (μ3-2-(hydroxymethyl)-2-(oxidomethyl)propane-1,3-bis(olato))-(μ6-oxo)-hexakis(μ2-oxo)-hexaoxo-hexavanadium(V) – dichloromethane (1/1), C27H60Cl2N6O23V6
- Crystal structure of bis(μ3-methanolato-κ3O:O:O)-bis(μ2-methanolato-κ2O:O)-dimethanol-bis{6,6′-(1,3-dihydroxyl-2-acetylpropane-1,3-diyl)bis(2-chloro-4-bromophenolato)}tetramanganese(III) C40H40Br4Cl4Mn4O16
- Synthesis and crystal structure of tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dibromo-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5)tetramanganese(III), C40H40Br8Mn4O16
- Synthesis and crystal structure tetrakis(μ2-methanolato)-dimethanol-bis(μ2-2-acetyl-1,3-bis(3,5-dichloro-2-oxidophenyl)propane-1,3-bis(olato)-κ5O1,O2,O3:O3,O4,O5), tetramanganese(III), C40H40Cl8Mn4O16
- Crystal structure of bis{5-methoxy-2-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O)}copper(II), C34H34CuN4O6
- Crystal structure of (E)-1-(2-hydroxy-3-{[2-hydroxybenzylidene]amino}phenyl)ethan-1-one, C15H13NO3
- Crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′′:O′′}tricobalt(II), C44H49Cl2Co3N6O12
- Crystal structure of bis{μ2-2,4-dichloro-6-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ5O:O,N,N′,O′}dicobalt(II) acetone solvate, C43H48Br4Co2N6O9
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis{μ2-4-chloro-2-(8-(4-(diethylamino)-2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenolato-κ6O:O,N,N′,O′:O′}trizinc(II), C44H49Cl2N6O12Zn3
- Crystal structure of bis{5-(N,N′-diethylamine)-5′-methoxy -2,2′-[ethylenediyldioxybis(nitrilomethylidyne)]diphenolato}-bis(μ2-acetato-κ2O:O′)trizinc(II), C46H56Zn3N6O14
- Crystal structure of tris(cyano-(hydrogen tris(3,5-dimethylpyrazolyl)borate))-iron(III) 4-methoxypyridinium monohydrate, C24H32BN10O2Fe
- Synthesis and crystal structure of bis{1-(((4-(1-(hydroxyimino)ethyl)phenyl)imino)methyl)naphthalen-2-olato-κ2O,N}copper(II), C38H30CuN4O4
- Crystal structure of (E)-1-(4-{[(E)-2-Hydroxy-1-naphthalenylmethylene] amino}phenyl)ethanone oxime, C19H16N2O2
- The pseudosymmetric crystal structure of 2-(2-naphthalenyl)-3-nitro-2H-1-benzopyran, C38H26N2O6
- Crystal structure of 2-benzoyl-3-(4-methoxyphenyl)cyclopropane-1,1-dicarbonitrile, C19H14N2O2
- N′-(5-ethoxycarbonyl-3,4-dimethyl-pyrrol-2-yl-methylidene)-4-hydroxybenzohydrazide monohydrate, C17H21N3O5
- The crystal structure of carbonyl-[4-(2,4-dichlorophenylamino)pent-3-en-2-onato-κ2N,O]-(triphenylphosphine-κP)rhodium(I), RhC30H25Cl2NO2P
- Crystal structure of (E)-5-(diethylamino)-2-(((1,1,2-trihydroxyethyl)iminio)methyl)phenolate, C15H24N2O4
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)(μ2-isophthalato-κ4O,O′:O′′,O′′′)cadmium(II)], C34H30N4O4Cd
- Crystal structure of 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]chinolizinium 3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate - methanol - water (1/1/1), C36H33NO10
- A single crystal study on 2-(methylcarbamoyl)benzoic acid, C9H9NO3
- Crystal structure of the salt 1,1′-(ethane-1,2-diyl)bis(1,4-diazabicyclo[2.2.2]octan-1-ium) diperchlorate, C14H28N4(ClO4)2
- Crystal structure of 2,5-bis((E)-2-(trifluoromethyl)benzylidene)cyclopentan-1-one, C21H14F6O
- Crystal structure of catena-poly[(μ2-benzene-1,4-dicarboxylato-κ2O:O′)-(1-ethyl-6-fluoro-7-(4-methylpiperazin-1-ium-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)zinc(II)] 1.25 hydrate, C25H26.5N3O8.25FZn
- Crystal structure of 1-methyl-1,4-diazabicyclo[2.2.2]octan-1-ium poly[aqua-bis(μ2-perchlorato-κ3O,O′:O′′)sodium], C7H17Cl2N2NaO9
- Crystal structure of trimethyammonium 2,6-dicarboxyisonicotinate monohydrate, C11H16N2O7
- Crystal structure of dodecaguanidinium bis(tetrapropylammonium) heptacarbonate, C43H128N38O21
- Crystal structure of poly[tetraaqua-bis(μ6-benzene-1,2,4,5-tetracarboxylato)nickel(II)diyttrium(III)]dihydrate, C20H16NiO22Y2
- Halogen bonds and π–π interactions in the crystal structure of 1,3,5-trifluoro-2,4,6-triiodobenzene–N,N-dimethylformamide (1/1), C9H7F3I3NO
- Crystal structure of guanidinium tetraethylammonium carbonate dihydrate, C10H30N4O5
- The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn
- Crystal structure of 8-((E)-((4-((E)-1-((benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one, C26H22N2O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}copper(II), C40H38CuN4O6
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri(p-tolyl)phosphine-κP)rhenium(I), C29H28O5PRe
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(benzyldiphenylphosphine-κP)rhenium(I), C27H24O5PRe
- Crystal structure of triethylammonium bis{3-(((3-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}manganese(III), C46H38MnN3O8
- Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14
- Crystal structure of bis(4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)-bis(2-(2-((2,6-difluorophenyl)amino)phenyl)acetate-κO)cadmium(II), C44H34N8CdCl4O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(pyridin-1-ium) catena-poly[diaqua-bis(μ2-3′,5′-dicarboxy-[1,1′-biphenyl]-2,5-dicarboxylato-κ2O:O′] dihydrate, C48H42O22N2Ca
- The crystal structure of (S)-2-benzylsuccinic acid, C11H12O4
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)yttrium(III)], C26H17N2O9Y
- Crystal structure of ethyl 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate, C14H17N3O2
- Crystal structure of tetrakis(methanol-κO)-bis{μ2-3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olate-κ4O,N;O′:O′}dizinc(II), C38H38Zn2N2O14
- Crystal structure of bromido(4,4′-dimethoxy-2,2′-bipyridine-κ2N,N′)(isopropyl(diphenyl)phosphane-κP)copper(I), C27H29BrCuN2O2P
- Crystal structure of bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene, C41H64B2O4
- Crystal structure of 11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinoline-10-carbaldehyde - a julolidine derivative, C16H15NO3
- Crystal structure of 1,3-dimethyl-5,5-dibenzylbarbituric acid, C20H20N2O3
- Crystal structure of bis(N,N,N-trimethylethanaminium) poly[bis(μ2-heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14
- Crystal structure of 1-{4-[(5-Chloro-2-hydroxy-benzylidene)amino]phenyl} ethanone O-ethyl-oxime, C17H17ClN2O2
- The crystal structure of methyl N-(4-bromophenyl)carbamate, C8H8BrNO2
- Synthesis and crystal structure of 1-{4-[(2-hydroxy-benzylidene)amino]phenyl}ethanone oxime, C15H14N2O2
- Crystal structure of 1,1′-butanebis(3-methyl-1H-imidazol-3-ium)bis(hexafluorophosphate), C12H20F12N4P2
- (E)-N-benzylidene-3-(benzylthio)-5-p-tolyl-4H-1,2,4-triazol-4-amine, C23H20N4S
- Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2
- Crystal structure of 5,5-difluoro-10-(4-fluorophenyl)-1,3,7,9-tetramethyl-5H-4l4,5l4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine - a Z′ = 3 structure, C19H18B2F3N2
- The crystal structure of the Matrine derivative: 12-(1H-indol-1-yl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one hydrate, C23H29N3O
- The crystal structure of tris(μ2-1,3-bis(4,4,4-trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-κ2O,O′)dilutetium(III), C50H38F18Lu2O16
- The crystal structure of (Z)-2-(3-(2-(4-chlorobenzoyl)hydrazono)-2-oxoindolin-1-yl) acetic acid, C17H12ClN3O4
- Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2
- The crystal structure of N,N-dimethyl-2,6-di-p-tolylpyrimidin-4-amine, C20H21N3
- The crystal structure (E)-4-methyl-N′-(2-nitrobenzylidene)benzenesulfonohydrazide, C14H13N3O4S
- Crystal structure of catena-poly[(μ2-1,3-bis(benzimidazol-1-yl)propane κ2N:N′)-(μ2-5-methoxyisophthalato-κ2O:O′)zinc(II)] hydrate, C26H24ZnN4O6
- The crystal structure of (E)-N′-(quinolin-2-ylmethylene)furan-2-carbohydrazide monohydrate, C15H13N3O3
- Crystal structure of 2,8-diphenyl-3,7,9-trioxa-1-azaspiro[4.5]dec-1-ene, C18H17N1O3
- Crystal structure of diethyl 2-(4-chlorophenyl)-1,3-dioxane-5,5-dicarboxylate, C16H19ClO6
- Crystal structure of 1-(carboxymethyl)-1H-benzo[d][1,2,3]triazole 3-oxide, C8H7N3O3
- Crystal structure of (acetylacetonato-κ2O,O′)-(2-amino-6-chlorobenzoato-κO)-oxido(1,10-phenanthroline-κ2N,N′)vanadium(IV) – trichloromethane (1/1)
- Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one, C17H12Cl2O
- The crystal structure of trans-dibromido-bis(pyridine-κN)platinum(II), C10H10Br2N2Pt

